Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.7 n.8 Texcoco Nov./Dec. 2016
Articles
Search and isolation of nematophagous fungi vs Meloidogyne spp. in northern Sinaloa, Mexico
1Universidad Autónoma de Sinaloa- Escuela Superior de Agricultura del Valle del Fuerte. Calle 16 y Av. Japaraqui, Juan José Ríos, Ahome, Sinaloa, C. P. 81110, Tel: 68 78 96 09 08. (gabriel_lugo9010@hotmail.com).
2Lab. de Nematología Agrícola- CIIDIR-IPN Unidad Sinaloa, Blvd. Juan de Dios Bátiz Paredes No. 250, Guasave, Sinaloa C. P. 81101, Tel. 68 71 58 52 81. (manuel.mundo@ucr.edu).
3Universidad de California- Departament of Nematology, 900 University Ave. 92521, Tel. 909 787 2691. (irma.deley@ucr.edu; obecker@ucr.edu).
Production of various horticultural crops in northern Sinaloa, is being limited by the attack of “root-knot nematode” Meloidogyne spp. The search for alternatives to manage this plant parasite rises as response to this problem; among the solutions is isolation and identification of native neumatophagous fungi from Valle del Fuerte. The aim of this research was to identify existing nematophagous fungi in soils where crops are grown under protected conditions and susceptible to Meloidogyne spp. The study was conducted from January 2013 to July 2014 in four producing areas of pepper c.v. Bell Pepper from Valle del Fuerte, where soil samples were collected, which were processed through the sprinkling plate method (wateragar). To purify and select nematophagous fungi, isolates were transferred to plates with agar cornmeal, identifying morphological structures for diagnostic at genus level. Identified genres were: Dactylella, Arthrobotrys and Nematoctonus.
Keywords: Dactylella; Arthrobotrys; Nematoctonus; nematophagous fungi
La producción de diversos cultivos hortícolas en el norte de Sinaloa, está siendo limitada por el ataque del “nematodo nodulador” Meloidogyne spp. La búsqueda de alternativas para el manejo de este fitoparasito surge como respuesta a esta problemática, entre estás el aislamiento e identificación de hongos nematófagos nativos del Valle del Fuerte. El objetivo de la presente investigación fue identificar hongos nematófagos existentes en suelos donde se producen cultivos en condiciones protegidas y susceptibles a Meloidogyne spp. El estudio se realizó de enero 2013 a julio 2014 en cuatro zonas productoras de chile c.v. Lorca Bell Pepper del Valle del Fuerte, donde se recolectaron muestras de suelo, las cuales fueron procesadas mediante el método de espolvoreado en placa (agua-agar). Para purificar y seleccionar hongos nematófagos, los aislamientos se transfirieron a placas con agar harina de maíz, identificándose las estructuras morfológicas para el diagnóstico a nivel género. Se identificaron a los generos: Dactylella, Arthrobotrys y Nematoctonus.
Palabras clave: Dactylella; Arthrobotrys; Nematoctonus; hongos nematófagos
Introduction
Biological control of nematodes through nematophagous fungi is a promising alternative for the management of plant parasites (Duponnois et al., 2001, Sorbo et al., 2003; Singh et al., 2007). Therefore have been conducted extensive studies of their taxonomy, phylogeny, biology and ecology (Cooke 1963; Kerry 1987; Sayre and Walter 1991; Sikora 1992; Morton et al., 2004; Dong and Zhang 2006). This are considered an important group of soil microorganisms that can suppress parasitic nematode populations of plants and animals. Four groups were classified overall based on the mechanisms of attack to nematodes a) trapping nematodes: these use mechanically nodules or adhesive hyphae, belong to a group of asexual ascomycete with defined species by its type of capture devices (Scholler et al., 1999; Li et al., 2005; Yang and Liu, 2006; Yang et al., 2007); b) endoparasitic fungi using its spores; c) fungi that use the tips of their hyphae to invade females and eggs; and d) fungi producing toxins that immobilizes nematodes (Kendrick, 2001; Liu et al., 2009).
Chemical control is still used as the fastest option to address phytosanitary problems. However, the environmental implication is currently a hot topic and in this scenario, biological control, emerges as a promising alternative in controlling pathogens and pests including plant parasitic nematodes. Nematophagous fungi are soil residents that use their spores or mycelium to infect worm-like nematodes. They are found in different types of substrates and are able to survive in extreme climatic or nutritional conditions in different geographic regions of the world (Gray and Soh, 1989). Its abundance varies from 1, 8 and 150 propagules per gram of soil and this is mainly due to the amount of organic matter, water content, soil type, temperature and presence of host nematodes (Persmark and Jansson, 1997).
Antagonists of these nematodes have been located in a wide range of organisms including fungi, bacteria, viruses, plants, protozoa, turbellaria, tardigrades, mites and even other nematodes. Jansson and López-Llorca (2001) state that one to five species of these microorganisms are recovered from a soil sample. Within this broad group of nematophagous fungi, the most important in the regulation of nematode populations are some species of the genera: Arthrobotrys, Dactylella, Dactylellina, Gamsylella, Drechslerella, Monacrosporium, Monacrosporiella, Nematoctonus, Stylopage, Stropharia, Didymozoophaga (Chen and Dickson, 2004). From the description of Arthobotrys oligospora by (Zopf, 1988), there have been many studies on the taxonomy, ecology and physiology of these fungi. More than 200 species of nematophagous fungi have been described; a group with a high potential to be used as biological control agents to suppress plant parasitic nematodes (Qadri, 1989; Qadri and Seleh, 1990; De Leij and Kerry, 1991, and Akram Khan, 2000; Kerry, 2000). This study contributes to the knowledge of fungi in nematophagous and the first report of several isolates, obtained in northern Sinaloa, as an alternative to the integrated management of the root knot nematode Meloidogyne spp.
Materials and methods
Study area
Four producing regions of Bell Pepper cv Lorca (Capsicum annuum L.) located in the Valle del Fuerte, Sinaloa (Figure 1) were sampled. Soil samples were collected from the rhizosphere in areas naturally infested with Meloidogyne species during the agricultural cycle spring-summer and autumn-winter 2013-2014, the crop to be sampled was determined by two factors: 1) production under greenhouse conditions; and 2) cooperating farmers to make and select sampling sites, each composite sample consisted of 2.5 kg of soil; crop sampling stages were in the developmental stages at (0-30 ddt) initial, (60 and 90 ddt- fruit maturity) intermediate and (120 ddt-harvest) end of the crop in the regions from Ejidos: the Panguitas (25° 54᾽ 56.65” north latitude, 108° 50᾽ 16.70” west longitude), Campo 35 ( 25° 49' 32.02” north latitude 108° 54᾿ 20.77” west longitude), Jiquilpan (25° 47᾿ 41.4” north latitude, 108° 55᾽ 52.8” west longitude) and Estacion Bamoa (25° 50᾽ 11.33” north latitude 108o 54᾽ 37.20” west longitude), under protected conditions whose agricultural sites had established pepper cv Lorca Bell Pepper susceptible to Meloidogyne spp. In order to obtain a greater number of isolates from nematophagous fungi at the study sites, these were processed under two separate methods Baermann funnel and Cobb sieving.
Sampling procedure
In total 20 field visits were conducted. For the isolation of parasitized larvae and nematophagous fungi from soil, were delimited in each greenhouse for each region, areas identified with root-knot nematode problems, within which two sampling areas of 140 m2 were selected, separated from each other by a distance of 1.6 m, recognized as highly infested; i.e. each area consisted of 28 m long and 5 m wide. In each region and specific area was sampled with the help of an auger model LS Soil sampler of 91.4cm with a sampling tube of 27.5 cm, 25 sub-samples were collected in zig-zag in the area recognized with root-knot nematode problems, at a depth of 25 cm, obtaining an average of 2 to 2.5 kg of soil per composed sample stored in polyethylene bags inside a cooler properly labeled and transferred to Nematology Laboratory of the Escuela Superior de Agricultura del Valle del Fuerte from the Autonomous University of Sinaloa , for processing. The geographical coordinates of each greenhouse were determined through a GPS ETrex®, Garmin.
Nematode collection
Soil samples from the root of pepper cv. Lorca Bell Pepper were collected within naturally infested areas by Meloidogyne species, during the spring-summer cycle. Soil samples were collected at fruit maturity (60 and 90 days after transplantation) and end of harvest (120 days), with the help of an auger model LS Soil Sampler, 25 sub-samples were collected in zig-zag within the problem area of root-knot nematode at a depth of 25 cm, obtaining an average of 2 to 2.5 kg of soil per composite sample. The method used for extraction of parasitized nematode larvae was Baermann funnel modified according to (Christie and Perry, 1951) consists in suspending soil in a given volume of water, and after a short rest period, the suspension is poured on a superimposed set of sieves of 100 and 400 mesh Duves®. The soil retained in the finest sieve is transferred to a cylinder with fabric bottom, which subsequently is placed in the funnel. After 24 to 48 h, all active forms of nematodes have passed through the cloth or paper and can be collected in a small volume of water when removing the mohr clamp from the hose and then proceeded to observation under a compound microscope Nikon Eclipse E200, nematodes that showed parasitism were collected with the help of a micropipette 10 to 100 μL Eppendorf, and place on plates with medium wateragar 5g L-1 (AA) (Barron, 1977).
Fungi collection
Soil samples were collected from the root of pepper c.v. Lorca Bell Pepper within areas naturally infested with Meloidogyne species at a depth of 25 cm during the fallwinter cycle (O-I) during initial maturity (0 and 30 days after transplantation) and intermediate (60 days), with the aid of an auger model LS soil sampler, 25 sub-samples were collected in zig-zag within the problem area of root-knot nematode, at a depth of 25 cm, obtaining an average of 2 to 2.5 kg of soil per composed sample, properly labeled were transported to the Nematology Laboratory. The method used was Cobb sieving (Staniland, 1954); i.e., each soil sample was homogenized with water in a 19 L bucket, then the soil volume was deposited in another bucket passing through a 80 mesh screen Duvesa®, discarding the sediment and that obtained in the sieve to remove stones and organic matter of great size difficult to handle in petri dishes, then the supernatant was passed through a 200 mesh screen Duvesa®, the material retained on the sieve was collected with the help of a wash bottle Cienceware®, to a beaker of 500 ml Pyrex®, removing as much water through a 500 mesh screen Duvesa® and with a spatula a striated with 1 g of sieved soil was performed on water-agar plates 5 g L-1 (AA).
Isolation of nematophagous fungi
This phase was carried out in the Nematology Laboratory at the Escuela Superior de Agricultura del Valle del Fuerte. Soil samples were processed through the "sprinkling plate" method described by Barron (1977) for the isolation of nematophagous fungi. The technique consisted on using for each sampling area, five replications placing in Petri dishes of 9 cm diameter water-agar (A-A) and 1 g of the sieved soil sample. With the help of a spatula a striate was performed in a plate (A-A). The plates were incubated at room temperature (27-30 °C) and fluorescent light. From the fourth day of incubation, plates were observed daily with a stereomicroscope Labomed Luxeo 4Z®, in search for hyphae, conidia, spores or parasitized nematodes (Figure 2).
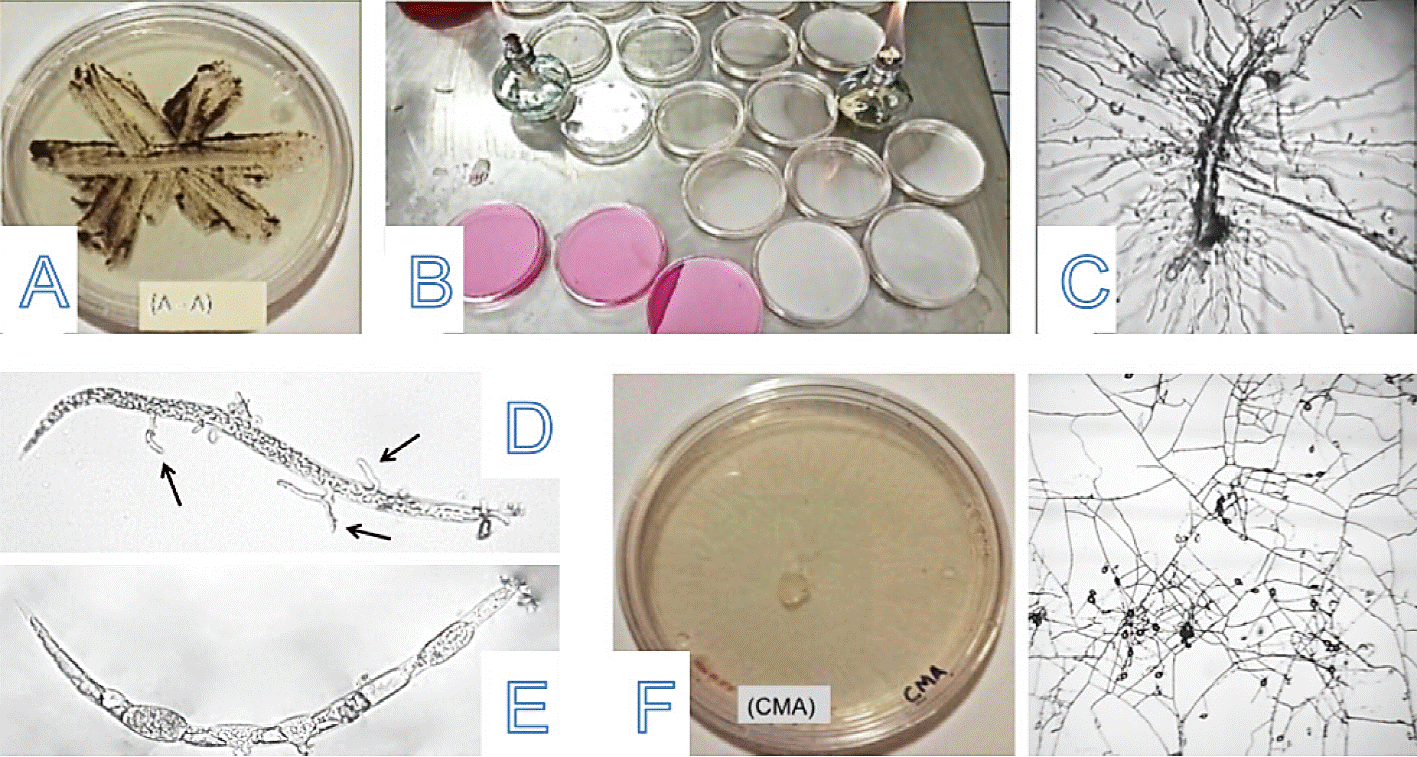
Figure 2 A) Striate plate (water-agar); B) two types of specific media for striate; C) free living nematode parasitized; D-E) Meloidogyne spp larvae, parasitized; and F) Dactylella fungus growth in specific mediumcornmeal agar (CMA).
The observations were made for another week in order to increase the possibility of finding some fungal structure. Once observed the possibility of presence of nematophagous fungi, it proceeded to its isolation and purification. With the help of a micropipette 10 to 100 μL Eppendorf and observed under a compound microscope, conidia, mycelium or parasitized nematodes were taken and placed on medium cornmeal agar (CMA) (corn-meal-agar) Bbl™ 17 g L-1, chloramphenicol Vixim® 0.1% (w/v) (Nuñez, 2002a), to prevent bacteria growth, additionally another specific medium CMA 17 g L-1 was prepared plus reagent rose bengal Faga -lab® to .5 g L-1, according to that described by (Perez et al., 2007) for the specific growth Pochonia chlamydosporia. The samples were incubated at room temperature (27 to 30 oC) and one week after fungal growth was compared to growth in medium (CMA + rose bengal) was verified and no growth was observed; however in plates with water-agar mycelium growth was observed, these colonies were transferred into medium (CMA + chloramphenicol Vixim® 0.1% (w / v) (Nuñez, 2002) for purification and then in medium CMA 17 g L-1, without antibiotic for replication. The identified fungi were stored in test tubes of 20 ml KIMAX®, with sloping CMA and mineral oil Faga-Lab® at 4 °C for later use in pathogenicity tests (Kerry , 2001). The times of appearance of nematophagous fungi vary considerably, and in order to recover most of the species in a particular sample, the plates must be examined daily for up to ten days to make sure to find potential nematophagous fungi in the soil including slow growth (Barron, 1978).
Identifying nematophagous fungi
Semipermanent mountings were prepared by sample between two glasses (Kohlmeyer and Kohlmeyer, 1972; Connell and Padgett, 1988), which consist placing on each slide Fisherfinest (25 x 75 x 1 mm) a thin section of agar containing the fungal structures of each isolated fungal covered by slides Fisherfinest® superslip (24 x 50 mm) (Shephered, 1995). Preparations and identifications of nematophagous fungi were studied by direct observation under a compound microscope Nikon Eclipse E200 at different magnifications because some structures are accessible in minor magnification, structures like growing mycelium, conidiophores and conidial were compared, trapping structures was only possible to observe when transferring and purifying fungi collected in plates with cornmeal agar without antibiotic (CMA) and add plant parasitic nematodes and "free living", taking micrographs with differential interference contrast microscopy Nomarski in the Laboratory from the University of California Riverside Campus.
Taxonomic identification of nematophagous fungi was performed using the codes proposed by (Cooke and Godfrey, 1964; Barnett and Hunter, 1998); which depends on conidia form, septa and size and presence or absence of chlamydospores (Drechsler, 1937). The collection of nematophagous fungi identified is deposited in the Nematology Laboratory of the Escuela Superior de Agricultura del Valle del Fuerte. . Preserved in test tubes 20 ml KIMAX®, with sloping CMA medium and mineral oil Faga-Lab® at 4 °C, technique that decreases medium dehydration, slows down metabolic activity and reduces the possibility of infection by mites (Jong and Atkins, 1985).
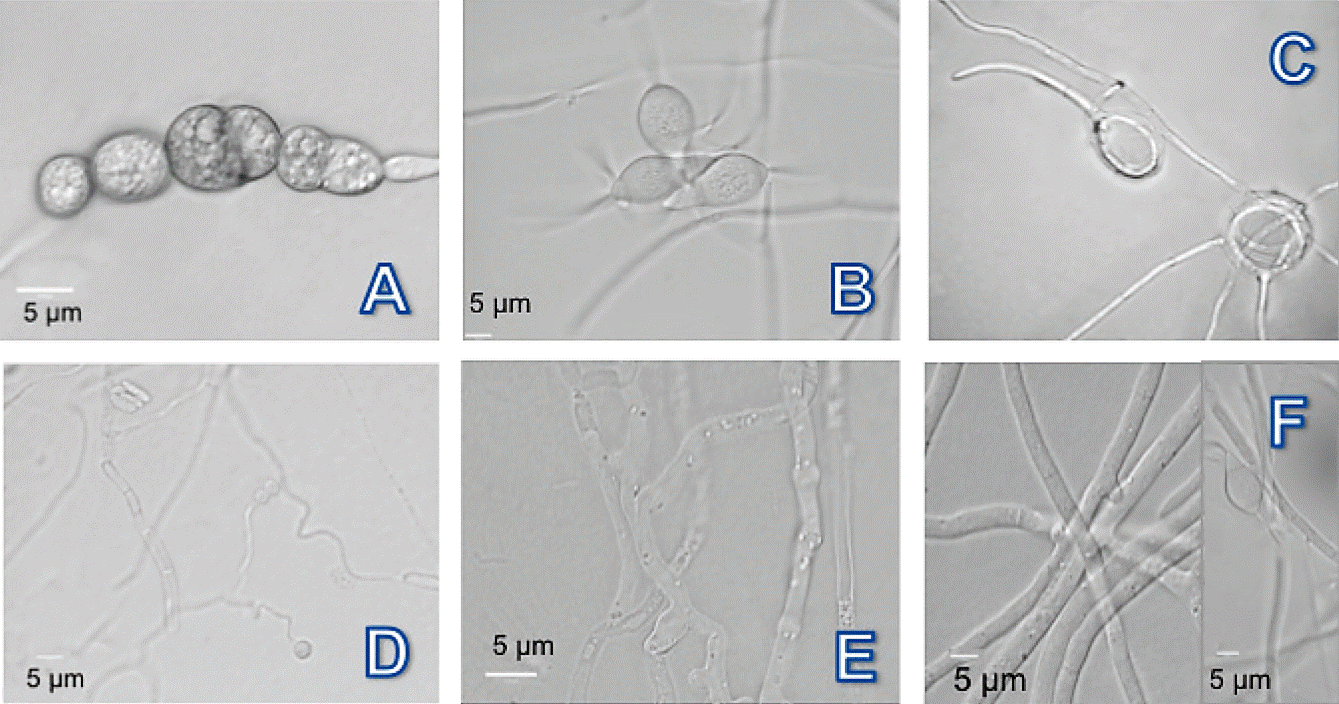
Figure 3 Photomicrographs of nematophagous fungi isolated from soil of producing areas of Bell Pepper in the Valle del Fuerte, Sinaloa. A-C) Dactylella chlamydospores, conidia and not constrictors rings; D and E) Nematoctonus nodules and adhesive mycelium; and F) Arthrobotrys hyphae network and conidia.
Results and discussion
15 isolates of fungi associated with the cultivation of Bell Pepper from four producing areas of Valle del Fuerte were obtained, 14 were identified and one more was classified as sterile mycelium since it did not produce reproductive structures that facilitate their identification, 11 genera were isolated, based on its microscopic descriptions and morphological aspects like plant parasitic. The most isolated genus was Fusarium, with 25%, followed by Phytophthora, 19%, Sclerotinia, 13%, F. oxysporum 9%, Pythium, 8%, Aspergillus, 6%, Trichoderma, 5%, Fusarium moniliforme 5%, Corynespora, 5%, Geotrichum, 3%, Rhizoctonia, 2%. Some of these genera such as Fusarium, Sclerotinia and Phytophthora are considered crop pathogens without deleterious action on nematodes. With respect to phytopathogenic nematodes management, nematophagous fungi associated to infested soils by Meloidogyne spp., three genera based on their microscopic descriptions and morphological aspects, the most common genus isolated and identified was Dactylella, 11%, Arthrobotrys 5% and Nematoctonus, 4%. In the ejido Las Panguitas was where the highest number of fungal isolates was present (57%), followed by "Campo 35" (21%), Jiquilpan (12%) and Estacion Bamoa 10%. In addition to the percentage of nematophagous fungi obtained from The Panguitas and "Campo 35" have been adopted cultural practices such as crop rotation at the end of the harvest, with forage grasses, crotalaria and radish. Meanwhile (Velasco, 2002), found that the addition of organic matter greatly improves soil properties.
By incorporating organic fertilizers it has been registered a positive effect on beneficial populations of bacteria, actinomycetes and fungi; these results coincide with those reported by other authors (Sánchez et al., 1987; Alvarez-Solís et al., 1992). (Hajieghrari et al., 2008) confirmed that pH, temperature and organic matter are key parameters in the growth, sporulation and saprophytic fungi capacity. Other works reported (Capstick et al., 1957; Giuma and Cooke, 1972) that nematodes living in soil will be colonized by parasitic fungi, therefore, can be isolated by extracting nematodes from the soil and transferred them into agar at low concentrations. In previous studies (Hidalgo et al., 2000; Flores et al., 2007; Perez et al., 2007; Flores et al., 2008; Franco et al., 2009) obtained isolates of nematophagous fungi with a high parasitism potential to reduce galls on tomato roots, (Huang et al., 2004; Gan et al., 2007) confirm that chitinase in nematophagus fungi degrade chitinolytic components in root nematodes eggs.
These isolates are similar to the work obtained by (Verdejo et al., 2002), finding Dactylella ovi-parasitic, Pochonia chlamydosporium and P. catenulatum associated with egg masses of Meloidogyne spp. (Nuñez, 2002) confirmed the presence of three species of Cladosporium spp., associated to cysts of Globodera rostochiensis in potato crop (Solanum tuberosum L.) in the region of Cofre de Perote, Veracruz, (Yang et al., 2012) reports Dactylella as a promising fungus in egg parasitism of Heteroderidae family. Similar results report Dactylella as viable in the parasitism of young females which would reduce oviposition of Meloidogyne spp. (Smith et al., 2011). Other works by (Timper et al., 1993) show low virulence of Nematoctonus that is why it is suggested to perform pathogenicity tests and corroborate its biological effectiveness on plant parasitic nematodes in the region.
(Persson et al., 1993) state that isolates of nematophagous fungi like Arthrobotrys dactyloides and A. superba have the ability to colonize tomato roots resulting in an effective biological control. Previous studies (Stirling and Mani, 1995; Galper et al., 1995; Jaffee and Muldoon, 1997; Jacobs, 1997; Stirling et al., 1998) reported the ability of Arthrobotrys to suppress Meloidogyne spp. The results obtained agree with jobs where Meloidogyne is associated with beneficial soil microorganisms (Gallegos et al., 2009).
Conclusions
The existence of nematophagous fungi associated to crops affected by Meloidogyne spp., was confirmed in three of the four regions studied. It is important to mention that to isolate nematophagous fungi there are other methods like the technique of differential centrifugation, molecular biology, which may favor future work for fungi diversity, particularly endoparasites, obligate or highly specific, promising in the search for biological management of Meloidogyne spp. populations reported for Sinaloa.
Literatura citada
Álvarez, S. J. D.; Ferrera, C. R. y Zebrowski, C. 1992. Análisis de la microflora asociada al manejo ecológico en la recuperación de tepetates. Terra (número especial). 10:419-424. [ Links ]
Barnett, H. L. and Hunter, B. B. 1998. Illustrated Genera of Imperfect Fungi, 4th Edition. The American Phytopathological Society, St. Paul, MN, USA. 218 p. [ Links ]
Barron, G. L. 1977. The nematode-destroying fungi. [Topics in Mycology No. 1.]. Guelph: Canadian Biological Publications. 140 p. [ Links ]
Barron, G. L. 1978. Nematophagous fungi: Endoparasites of Rhabditis terricola. Microbial Ecology, 4:157-163. [ Links ]
Capstick, C.; Twinn, D. and Waid, J. 1957. Predation of natural populations of free Iiving nematodes by fungi. Nematologica, 2:193-201. [ Links ]
Chen, S. and Dickson, D. W. 2004. Biological control of nematodes by fungal antagonist. Editorial Nematology. Advances and perspectives, Volume II. Beijing, China. 979-1039 p. [ Links ]
Cooke, R. C. and Godfrey, B. E. 1964. A key to the nematode-destroying fungi. Transactions in British Mycology Society 47: 61-74. [ Links ]
Cooke, R. C. 1963. Ecological characteristics of nematode-trapping hyphomycetes I. Preliminary studies. Annals of Applied Biology, 52:431-437. [ Links ]
Connell, S. L. and Padgett, D. E. 1988. An improved technique for making permanent slide cultures of fungi. Mycopathologia. 101:165-166. [ Links ]
Cristie, J. R. and Perry, V. G. 1951. Removing nematodes form soil. Proceedings of the Helminthological Society of Washington 18:106-108. [ Links ]
Dong, L. Q. and Zhang, K. Q. 2006. Microbial control of plant-parasitic nematodes: A five-party interaction. Plant and Soil. 288:31-45. [ Links ]
De Leij, F. A. and Kerry, R. B. 1991. The nematophagus fungus Verticillium chlamidosporium as a potential biological control agent for Meloidogyne arenaria. Revue Nematol. 14: 157-164. [ Links ]
Drechsler, C. 1937. Some hyphomycetes that prey on free-living terricolous nematodes. Mycologia, 29, 447-552. [ Links ]
Duponnois, R.; Chotte, J. L.; Sall, S. and Cadet, P. 2001. The effects of organic amendments on the interactions between a nematophagous fungus Arthrobotrys oligospora and the rootknot nematode Meloidogyne mayaguensis parasitizing tomato plants. Biology and Fertility of Soils, 34: 1-6. [ Links ]
Flores, C. R.; Manzanilla L. R. H.; Cid, del P. I. y Martínez, G. A. 2007. Control of Nacobbus aberrans (Thorne, 1935) Thorne y Allen, 1944 with Pochonia chlamydosporia (Verticillium chlamydosporium) (Goddard) Zare and W. Gams. Revista Mexicana de Fitopatología. 25:26-34. [ Links ]
Flores, C. R.; Atkins, S. D; Manzanilla, L. R. H; Cid, Del P. I. and Martínez, G. A. 2008. Caracterización de aislamientos mexicanos de Pochonia chlamydosporia var. chlamydosporia (Goddard) Gams and Zare para el control biológico de Nacobbus aberrans (Thorne). Revista Mexicana de Fitopatología. 26:93-104. [ Links ]
Franco, N. F.; Vilchis, M. K. and Miranda, D. J. 2009. New records of Pochonia chlamydosporia from Mexico: isolation, root colonization and parasitism of Nacobbus aberrans. Nematropica. 39:133-142. [ Links ]
Galper, S.; Edén, L. M.; Stirlíng, G. R. and Smith, L. J. 1995. Simple screening methods for assessing the predacious activity of nematode-trapping fungí. Nematologica. 41:130-140. [ Links ]
Gallegos, G. M; Cepeda, M. S; Hernández, F. D. C; Zamarripa, A. A. M; Velásquez, V. R; González, G. E. and Sánchez, J. M. 2009. Microorganismos benéficos asociados a Meloidogyne incognita (Kofoid y White) Chitwood en Guayabo (Psidium guajava L.) de Calvillo, Aguascalientes, México. Revista Mexicana de Fitopatología. 27(2):106-112. [ Links ]
Gan, Z.; Yang, J.; Tao, N.; Liang, L.; Mi, Q.; Li,, J. and Zhang, K. Q. 2007. Cloning of the gene Lecanicillium psalliotae chitinase Lpchil and ¡dentification of its potential role in the biocontrol of root knot nematode Meloidogyne incógnita. Applied Microbiology and Biotechnology. 76:1309-1317. [ Links ]
Giuma, A. and Cooke, R. 1972. Some endozoic fungi parasitic on soil nematodes. Transactions of the British Mycological Society. 59:213-218. [ Links ]
Gray, A. F. and Soh, H. D. 1989. A nematicide seed treatment to control Dytlienchus dipsaci on seedling alfalfa. Journal of Nematology. 21:184-188. [ Links ]
Hidalgo, D. L.; Bourne, J. M.; Kerry, B. R. and Rodríguez, M. G. 2000. Nematophagus Verticillium spp. in soil infested with Meloidogyne spp. in Cuba: isolation and screening. International Journal of Pest Management. 46:277-284. [ Links ]
Huang, X.; Zhao, N. and Zhang, T. K. Q. 2004. Extracellular enzymes serving as virulence factors in nematophagous fungi involved in infection of the host. Research in Microbiology. 155:811-816. [ Links ]
Jacobs, P. 1997. Untersuchungen ifber die Wirkung nematophager Pilze auf Meloidogyne sp. in vitro und auf deren Befall an Lycopersicon esculentum. Zeitschrift fu"r Pflanzenkrankheiten undPflanzenschutz. 104:153-165. [ Links ]
Jaffee, B. A.; Muldoon, A. A. E. and Didden, W. A. M. 1997. Enchytraeíds and nematophagous fungi in soil microcosms. Biology and Fertility of Soils. 25:382-388. [ Links ]
Jansson, H. B. and Lopez, Ll. L. V. 2001. Biology of Nematophagous fungi. In J. K. Misra y B. W. Horn (Eds.) Trichomycetes and other fungal groups. Plymouth: Science Publishers. 145-172 p. [ Links ]
Jong, S. C. and Atkins, W. B. 1985. Conservation, Collection and distribution of cultures. In: Fungi Pathogenic for Humans and Animals. Pt B: Pathogenicity and Detection II (HDH Howard, ed), Marcel Dekker, Inc, New York, NY, USA. 153-194 pp. [ Links ]
Kendrick, B. 2001. The fifth kingdom (3rd ed). Canada: Mycologue Publications. [ Links ]
Kerry, B. R. 1987. Biological control. In R. H. Brown & B. R. Kerry (Eds.) Principles and practice of nematode control in crops New York: Academic Press. 233-263 pp. [ Links ]
Kerry B. R. 2000. Rhizosphere interacctions and the exploitation of microbial agents for the biological control of plant parasitic nematodes. Annual Review Phytopathology. 38:423-441. [ Links ]
Kerry, B. R. 2001. Exploration of the nematophagus fungus Verticillium chlamidosporium Goddard for the bio-logical control of rootknot nematodes (Meloidogyne spp.) In fungi as Biocontrol Agents: Progress problems and potential. CABI International, Wallingford, UK. 55-168 pp. [ Links ]
Khan, B. R. and Akram, M. 2000. Effect of certain antagonistic fungi and rhizobacteria on wilt complex caused by Meloidogyne incognita and Fusarium oxysporum on tomato. Nematologica Mediterranea. 28:139-144. [ Links ]
Kohlmeyer, J. and Kohlmeyer, E. 1972. Permanent microscopic mounts. Mycologia. 64: 666-669. [ Links ]
Li, Y.; Hyde, K. D.; Jeewon, R.; Lei, C.; Vijaykrishna, D. and Zhang, K. Q. 2005. Phylogenetics and evolution of nematode-trapping fungi (Orbiliales) estimated from nuclear and protein coding genes. Mycologia. 97:1034-1046. [ Links ]
Liu, X.; Xiang, M. and Che, Y. 2009. The living strategy of nematophagous fungi. Mycoscience. 50:20-25. [ Links ]
Morton, C. O.; Hirsch, P. R. and Kerry, B. R. 2004. Infection of plantparasitic nematodes by nematophagous fungi. A review of the application of molecular biology to understand infection processes and to improve biological control. Nematology. 6:161-170. [ Links ]
Nuñez, E. A. 2002. Aislamiento y evaluación de hongos nematófagos asociados a quistes de Globodera roschotiensis (Woll.) en la región del Cofre de Perote Veracruz. Tesis maestría. Universidad de Colima. México. 80-91 pp. [ Links ]
Olatinwo, R.; Borneman, J. and Becker, J. O. 2006. Induction of beetcyst nematode suppressiveness by the fungi Dactylella oviparasitica and Fusarium oxysporum in field microplots. Phytopathology. 96:855-859. [ Links ]
Peréz, R. I.; Doroteo, A.; Franco, F. N.; Santiago, V. S. and Montero, A. P. 2007. Isolates of Pochonia chlamydosporia Var. chlamydosporia from México as potencial biological control agent of Nacobbus aberrans. Nematropica. 37:127-134. [ Links ]
Persmark, L. and Jansson, H. B. 1997. Nematophagous fungi in the rhizosphere of agricultural crops. FEMS Microbiology Ecology. 22:303-312. [ Links ]
Persson, C. and Jansson, H. 1993. Rhizosphere Colonizaron and Control of Meloidogyne spp. By Nematode-trapping Fungi. Journal of Nematology. 31(2):164-171. [ Links ]
Qadri, A. N. 1989. Fungi associated with sugar beet cyst nematode in Jerash Jordan. MSc Thesis, Univerisity Of Jordan. 126 p. [ Links ]
Qadri, A. N. and Saleh, H. M. 1990. Fungi associated with Heterodera schachtii (Nematoda) in Jordan. Nematologica. 36:104-113. [ Links ]
Sánchez, J. M.; Ruíz, J. F. and Cuautle, F. 1987. Comportamiento de dos tipos de Tepetates bajo la adición de abonos orgánicos y abonos verdes en condiciones de invernaderos. En: Uso y Manejo de Tepetates para el Desarrollo Rural. Universidad Autónoma de Chapingo, Texcoco: 50-68 pp. [ Links ]
Sayre, R. M. and Walter, D. E. 1991. Factors affecting the efficacy of natural enemies of nematodes. Annual Review of Phytopathology. 29:149-166. [ Links ]
Scholler, M.; Hagedorn, G. and Rubner, A. 1999. A reevaluation of predatory orbiliaceous fungi. II. A new generic concept. Sydowia. 51: 89-113. [ Links ]
Shepherd, A. M. 1955. Some observations on the distributíon and biology of fungi predaceous onnematodes. Ph. D. Thesis, University of London. [ Links ]
Sikora, R. A. 1992. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Annual Review of Phytopathology. 30:245-270. [ Links ]
Smith, B. J.; Yang, J.; Borneman, J.; Timper, P.; Riggs, R. R. and Becker, J. O. 2011. Investigations into the relatedness of the nematophagous fungi Dactylella oviparasitica and ARF-L. Journal of Nematology. 43:288. [ Links ]
Singh, K. P.; Jaiswal, R. K.; Kumar, N. and Kumar, D. 2007. Nematophagous fungi associated with root galls of rice caused by Meloidogyne graminicola and its control by Arthrobotrys dactyloides and Dactylaria brochopaga. Journal of Phytopathology. 155:193-197. [ Links ]
Sorbo, D. G.; Marziano, F. and D’Errico, F. P. 2003. Diffusion and effectiveness of the nematophagous fungus Hirsutella rhossiliensis in control of the cyst nematode Heterodera daverti under field conditions. Journal of Plant Pathology. 85: 219-221. [ Links ]
Stirling, G. R. and Mani. A. 1995. The actívity of nematode-trapping fungi following their encapsulation in alginate. Nernatologica. 41:240-250. [ Links ]
Stirling, G. R.; Smith, L. J.; Licastro, K. A. and Edén, L. M. 1998. Control of root-knot nematode with formulations of the nematode-trapping fungus Arthrobotrys dactyloides. Biological Control. 11:224-230. [ Links ]
Staniland, L. 1954. A modification of the Baermann funnel technique for the collection of nematodes from plant material. Journal of Helminthology, 28: 115-118. [ Links ]
Timper, P. and Brodie, B. B. 1993. Infection of Pratylenchus penetrans by Nematode-pathogenic Fungi. Journal of Nematology. 25(2):297-302. [ Links ]
Velasco, V. J. 2002. Alternativa tecnológica de reciclaje de los desechos orgánicos del Colegio de Postgraduados. Tesis de Maestría. Colegio de Postgraduados, Montecillo, Estado de México. 91 p. [ Links ]
Verdejo, S.; Ornat, C.; Sorribas, F. J. and Stchiegel, A. 2002. Species of root-knot nematodes and fungal egg parasites recovered from vegetables in Almeria and Barcelona, Spain. Journal of Nematology. 34: 405-408. [ Links ]
Yang, Y. and Lui, X. Z. 2006. A new generic approach to the taxonomy of predatory anamorphic Orbiliaceae (Ascomycotina). Mycotaxon. 97:153-161. [ Links ]
Yang, Y.; Yang, E.; An, Z. and Liu, X. 2007. Evolution of nematode-trapping cells of predatory fungi of the Orbiliaceae based on evidence from rRNA-encoding DNA and multiprotein sequences. Proceedings of the National Academy of Sciences. 104: 83-79. [ Links ]
Yang, J.; Benecke, S.; Jeske, R. D.; Rocha, F. S.; Becker, J. S. and Timper, P. 2012. Population Dynamics of Dactylella oviparasitica and Heterodera schachtü: Toward a Decisión Model for Sugar BeetPlanting. Journalof Nematology. 44(3):237-244. [ Links ]
Zopf, W. 1888. Zur Kenntnis der Infektionskrankheiten niederer Thiere und Pflanzen. Nova Acadamy of Caes. Leop. Germán. Naí. Cur. 52:314-376. [ Links ]
Received: June 2016; Accepted: September 2016











 text in
text in