Introduction
Breast cancer is the most common type of cancer that currently affects women in the world. One of the main problems of breast cancer, like many solid tumors, is its ability to metastasize, this being the leading cause of death in cancer patients. One of the characteristics of metastasis is increased cell migration, which is the main process for cancer cells to invade and metastasize1.
Metastasis is the spread of cancer cells from the original site (primary tumor) to other sites in the body to form a secondary tumor; if metastasis did not occur, surgical removal of the tumor would be enough to control the majority of malignant tumors2. The ability to metastasize requires at least five steps: (1) invasion by tumor cell into adjacent structures, such as basement membranes; (2) passage into the blood or lymphatic vessels, with the release of tumor cells into the circulation (intravasation); (3) survival of tumor cells in circulating blood and overcoming immune surveillance; (4) escape from circulation (extravasation); and (5) implantation in a different tissue with the formation of a new tumor focus2. Increased migration is the main process for cancer cells to invade and metastasize during the development of the disease1.
Cell migration is the process by which cells move through tissues or on the surface of a culture dish, in which cytoplasmic expansions take part, it is a cyclical process that results from the combination of extension-contraction and adhesion-disengagement cycles3. It involves the spatial and temporal coordination of cellular components and is involved in multiple processes such as inflammatory responses, embryogenesis, organogenesis, and wound healing. However, aberrant cell mobility contributes to the development of diseases such as metastatic cancer4.
There are two main types of cell migration: (1) individual cell migration, where cells from the epithelium undergo epithelial-mesenchymal transition (EMT) to become mesenchymal cells with the ability to migrate, and (2) collective cell migration, where several cells move together maintaining characteristics similar to epithelial cells which are not governed by EMT5.
EMT is the process by which cells lose their epithelial characteristics and gain mesenchymal properties6. Among the changes involved are: loss of cell polarity, acquisition of a migratory capacity, invasive capacity, resistance to apoptosis, and increased production of the extra cellular matrix (ECM) components7. Epithelial cells form polarized sheets that anchor to the basement membrane to maintain apical-basal polarity. In contrast, mesenchymal cells are embedded within the ECM8. This change in cellular behavior is mediated by a complex molecular regulation involving a large number of signaling pathways, some acting independently and others interconnected; the majority converge on the control of the expression of E-cadherin, whose down-regulation is the key molecular event in this process9. E-cadherin is a glycoprotein whose function is to help in calcium-dependent cell adhesion to form organized tissues due to the fact that it forms complexes with cytosolic proteins called catenins10. Several molecular differences have been observed between epithelial and mesenchymal cells. For example, mesenchymal cells express less E-cadherin compared to epithelial cells11. Based on this observation, one of the most studied markers to evaluate EMT is the decrease in the expression of E-cadherin12. In other words, a decrease in the expression of E-cadherin would be related to an individual cell migration and if the expression of E-cadherin is kept constant it can be related to a collective cell migration. On the other hand, it has been shown that prolactin (PRL) is capable of favoring the destruction of tumor cells in breast cancer through the activation of its receptor13. It was also reported that when some breast cancer cell lines, one of them MCF-7, were stimulated with PRL, a significant increase in cell migration was demonstrated14. Therefore, the objective of this study was to analyze the effect of PRL on the migration of MCF-7 breast cancer cells and on the expression of E-cadherin, to elucidate whether the migration of MCF-7 cells, by effect of the PRL, is individual or collective.
Methods
Cell culture
The MCF-7 breast cancer cell line (ATCC) was routinely cultured in sterile 90 × 20 mm Petri dishes (46 cm2 growth area; Corning) and using RPMI-1640 culture medium (Lonza), supplemented with 8% (v/v) of fetal bovine serum (FBS) (Biowest), 2 mM glutamine (Biowest), 1 mM sodium pyruvate (Biowest), and 1% (v/v) penicillin/streptomycin (Sigma-Aldrich) at 37°C in a humidified atmosphere with 5% CO2.
Cell migration assay
MCF-7 cells were seeded in 60 mm culture plates (21 cm2 growth area; Corning), at a density of 1 × 105 cells/cm2 in RPMI-1640 medium supplemented with 8% (v/v) of FBS (Biowest) and 1% (v/v) of penicillin/streptomycin (Sigma-Aldrich), and they were incubated at 37°C in an atmosphere of 5% CO2 until total confluence. Pressure was applied to the confluent monolayer of cells with a sterile razor to mark the start line, the cells were scraped to one side of that line and the cells were washed 3 times with PBS. Subsequently, the cells were maintained in RPMI-1640 medium supplemented with 1% (v/v) of FBS (Biowest), with or without PRL (Sigma-Aldrich) at a concentration of 2 nM. The medium and treatment were replaced with fresh medium every 24 h. Cell migration was monitored for 72 h, photos were taken of each well in a delimited area with a reflex camera (CANON t6) adapted to an inverted microscope. The migration area in square micrometers was measured with ImageJ software15.
Western Blot
Protein expression in cells was analyzed by western blot. Briefly, at the end of the cell migration assay at 72 h, the cells were lysed with a buffer containing 1% (v/v) NP-40, 10 % (v/v) glycerol, 5 M NaCl, 1 M Tris pH 8, and cOmplete Mini Protease Inhibitor Cocktail (Roche), the samples were kept at −80°C until their analysis. Protein concentration was determined spectrophotometrically using the Pierce BCA Protein Assay Kit (Thermo Scientific). 20 μg of proteins treated under denaturing conditions with Laemmli buffer were electrophoresed in denaturing polyacrylamide gels (SDS-PAGE) at 10% and transferred to a nitrocellulose membrane (Bio-Rad). The membranes were blocked for 1 h with 5% skim milk in 1% TBS-Tween. Subsequently, they were incubated for 18 h at 4°C with the corresponding primary antibodies: anti-E-cadherin dilution 1/200 (sc-8426 Santa Cruz Biotechnology) and anti-GAPDH dilution 1/500 (sc-25778 Santa Cruz Biotechnology). Subsequently, they were incubated with biotinylated anti-mouse secondary antibody (BA-2000, Vector Laboratories) and anti-rabbit (BA-1100, Vector Laboratories), both at a 1:200 dilution. Finally, the proteins were detected using avidin and biotinylated HRP from the Vectastain ABC kit (Vector Laboratories) and scanned using the ChemiDoc XRS + equipment (Bio Rad), the densitometry was analyzed with the software Image Lab 6.0.1. and the expression of the proteins was reported as relative density normalized to the loading control.
Results
PRL increases cell migration in MCF-7 cells
Stimulation of MCF-7 cells with PRL at a concentration of 2 nM significantly increased the area (square microns) of migrating cells after 72 h of treatment (Fig. 1).
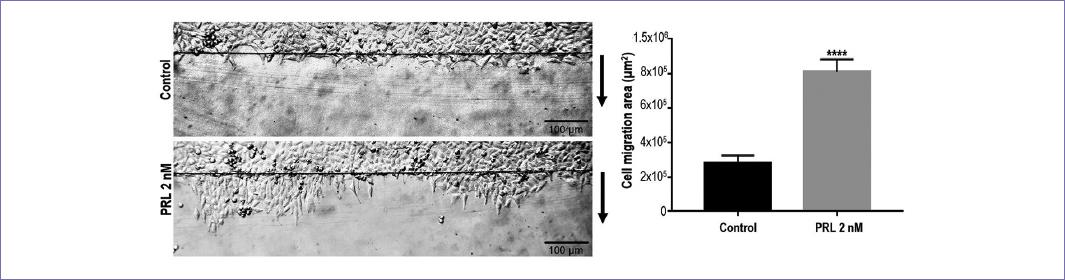
Figure 1 Migration of MCF-7 cells after 72 h of 2 nM PRL administration. Arrows indicate the direction of migration. The top black line indicates where the migration begins. The area covered by the migrating cells was measured in μm2. Images are representative of three separate experiments and were converted to gray scale. p < 0.0001 against control.
PRL does not induce the epithelium-mesenchyme transition in vitro in MCF-7 cells
Once it was shown that PRL increases the migration of MCF-7 cells, it was analyzed whether this same treatment could induce EMT in the same cells. EMT was evaluated through the expression of E-cadherin using the Western blot technique (Fig. 2). The results show that stimulation with PRL (2 nM) for 72 h did not induce a decrease in the expression of E-cadherin with respect to the control group, for which it can be considered that the migration of MCF-7 cells was carried out without inducing an EMT.
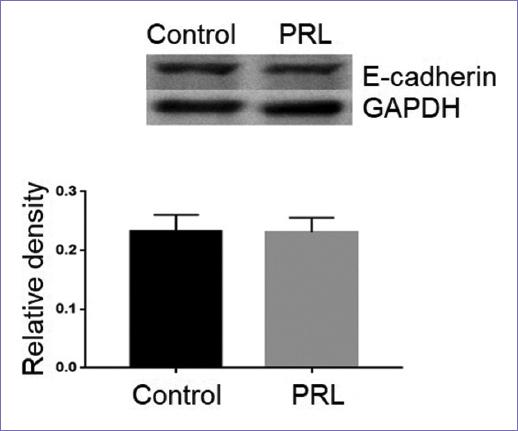
Figure 2 Western blot for E-cadherin expression. It is observed that PRL does not induce the loss of E-cadherin expression after a 72 h treatment with 2 nM PRL. GAPDH was used as a loading control, the density was normalized against it. The images are representative, the experiment was repeated 3 times. No significant difference was found.
Discussion
In this article, we demonstrate that PRL increases cell migration without inducing EMT in MCF-7 breast cancer cells. When MCF-7 cells were treated with PRL for 72 h, they significantly increased the cell migration area with respect to the control and did not induce a decrease in the expression of E-cadherin, which indicates that an EMT was not carried out.
PRL is a hormone that is involved in the tumorigenesis of the mammary gland and in the migration of cancer cells14,16,17; however, the existing information does not indicate whether the migration induced by PRL is individual or collective. Although there is information on the effect of PRL on vascular endothelial cadherin to carry out the development of the corpus luteum18 or on the expression levels of mRNA and E-cadherin protein in the growth of mammary gland epithelial cells (MECs)19, the relationship between PRL induced cell migration and E-cadherin levels had not previously been analyzed.
At present, there are some studies that report the role of PRL in the migration of the MCF-7 cell line. PRL at doses of 50 ng/ml (2.17 nM) and 100 ng/ml (4.35 nM) has been reported to increase migration in breast cancer cell lines T47D, ZR75-I, and MCF-7 through inducing changes in actin cytoskeleton remodeling, which was demonstrated by the increase in the expression levels of the proteins involved (c-Src, moesin, FAK, and their phosphorylated forms)14. Although the concentration used in this study is similar to that used in our study (2 nM), there are differences regarding the type of PRL used, as well as the type of incubation. Other studies have also shown that PRL induces migration in MCF-7 cells through different regular mechanisms such as PAK116, sphingosine kinase 120, PI3K21; however, none of these studies have evaluated the effect of PRL on intercellular junctions. Although PRL acts in the actin cytoskeleton remodeling to induce cell migration14, its effect on molecules involved in adherent junctions (important for cell migration) such as E-cadherin, is practically unknown.
EMT has been directly related to the type of cell migration that can occur. The cells that migrate individually are those coming from epithelia that, through an EMT, are delaminated and become mesenchymal cells with the capacity to migrate. On the other hand, collective migration involves the movement of several cells, forming part of a group, row or layer, which maintain characteristics similar to those of epithelial cells, which is why it is considered that an EMT itself is not present22. Of these two types of migration, collective migration is the one with the greatest potential to cause metastasis, this potential being 23-50 times greater than individual cell migration23. In this regard, various markers involved in EMT have been described; one of the most studied is the decrease in the expression of E-cadherin12.
The loss of E-cadherin is a key characteristic of EMT, which is why it is considered as a marker, during which cancer cells lose their epithelial phenotype and acquire a mesenchymal phenotype that gives them greater migratory and invasive capacity24,25. Thus, for cancer cells to metastasize, they must first detach from the primary tumor, which is facilitated by the EMT process. The functional loss of cell adhesion, mediated by the loss of E-cadherin, allows cells to detach from the primary tumor, invade adjacent tissues, and migrate to distant sites where they establish to form metastatic tumors25. Therefore, a decrease in the expression of E-cadherin can be related to an individual type migration. However, the results obtained show that PRL, despite increasing cell migration, did not decrease E-cadherin levels. Accordingly, the type of cell migration induced by this hormone could be related to collective migration. To date, the relationship between the type of migration induced by PRL and the levels of E-cadherin expression is not fully known. Nevertheless, there is information that supports that PRL can upregulate the expression levels of mRNA and E-cadherin protein to efficiently carry out the proliferation of MECs19, even though these studies are focused on cell proliferation.
Finally, we can conclude that PRL significantly increased the migration of the MCF-7 breast cancer cell line without inducing EMT, which could be linked to a collective type migration.











 text new page (beta)
text new page (beta)


