Restoration actions may include different degrees of intervention to recover the structure and function of degraded tropical rain forest. Minimal intervention includes actions to stop disturbance so natural succession may take place whereas maximal intervention involves the establishment of restoration plantings (SER 2004, Morrison & Lindell 2011, Martínez-Garza et al. 2016). Many studies have quantified the natural recovery of vegetation of the rain forest after disturbance (e.g. Uhl 1987, Martínez-Ramos & García-Orth 2007) whereas other studies have evaluated the performance of tree species in plantings (e.g. Davidson et al. 1998, Hooper et al. 2002, Carpenter et al. 2004, dos Santos et al. 2006, Douterlungne et al. 2010). However, we are not aware of any study that compares the performance of naturally regenerating seedlings (i.e. recruits) and transplants in the same restoration setting to evaluate the success of minimal versus maximal restoration intervention.
Under minimal restoration intervention, natural succession takes place and pioneer species may establish. Pioneer tree species are those that colonize naturally in early successional environments, due to the high dispersal capacity of their small seeds and their rapid growth rates associated to high availability of resources (Swaine & Whitmore 1988, Whitmore 1989). Given that restoration plantings are expensive, pioneers are frequently selected because of high survival in such conditions (Davidson et al. 1998, dos Santos et al. 2006). On the other hand, late-successional non-pioneer species do not naturally recruit in early successional environments: they have large seeds dispersed by animals that do not cross open areas (Westoby 1998, & Howe 2003). However, some non-pioneers may perform as well as pioneers when transplanted to restoration areas in the tropics (Hooper et al. 2002, Carpenter et al. 2004) and they may arrive at older restoration plantings (De la Peña et al. 2013). Given that pioneer species naturally establish in early successional habitats, it is expected that they outperform non-pioneer species early in restoration settings. However, as succession takes place, may pioneers continue to outperform non-pioneers irrespective of their origin, recruited or transplanted?
When nursery-raised seedlings are transplanted, they may experience stress known as trans- plant shock. Transplant shock is defined as low survival and growth of seedlings transplanted to the field compared to naturally recruited seedlings (Close et al. 2005), usually due to the low contact between the roots of the seedlings and the soil (Burdett 1990). Transplant shock had been recorded in temperate forest (see for example, Bernier 1993); however, in the rain forest of Chiapas, Mexico, transplanted individuals realized higher survival than recruits established after direct seeding (Douterlungne et al. 2010). Transplant shock has never been evaluated for tropical tree species exposed to severely adverse environmental conditions of degraded areas; there, smaller recruits may be more affected by adverse soil conditions than larger transplants even after experiencing transplant shock.
Planning for restoration plantings in tropical rain forests involve the challenge of selecting appropriate species from around 53,000 species (Slik et al. 2015). Species selected for restoration plantings are usually those with high survival and growth rates in early successional environments (Vázquez-Yanes et al. 1999); however, evaluation of performance have been done for few species, mainly for forestry use (Evans & Turnbull 2004). Survival and growth of species in early successional environments may be predicted by their functional traits; a functional trait is a measurable property of the organisms that strongly influences its performance (McGrill et al. 2006). For example, in the cloud forest, transplanted species with large leaves and high dry matter content (Saldaña-Acosta et al. 2009) or in the rain forest, transplanted species with deep canopies (Martínez-Garza et al. 2013b) showed higher performance in restoration plantings. On the other hand, tree species that recruit naturally early in succession have functional traits associated to high dispersal capacity, as small seed size (Lohbeck et al. 2013). Also, seed size is related to establishment: larger seeds have higher establishment capacity (Coomes & Grubb 2003, Poorter et al. 2008). Plant strategies of both, transplants and naturally recruited trees include multiple functional traits (multivariate plant strategies; Weiher et al. 1999, Violle et al. 2007) which may coincide, so both successfully establish in early successional environments irrespective of their origin. Alternatively, individual functional traits that predict performance may be different among transplanted and naturally recruited trees, therefore individual traits may better predict performance of tree species than multivariate plant strategies.
The main objective of this study is to propose management and restorationfor restoration plantings of tropical tree species by using life-history, the origin of the seedlings and plant functional traits to predict survival and growth rates of 15 tropical tree species in 5 years old restoration settings. We tested the following hypotheses: 1) pioneer species (both recruits and transplants) perform better than non-pioneers; 2) transplants have lower performance than recruits because of “transplant shock”; 3) multivariate functional traits predict performance of tree species better than individual traits irrespective of seedling origin; multivariate plant strategy for high performance in early successional environments include larger leaves, deep canopies and small seeds. Finally, we give recommendations to select tree species for restoration plantings under different scenarios based in the life history category, functional traits, number of recruits registered during 5 years, and performance of tree species.
Methods
Research site. The Los Tuxtlas Biological Station (LTBS) lies within a reserve of 640 ha of lowland tropical rain forest in the state of Veracruz, southeast Mexico. The forest has a closed canopy ~35 m high where Nectandra ambigens (S.F. Blake) C.K. Allen (Lauraceae) is the most common species in the canopy and Pseudolmedia oxyphyllaria Donn. Sm. (Moraceae) and Astrocaryum mexicanum Liebm (Arecaceae) are abundant in the mid-canopy and understory, respectively (Bongers et al. 1988). Mean annual rainfall at the station from 1997 to 2007 was 4,275 ± 404 mm; the dry season extends from March to May and the rainy season from June to February; the mean annual maximum temperature was 28 °C (R. Coates, National University of Mexico, Veracruz, personal communication). Our site is a cow pasture that was grazed intensively for 30-40 years, and is embedded in primary and secondary forest on a hill 180-260 m above sea level, facing north-east to the Gulf of Mexico. Where cattle have access, pasture grasses are a closely cropped, 3-10 cm high mix of exotic [Cynodon plectostachyus (K. Schum.) Pilg., Capriola dactylon (L.) Kuntze, Brachiaria decumbens Stapf and Brachiaria brizantha (A. Rich.) Stapf] and native grasses [Axonopus compressus (Sw.) P. Beauv., Panicum spp., Paspalum conjugatum P.J. Bergius]. The soil is sandy loam classified as Vitric Andosol originating from basalt and andesite mixed with volcanic ash; the texture is mainly clay (48.5 %) with an acidic pH (4.9) (González-Soriano et al. 1997).
Experimental settings. A 3 × 8 grid of 24 fenced plots (30 × 30 m, each plot separated by 35 m) was established along an altitudinal gradient in a 12-ha pasture of the agricultural colony of Ruiz Cortines in August 2006, adjacent to the LTBS. Barbed wire fences were held up by living poles of Gliricidia sepium (Jaq.) Kunz (Fabaceae) every two meters. Plots on the grid are within 500-1,200 m of the edge of the LTBS and 90 m from the nearby secondary forest. Standing trees within the 12 ha were cut in 2006 (Howe et al. 2010). From September to December 2006, 144 seedlings of each of 24 native tree species were transplanted to 16 of the 24 plots (see details in Martínez-Garza et al. 2013a). Seedlings were 4-7 months old at the time of planting, and their average height was 17.8 cm (range 5-40 cm across species). The entire area for the plantings was 0.92 ha (16 plots), and the area where natural recruitment could occur was 1.38 ha, includ- ing eight additional plots where no plantings were established.
Data collection. For this study, we selected 15 species following three criteria: 1) given that individuals were considered replicates, only species with more than 17 individuals across all plots were selected; 2) transplanted species that were also recorded as recruited and 3) a similar number of pioneer and non-pioneer species. We selected 11 species transplanted and eight registered as naturally recruited; four species had individuals from both origins (Table 1). A priori distinction between pioneer and non-pioneer species was based on the literature (Martínez-Ramos 1985, Popma et al. 1992, Ibarra-Manríquez & Oyama 1992). This study includes two censuses: June 2007 and 2011; at each census, we measured height and diameter at the base of all individuals transplanted and recruited of the 15 tree species selected. Growth rates were calculated as the difference between the first measurement and the last, divided by the number of months elapsed. Performance of all tree species transplanted (Martínez-Garza et al. 2013b) and richness and abundance of recruits (de la Peña-Domene et al. 2013) has been evaluated before. The present study adds an explicit comparison of survival and growth rates of recruits with a pertinent subset of the transplanted species (see above) and their functional traits.
Table 1 Family, Life history and the Origin of the seedling of 15 tropical tree species in restoration settings at Los Tuxtlas, Veracruz, Mexico.
Data Analysis. Performance of pioneer and non-pioneer species.- Two one-way analyses of variance (ANOVA) were carried out to test for differences in diameter and height growth between pioneer and non-pioneer species. The life history category was the independent variable. The proportion of survivors for each species at all the plots (seven pioneer species and eight non-pioneer species) was analyzed with a General Linear Model (GLM). For the analysis of growth rates, individuals were used as replicates (N = 319 pioneers and N = 277 non-pioneers): given that they were growing in mixed stands at each plot under variable soil conditions, we consider the performance of each plant to be independent.
Performance of transplants and recruits.- To evaluate transplant shock we tested survival, diameter and height growth rates of recruits and transplants for the four species with individuals in both groups (Cecropia obtusifolia, Cedrela odorata, Heliocarpus appendiculatus and Albizia purpusii). For these species, we had more transplants than recruits; therefore, we randomly selected the number of transplants necessary to balance the data points we had for recruits of each species (up to 15 individuals for each species; see Appendix 2). T-tests were used to evaluate survival and growth rates in diameter and height for recruits and transplants. Height and diameter growth rates of Albizia purpusii and Cecropia obtusifolia were transformed with the natural logarithm to homogenize variances. Overall species, life-history was not included in the analysis because variances for the interaction with origin of the seedling could not be homogenized. To evaluate survival overall recruits (N = 8 species) and transplants (N = 11 species), species were used as replicates; individuals at all the plots were considered as one population. To evaluate growth rates overall recruited and transplanted species, a subset of individuals (up to 15 individuals for each species) were chosen randomly to balance sample size (see above; N = 91 recruits and N = 138 transplants).
Functional traits as predictors of species performance.- To evaluate multivariate plant strategies, a principal components analysis (PCA; Dunteman 1989) was ran using 12 functional traits related to leaf display (Leaf Area, Leaf Mass per unit Area [LMA], Leaf Dry Matter Content [LDMC]), tree architecture (Crown Area, Crown Length, Height to the First Branch [Height 1st B], Maximal tree height), and reproduction (Flower size [Flower S], Fruit size [Fruit S], Fruit Weight [Fruit W], Seed Weight [Seed W] and Seed Number per Fruit [Seed Number]) (Appendix 1). Leaf and architectural traits were measured in the same plants measured for performance whereas reproductive traits were taken from Ibarra-Manríquez & Oyama (1992). Given that tree architecture changes when trees increase in size, plants were compared at a standardized size of 30 mm of stem diameter at the base. To this end, species-specific regression curves were obtained relating the architectural trait of interest to stem diameter. When the regression of a trait was not significant we used the average of stem diameter calculated with the five closest values to 30 mm stem diameter. We used all the single functional traits and the two first axes of the PCA to predict tree performance using regressions.
Species for different restoration strategies.- to give restoration and management recommen- dations for different scenarios, tree species were grouped based in their life-history category, survival, growth rates and number of individuals naturally recruited during 5 years in the res- toration settings.
All analyses were done with STATISTICA (StatSoft 2004). Variables were back transformed to report original values in the result section. Means and standard deviation of variables are shown throughout results. From now on, species are mentioned by genus only.
Results
Performance of pioneer and non-pioneer species. Survival varied six times among species. The non-pioneer Albizia showed the highest survival (100 %) whereas the non-pioneer Brosimum showed the lowest (15.6 %; Appendix 1). Overall recruits and transplants, pioneer species showed higher survival (75.61 ± 8.43 %, N = 7) than non-pioneer species (50.45 ± 7.88 %, N = 8); the GLM reveled significantly higher survival of pioneer species (F(1,13) = 4.75, P < 0.05).
Diameter growth varied an order of magnitude among species. The pioneer Ochroma had the highest diameter growth rates (11.6 ± 6.4mm/month) whereas the non-pioneer Brosimum realized the lowest (0.5 ± 0.2 mm/month; Appendix 1). Pioneer species showed two times higher diameter growth rates (3.8 ± 3.2 mm/month) than non-pioneer species (1.59 ± 1.03 mm/month). The analysis of variance reveled significantly higher growth rates in diameter of pioneer species compared with non-pioneers (F(1,595) = 6.44, P < 0.03).
Height growth rates varied three times among tree species. The pioneer Heliocarpus showed the highest growth rates in height (11.0 ± 3.0 cm/month) whereas the non-pioneer Amphitecna showed the lowest (3.40 ± 1.09 cm/month). Pioneers showed significantly higher growth rates in height (9.39 ± 3.66 cm/month) than non-pioneer species (5.85 ± 1.59 cm/month; F(1,595) = 5.98, P < 0.02).
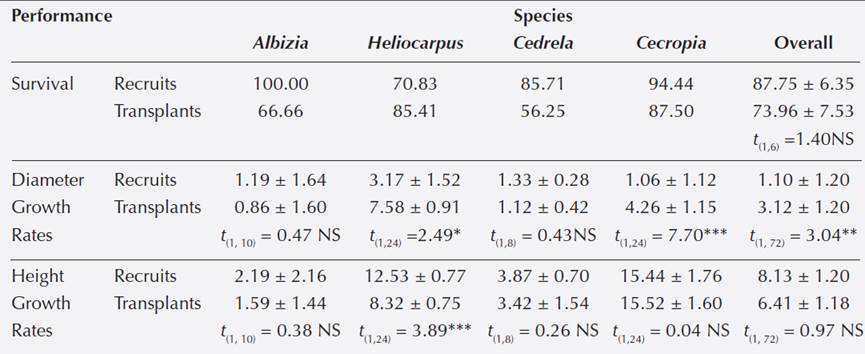
Performance of recruits and transplants. Species with recruits and transplants (4 spp).- For three pioneer species (Heliocarpus, Cecropia and Cedrela) and one non-pioneer species (Al- bizia), we recorded performance of recruits and transplants. Transplants of Cedrela and Albizia suffered > 30 % decrease in its survival compared to naturally recruited individuals whereas Cecropia experienced a 7 % reduction of survival when transplanted (Table 3). On the other hand, Heliocarpus showed an increment of 17 % in survival when transplanted (Table 3). Over- all these four species, recruits showed higher survival (87.75 ± 6.35 %) than transplants (73.96 ± 7.53 %), however, analysis revealed that differences in survival were not statistically significant (t (1,6) = 1.40, P > 0.74).
Table 2 Pearson correlation coefficients (r) between 12 functional traits and survival (%), growth rate in height (cm/month) and diameter (mm/month) for 15 tree species recruited and transplanted to restoration settings at Los Tuxtlas, Veracruz, Mexico. Correlations with the axes 1 and 2 from the PCA (multivariate trait axes; see Fig. 2) are also shown. LMA refers to leaf mass per unit area, and LDMC to leaf dry matter content. Coefficients of determination (r2) are shown in the figures for selected correlations.
* P < 0.05, ** P < 0.01, *** P < 0.001
Table 3 T-test for Survival (%), growth rates in Diameter (mm/month) and Height (cm/month) of individuals naturally recruited and transplanted to restoration settings at Los Tuxtlas, Veracruz, Mexico. A t-test of perfor- mance overall the four species is also shown. Means and standard errors are shown.
* P < 0.05, ** P < 0.01, *** P < 0.001; NS = Not significant
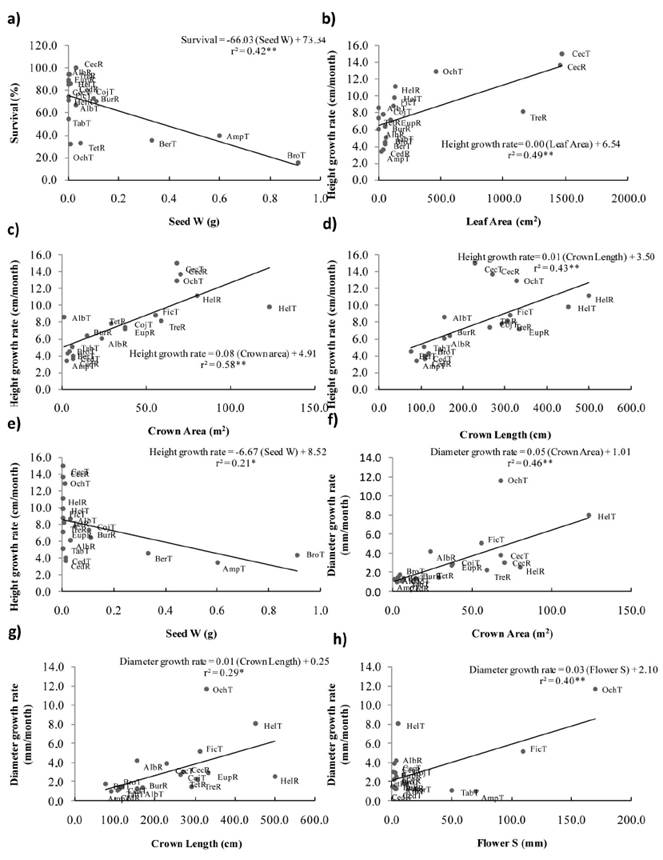
Figure 1 Correlations between performance and individual functional traits: Survival with (a) Seed weight; Height growth rates with (b) Leaf Area, (c) Crown Area, (d) Crown Length and, (e) Seed Weight. Diameter growth rates with (f) Crown Area, (g) Crown Length and (h) Flower Size. Values of r2, regression lines and equations are shown. Acronyms refer to the first three letter of the genus name of 15 species recruited (R) or transplanted (T) on restoration settings at Los Tuxtlas, Veracruz, Mexico.
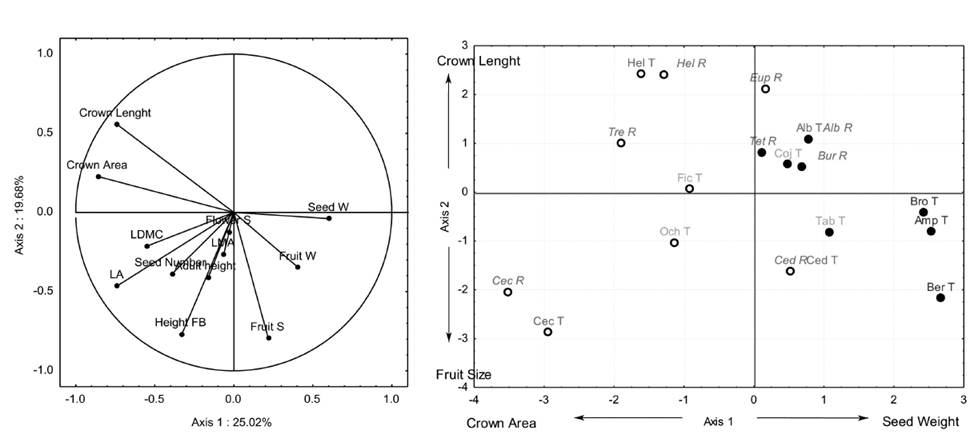
Figure 2 (a) Trait loading and (b) species scores of PCA axes 1 and 2 of an ordination based on 12 functional traits of 15 tree pioneer (open symbols) and non-pioneer (close symbols) species recruited (R) or transplanted (T) on restoration settings at Los Tuxtlas, Veracruz, Mexico. Groups of species for different restoration strategies are shown: good recruiters (red letters), good transplants (green letters) and poor transplants (blue letters). Acronyms refer to the first three letter of the genus name.
The non-pioneer Albizia and the pioneer Cedrela showed a similar 27 % decrease in diameter growth rates when transplanted, however, diameter growth rate were not statistically different by origin for both species (Table 3). On the other hand, transplanted individuals of Heliocarpus and Cecropia showed significantly higher diameter growth rates (7.58 and 4.26 mm/month respectively) than recruits (3.17 and 1.06 mm/month; Table 3). Overall the four species, recruits showed significantly lower diameter growth rates (1.10 ± 1.20 mm/month; N = 36) than transplants (3.13 ± 1.20 mm/month; N = 36) (t (1,72) = 3.04, P < 0.01; Table 3).
Transplants of Albizia, Cedrela and Cecropia showed 30, 10 and 9 % higher growth rates in height respectively, than naturally recruited individuals; however, height growth rates were statistically similar for these species (Table 3). Heliocarpus transplants decreased significantly its height growth rates by 11 % compared to recruits (Table 3). Overall the four species, recruits showed higher height growth rates (8.13 ± 1.20 cm/month) than transplants (6.41 ± 1.18 cm/ month) but differences were not statistically significant (t (1,72) = 0.97, P > 0.73).
All species (11 spp).- Survival of 11 transplanted species (5 pioneer and 6 non-pioneer species) varied 5 times: from 15 % (Bernoullia) to 87.5 % (Cecropia). Survival of naturally recruited species (5 pioneer and 3 non-pioneer species) was > 70 % excepting for the non-pioneer Tetrorchidium (33 %; Appendix 2). Overall species, recruits showed significantly higher survival (80.51 ± 19.25 %) than transplants (56.32 ± 22.31 %; F(1,18) = 5.43, P < 0.03).
Diameter growth rates of transplanted individuals of 11 species varied 12 times, from 0.93 ± 0.72 mm/month (Amphitecna) to 11.6 ± 5.42 mm/month (Ochroma) whereas diameter growth rates of recruits (eight species) varied 4 times from 1.19 ± 0.61 mm/month (Cedrela) to 4.16 ± 3.29 mm/month (Albizia; Appendix 2). Overall species, transplanted individuals showed higher diameter growth rate (1.88 ± 1.10 mm/month; N = 138) than recruits (1.51 ± 1.12 mm/month; N = 91); however, analysis revealed that differences in diameter were not statistically significant; F(1, 227) = 2.12, P > 0.15).
Height growth rates of recruits and transplants had similar variation among species, ca. 4 times; Cecropia showed the highest height growth rates of recruits (13.64 ± 5.84 cm/month) and transplants (14.98 ± 60.32 cm/month) whereas Cedrela (3.59 ± 1.55 cm/month) and Amphitecna (3.4 ± 1.09 cm/month) showed the lowest height growth rates for recruits and transplants respectively (Appendix 2). Overall species, recruits realized significantly higher growth rates in height (7.73 ± 1.08 cm/month) than transplants (5.70 ± 1.07 cm/month; F(1,227) = 7.58, P < 0.001).
Functional traits as predictors of species performance. Considering 8 species of recruits and 11 species of transplants, survival was negatively correlated with Seed Weight (r = -0.65, P < 0.01; Figure 1a; Table 2). Height growth rate was positively correlated with Leaf Area (r = 0.70, P < 0.01; Figure 1b), Crown Area (r = 0.76, P < 0.001; Figure 1c), Crown Length (r = 0.66, P < 0.01; Figure 1d) and negatively with Seed Weight (r = -0.46, P < 0.05; Figure 1e). Diameter growth rate was positively correlated with Crown Area (r = 0.68, P < 0.01; Figure 1f), Crown Length (r = 0.54, P < 0.05; Figure 1g), and Flower Size (r = 0.63, P < 0.01; Figure 1h).
The first two axes of the PCA explained 44.70 % of the variation among species (Figure 2a). The PCA axis 1 was related to decreasing Crown Area and increasing Seed Weight while axis 2 was related to increasing Crown Length and decreasing Fruit Size (Figure 2b). Axis 1 of the PCA was positively correlated with survival (r = 0.56, P < 0.05; Figure 3a), Height Growth rates (r = 0.86, P < 0.001; Figure 3b) and Diameter Growth rates (r = 0.47, P < 0.05; Figure 3c; Table 2). Axis 2 of the PCA was not correlated with tree performance (Table 2).
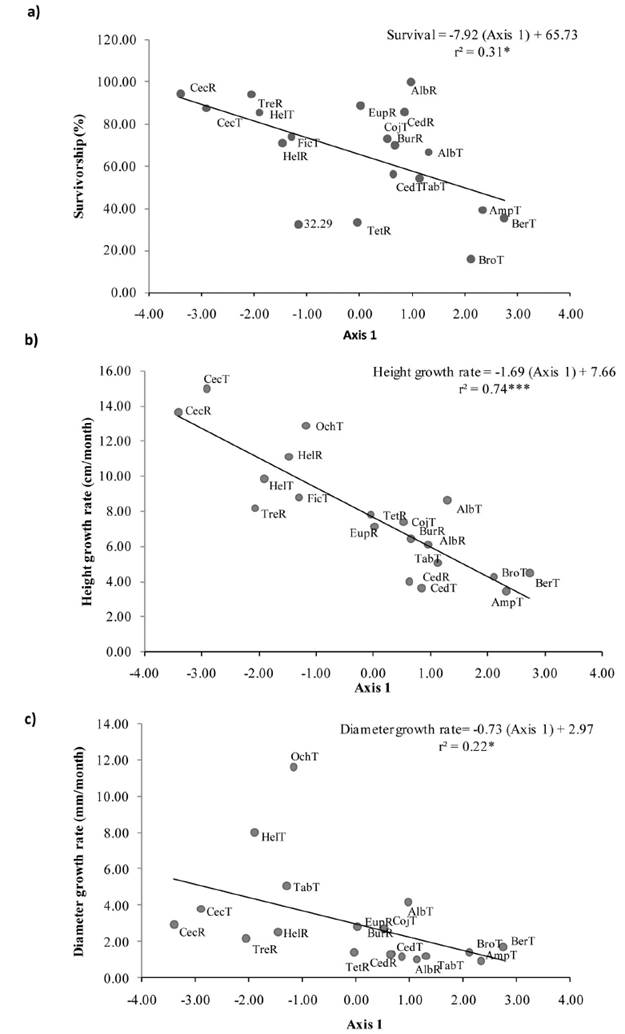
Figure 3 Correlations between performance and multivariate functional traits. PCA axis 1 with (a) Survival, (b) Height Growth rates and, (c) Diameter growth rates. Values of r2, regression lines and equations are shown. Acronyms refer to the fifirst three letter of the genus name of 15 species recruited (R) or transplanted (T) on restoration setting at Los Tuxtlas, Veracruz, Mexico.
Groups of species for different restoration strategies. Tree species were divided in three groups based on their life history category, performance of recruits and transplants, functional traits and the number of new recruits recorded during the first five years of the experiment (Appendix 2). The first group was named “Good recruiters” and it included five pioneer and three non-pioneer species. For these eight species, we recorded, 31 ± 17 recruits in average, excluding Bursera which recruited most individuals (337 individuals; Appendix 2). These species showed high sur- vival (77 ± 19.19 %), height growth rates (8.44 ± 3.49 cm/month; Appendix 2) and long crowns (Figure 2b). The second group was named “Good transplants” and included two pioneer and two non-pioneer species. The species in this group had intermediate values of survival (58.32 ± 19.59 %), height growth rates (8.50 ± 3.29 cm/month) and functional traits (Figure 2b). They showed high diameter growth rates (5.10 ± 4.01 mm/month) and only one recruit was registered for this group (Appendix 2). The third group was named “Poor transplants” and included three non-pioneer species. Species in this group had the lowest survival (30.13 ± 12.72 %) and height growth rates (4.03 ± 0.55 cm/month) and the heaviest seeds (Figure 2b). No recruits were registered for this group (Appendix 2).
Discussion
To guide the selection of species for restoration plantings, the life-history, the origin of the seedlings (recruits or transplants), and plant functional traits were used to predict performance of 15 tropical tree species. Overall 15 species, pioneer species had higher performance than non-pioneers whereas recruits had higher performance than transplants. The crown area was the individual functional traits most powerful to predict height growth rates whereas the multivariate plant trait axis 1 related to seed weight and crown area showed even higher power of prediction of height growth rates. Species that recruit well in early successional environments do not need to be planted whereas transplants with low survival and growth rates should be introduce at later stages of succession.
Performance of pioneer and non-pioneer species. According to our predictions, pioneer showed higher survival and growth rates than non-pioneers after 5 years. Our results agree with what has been reported in other studies: for example, in a rain forest in Brazil, seven pioneer species showed higher survival (95 %) compared with one non-pioneer species (65 %) after 2 months of planting (dos Santos et al. 2006). In the humid forest of Ecuador’s Amazonia, seven pioneers showed twice the survival (90 %) of eight non-pioneers (45 %) after 2.5 years of planting (Davidson et al. 1998). In plantings located in Ecuador, Brazil and Mexico, at the short term (< 3 years of planting) pioneer species had higher growth rates than non-pioneer species (Davidson et al. 1998, dos Santos et al. 2006, Román-Dañobeytia et al. 2012). Pioneer species seem to realize higher survival and growth rates at the short-term (< 5 years of planting) under the aggressive environmental conditions of pastures.
Performance of recruits and transplants. According to studies in the temperate forest, plantings may experience transplant shock which is observed as a decrease in performance when planted in the field (Close et al. 2005). This negative effect may last from a couple of years to decades (South & Zwolinski 1997). For this study, overall the four species with naturally recruited individuals and transplants, recruits showed similar survival and height growth rates compared to transplants. Therefore, it seems that, transplant shock in terms of these two measures of performance had been overcome after 5 years. This is the first record of transplant shock length evaluated for native tree species in the tropics.
Transplants may suffer from a low contact between the roots and the soil which causes water limitation (Burdett 1990). In this scenario, drought stress may trigger an increment in the growth of roots into deeper soil layers to improve uptake of water (Larcher 1985). In our study, transplants might have suffered some water limitation. This is supported by higher diameter growth rates of transplant compared to recruits; basal diameter has been related to root biomass for juvenile trees growing in experimental plantings (Martínez-Garza et al. 2013a). Evaluation at species level revealed a contrasting response of Heliocarpus: recruits of this species showed higher height growth rates than transplants suggesting that this species have not overcome trans- plant shock. For the other species, the increment in stem diameter of transplants in response to drought stress may have allowed them to reach similar survival and height growth rates com- pared to recruits, ending transplant shock after 5 years.
Under minimal intervention, pioneer species arriving by dispersal events initiate succession (Holl 1999, Martínez-Garza et al. 2009, Chazdon 2014) whereas plantings, as a maximal restoration intervention are frequently established with pioneer transplants (Lamb et al. 2005, Duterlounge et al. 2008). Others suggest to plant as many pioneer and late-successional species as possible (framework species; Tucker & Murphy 1997) or mostly late-successional species to skip early successional stages (Martínez-Garza & Howe 2003). If recruited tree species may outperform transplants, why spend resources establishing plantings? Far away from old growth forest, composition of regenerating forest may remain with few species for decades (Finegan 1996; pioneer desert, Martínez-Garza & Howe 2003), on the other hand, plantings implicate choosing tree species to accelerate and somehow guide succession. Further, plantings ameliorate environmental conditions and favor recruitment of mostly pioneers at the beginning (de la Peña et al. 2013) and also non-pioneer species at later stages (de la Peña et al. 2014). Plantings in island arrangements have also proven useful to favor natural recruitment in large areas at lower cost (Zahawi et al. 2013). To maximize natural recruitment at the lowest cost, different densities and arrangement of plantings should be tested; however, selection of successful species from a large pool (~53,000 species) for different scenarios is still a challenge.
Functional traits as predictors of the species performance. The multivariate plant strategy related to seed weight and crown area predicted performance better than individual functional traits, according to our expectation. By individual traits, species with smaller seed weight had higher survival and height growth rates whereas species with high crown area had higher diameter growth rates. In the multivariate trait space, species with high scores on the first strategy axis were the ones with large seeds contained in heavy fruits and smaller crown areas as Bernoullia, Amphitecna and Brosimum. Reproductive traits as high seed mass and few seeds per fruit are characteristics of non-pioneers species that establish in small gaps within the forest; they show lower inherent growth rates and poor dispersal (Martínez-Ramos 1985, Denslow 1987, Paine et al. 2015) and therefore, they do not recruit in early successional environments (de la Peña et al. 2013). Further, they show a slow life history which refers to low population growth rates strongly influenced by survival (Adler et al. 2014). The second axis separated species that developed deep crowns versus species with small fruits as Cecropia and Ochroma. These two species are well-known pioneers (Martínez-Ramos 1985), they have small seeds which move easily in fragmented landscapes (Estrada et al. 1984) and fast life histories with high population growth rates influenced by fecundity (Adler et al. 2014). Individual traits and multivariate strategies showed that pioneer or non-pioneer species with small seeds and large crowns may show higher performance in early successional environments as natural recruits or if transplanted, however, not all species need to be transplanted.
Groups of species for different restoration strategies. Tropical deforestation has multiple origins and causes corresponding to various biophysical and socio-economic conditions and therefore, it generates different scenarios for restoration (reviewed in Ceccon et al. 2015). Minimal intervention may be enough in some cases (Holl & Aide 2011) whereas other situation may call for maximal intervention with the use of plantings. To select successful tree species for restoration plantings, we usually choose species known to have higher performance in early successional environments. However, those few pioneer species with well-known performance in early successional environments may not need to be planted: in our study, the species in the Good recruiters category are natural colonizers (Dalling et al. 1997). They produce many seeds with high dispersal ability; for example, an average of 0.64 seeds/m2 of these species where recorded arriving to experimental plots (Martínez-Garza et al. 2009). Even when all these species are also successful if planted (Vázquez-Yanes et al. 1999), we suggest favoring its natural recruitment in landscapes including forest fragments. Plantings (maximal intervention) are more expensive than establishing cattle exclusions to favor recruitment (minimal intervention). For example, each individual of Cedrela odorata propagated in a Mexican nursery costs $0.84 USD (reviewed in Guzman-Luna 2012); to establish the plantings, additional cost of transplant should be added. Further, the Good recruiters showed a seed size that varied 2 orders of magnitude (Appendix 1); given that only small seeds arrive more than 10 m from the forest border to open areas (Aide & Cavelier 1994, Martínez-Garza & Gonzalez-Montagut 1999), direct seeding should be considered to favor recruitment of larger seeded species (Camargo et al. 2002, Cole et al. 2011). Finally, these species develop large crowns that shade out the grasses and may serve as attractors of frugivorous fauna that bring large forest seeds from the mature forest.
The species in the Good transplants category are pioneers or non-pioneer species with intermediate values of all functional traits (Figure 2b); they were not registered in the seed rain for the first year of the experiment except for Ochroma, for which a seed rain of 0.01 seeds/ m2 was recorded (Martínez-Garza et al. 2009). These species may show higher performance as transplant than by direct seeding (Douterlungne et al. 2010). Therefore, even when the species in this group are not usually recorded as establishing in early successional environments, they are successful if transplanted. Finally, the species in the Poor transplants category are all non-pioneers with large seeds (≥ 0.3 g; Appendix 1) and they have never been recorded in the seed rain of early successional environments (Martínez-Garza et al. 2009). We suggest transplanting these species or testing its introduction by direct seeding once a canopy has developed.
To recover the structure and processes of tropical forest, we recommend taking into account landscape matrix: restoration areas in complex matrices, those than include isolated trees, living fences or forest fragments should be excluded from disturbance to favor natural recruitment. In degraded isolated areas invaded by exotic grasses, plantings of a mix of small-seeded pioneer and non-pioneer species should be established for a rapid recovery of a canopy to suppress grasses. In any scenario, we recommend further augmentation of tree diversity with direct seeding or planting of non-pioneer species with large seeds after a canopy develops.











 text new page (beta)
text new page (beta)