Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Archivos de cardiología de México
On-line version ISSN 1665-1731Print version ISSN 1405-9940
Arch. Cardiol. Méx. vol.82 n.1 Ciudad de México Jan./Mar. 2012
Artículos de revisión
Multiplanar transesophageal echocardiography for the evaluation and percutaneous management of ostium secundum atrial septal defects in the adult
Ecocardiografía transesofágica multiplanar para la evaluación y el tratamiento percutáneo de la comunicación interauricular tipo ostium secundum en el adulto
Ayax Sobrino,1,2 Arsène J. Basmadjian,1 Anique Ducharme,1 Reda Ibrahim,1 Lise-Andrée Mercier,1 Guy B. Pelletier,1 François Marcotte,1 Patrick Garceau,1 Denis Burelle,1 Eileen O'Meara,1 Annie Dore.1
1 Department of Medicine. Montréal Heart Institute and Université de Montréal. Montréal, Québec, Canada.
2 División Académica de Ciencias de la Salud. Universidad Juárez Autónoma de Tabasco. Tabasco, México.
Corresponding author:
Annie Dore.
Montreal Heart Institute,
5000 Rue Belanger Montreal, QC H1T 1C8.
Telephone: (514) 376 3330.
E-mail: annie.dore@icm-mhi.org.
Received on February 1, 2011;
Accepted on October 3, 2011.
Abstract
The purpose of this paper is to review the usefulness of multiplanar transesophageal echocardiography before, during and after percutaneous transcatheter closure of secundum atrial septal defects.
Transesophageal echocardiography imaging techniques, including their role in patient selection, procedural guidance and immediate assessment of technical success and complications are described and discussed in this review.
Percutaneous transcatheter closure is indicated for ostium secundum atrial septal defects of less than 40 mm in maximal diameter. The defect must have a favorable anatomy, with adequate rims of at least 5 mm to anchor the prosthesis. Transesophageal echocardiography plays a critical role before the procedure in identifying potential candidates for percutaneous closure and to exclude those with unfavorable anatomy or associated lesions, which could not be addressed percutaneously. Transesophageal echocardiography is also important during the procedure to guide the deployment of the device. After device deployment, the echocardiographer must assess the device (integrity, position and stability), residual shunt, atrio-ventricular valve regurgitation, obstruction to systemic or venous return and pericardial effusion, in order to determine procedural success and diagnose immediate complications.
Key words: Transesophageal echocardiography; Percutaneous closure; Atrial septal defect; Canada.
Resumen
El propósito de esta revisión es analizar la utilidad de la ecocardiografía transesofágica multiplanar antes, durante y después del cierre percutáneo de la comunicación interauricular tipo ostium secundum. Las consideraciones técnicas de imagen durante la ecocardiografía transesofágica multiplanar, su utilidad en la evaluación de los pacientes, la guía peri-procedimiento, la evaluación del éxito técnico y las complicaciones son descritas y discutidas en esta revisión.
El cierre percutáneo está indicado en la comunicación interauricular tipo ostium secundum con diámetro máximo de 40 mm. El defecto debe tener una anatomía favorable con bordes de al menos 5 mm.
La ecocardiografía transesofágica multiplanar tiene un papel determinante antes del procedimiento para identificar a candidatos potenciales para el cierre percutáneo y para excluir aquéllos con anatomía no favorable o lesiones asociadas que no pueden ser manejados vía percutánea.
La ecocardiografía transesofágica multiplanar es importante durante el procedimiento para guiar la liberación del dispositivo. Después de la liberación del dispositivo el ecocardiografista debe evaluar la posición y estabilidad del dispositivo, la presencia de corto-circuito residual, la regurgitación de las válvulas A-V, el retorno venoso sistémico y pulmonar, y el pericardio, a fin de determinar el éxito del procedimiento y descartar complicaciones asociadas.
Palabras clave: Ecocardiografía transesofágica; Cierre percutáneo; Comunicación interauricular; Canadá.
Introduction
Atrial septal defects (ASD) represent 10% of all congenital cardiac abnormalities. Ostium secundum (OS) ASD is the 4th most common congenital heart disease with an incidence of 3.78 per 10,000 live births.1 Surgical repair of ASD using a median sternotomy is safe, however it is associated with significant morbidity, discomfort and scarring.2 Percutaneous transcatheter closure (PTC) of ostium secundum ASD is an attractive alternative.
The role of echocardiography during interventional procedures is well documented 3,4 and several techniques have been described for the guidance of PTC of ASD.5 The purpose of this review is to provide an illustrated echocardiographic guide on the use of multiplane 2D transesophageal echocardiography (TEE) before, during and after percutaneous ostium secundum ASD closure. Current indications for ASD closure are out of the scope of this paper and can be reviewed elsewhere.6,7
TEE assessment of ASD includes evaluation of the number and localization of the defect(s), dimensions and adequacy of the rims, direction and severity of the shunt, and the presence of possible associated defects.
Measurement of ASD size
The ideal scenario for PTC is a single ASD with a maximal diameter of less than 20 mm,8 with firm and adequately sized rims. Defects up to 40 mm in diameter with firm and adequate rims have been closed successfully via PTC, as have multiple ASDs and those associated with atrial septal aneurysms.9 PTC cannot presently be recommended for ASD larger than 40 mm.
The size of the ASD changes during the cardiac cycle; the maximal ASD diameter must be measured at the end of ventricular systole.10 The maximal two-dimensional measurements are taken in two orthogonal views as, for example, the maximal transverse defect diameter at 0° in the 4-chamber view and the maximal longitudinal diameter at 70-90° in the bi-caval view (Figure 1). The defect should not be measured with Color Doppler (CD), as this technique leads to overestimation of true ASD size.
Measurement of the ASD rims
It is critical to recognize the nomenclature and understand the anatomical disposition of the rims or edges bordering the ASD (Figure 2). Rims must be measured on TEE examination to determine if the patient is a candidate for PTC. The minimal two-dimensional measurement is taken.11 Rims must measure at least 5 mm in length, the most important being the border of the inferior vena cava (IVC).

Several authors have referred to these edges with anatomical connotations and others with spatial connotations. For example, some authors describe the "antero-septal rim", which corresponds anatomically to the aortic rim (Ao). To simplify this classification we refer to Table 1. For reasons of clarity, anatomic connotations are used herein.

TEE evaluation should begin in the mid-esophageal 4-chamber view at 0°.12,13 This view permits localization of the posterior rim (Figure 3), and the AV rim (Figure 4), which does not need to be large to permit PCT.


From the mid-esophageal 4-chamber view, the probe should be pulled out with a slight right rotation to permit the localization of the right upper pulmonary vein (RUPV) rim at the upper-esophageal level (Figure 5).

To achieve proper visualization of the Ao (Figure 6), the probe must be at a mid-esophageal level, at 45° with a leftward (counter-clockwise) rotation of the probe to allow simultaneous visualization of the aorta (AA) and the ASD. Sometimes the Ao is very small, or even absent (Figure 7), this finding makes the procedure more challenging but does not, preclude PTC of the defect.


From the mid-esophageal four-chamber view, the multiplane angle is rotated approximately 90° to the 2-chamber view. With slight probe rotation to the right (clockwise rotation of the shaft of the probe), the IVC and the superior vena cava (SVC) are seen. The SVC rim is measured from the superior lip of the ASD to the insertion of the rim into the SVC (Figure 8A). The mid-esophageal bi-caval view provides an excellent view of the inter-atrial septum, allowing interrogation of the septum with CD.

The evaluation of the IVC rim is fundamental (Figure 8B), because PTC would be very challenging in its absence,14 it is, however, usually the most diffcult to visualize and measure, and retrofexion of the probe may help when it is not visible in the standard bi-caval view.15 From the bi-caval view, the TEE probe is advanced into the stomach, retrofexed and slowly pulled back to the lower esophagus. At this point, the IVC rim is measured from the inferior lip of the ASD to the insertion of the rim into the IVC.
Measurement of the CS rim (Figure 9) requires a lower esophageal position, near or at the level of the gastroesophageal junction at 0°, where the CS will be seen on the right of the monitor screen. It is necessary to perform a slight retroflexion of the probe to obtain a view of both the lower end of the ASD and the CS.

Anatomic variants
It is fundamental to scan the atrial septum with CD to rule-out the presence of multiple ASDs. The presence of multiple defects of the inter-atrial septum have been reported in 7.3% of patients with ostium secundum ASD.11
In cases of multiple ASDs in close proximity to one another, PTC with a single device can be attempted. In such cases, the device should be implanted in the largest defect, with the smaller adjacent septal defect being enclosed in the area covered by the two disks, hence being occluded by the same device. A major concern in the presence of two separate septal defects (Figure 10) is the possibility of missing other supplementary defects. In these cases, it has been suggested to infate two balloons simultaneously under TEE guidance and to exclude a possible third atrial septal defect with CD assessment.9,16

Aneurysm of the inter-atrial septum is defined as: 1) diameter of the base of the aneurysmatic portion of the inter atrial septum (IAS) measuring ≥ 15 mm and either 2) protrusion of the IAS, or part of it ≥15 mm beyond the plane of the IAS or 3) phasic excursion of the IAS during the cardio respiratory cycle ≥15 mm in total amplitude.17 Atrial septal aneurysms have a prevalence of 2.2% and are associated with cerebral ischemic events.18 They are associated with a patent foramen ovale (PFO), an ASD or fenestrations of the atrial septum. It is important to cautiously measure the size of an ASD associated with a septal aneurysm since a small ASD (< 20 mm) associated with a septal aneurysm can be potentially, although not ideally, closed with a PFO occluder device whereas an ASD occluder device should be used for PTC when the associated defect is larger than 20 mm in maximal diameter.19
In summary, the baseline TEE must meet the criteria described in Table 2 in order for the patient to be eligible for percutaneous closure.

Pre-procedural TEE guidance of PTC
In most centers, PTC is performed under general anesthesia with echocardiographic TEE guidance because intracardiac echo without anesthesia remains an expensive option. Before the ASD closure procedure, TEE measurements could be performed in two ways; i.e. dynamic balloon measurement or static balloon measurement.
1) Dynamic balloon measurement. Under TEE visualization and guidance, in the 4-chamber mid-esophageal view, at 0°, a defated balloon catheter is passed through the ASD, into the left atrium over a guidewire and inflated to a diameter of approximately 5 mm greater than the estimated ASD diameter, using a mixture of 30% radiographic contrast and 70% saline. CD is used to image fow through the ASD and the balloon is then gently pulled back, at which stage color fow on the TEE will disappear when balloon occlusion is complete. If color fow persists it is likely that the ASD is very large or a second ASD (or PFO) exists, requiring more detailed TEE analysis. While maintaining firm but not undue pressure on the septum and under continuous TEE guidance, the balloon is slowly defated until it pops through the defect into the right atrium. The diameter at which this occurs is the stretched balloon diameter (SBD) of the ASD.20 The SBD is measured on TEE. In essence, the SBD represents the diameter of the firm margins of the ASD. The amount of contrast needed to infate the balloon to this diameter is carefully recorded and the balloon is then completely defated and withdrawn from the patient. Afterwards, it is re-infated to the SBD volume and measured against a sizing plate.
It is recommended to repeat the procedure if there is a discrepancy greater than 1 mm between the TEE measurement of the ASD diameter and the SBD. It is important to have a good alignment when doing the measurement of the SBD, because misalignment will produce incorrect measurements. The ideal image is that of a figure "8" (see below).
2) Static balloon measurement. In most centers, the static balloon measurement technique is used.21 A highly compliant sizing balloon (i.e., Amplatzer sizing balloon) is infated across the ASD until fow across the defect disappears (stop-fow technique). This typically creates an indentation (sometimes minimal) on the balloon (Figure 11). Overstretching of the ASD should be avoided to prevent erosion related to the utilization of oversized devices.

The main advantage of this technique is its short inflation-deflation cycle, making the procedure much simpler. It is important to recognize that only when the largest diameter is strictly craneo-caudal in direction, will it truly estimate the full size of the defect, achieving a figure "8" pattern view.
The diameter of the indentation can also be measured with fuoroscopy (Figure 12) using calibration markers on the balloon catheter. SBDs by both methods are compared and measurements are repeated if there is a greater than 1 mm discrepancy.22

It is recommended to choose a device that is the same size of the SBP to prevent oversizing and erosions. However, some operators prefer devices 1-2 mm greater than the measured SBD22 and up to 2-4 mm greater than the SBD in the presence of large defects, in defects with a deficient or absent Ao, in defects with an aneurismal septum or in the presence of multiple defects.
Special considerations
In older patients, left diastolic ventricular dysfunction associated with elevated flling pressures is observed and may lead to secondary pulmonary hypertension. In these cases, the atrial septal defect, functioning as an over-fow, may mask the presence of left ventricular diastolic dysfunction by an enhanced left-to-right shunt. If such a mechanism is suspected, temporary balloon occlusion of the defect should permit its unmasking. This condition is characterized by a marked increase of the left atrial pressure, particularly of the v-wave, and a change of the infow pattern across the mitral valve resulting in a pathological increase of the E/A ratio.23 In these patients, closure of the ASD may lead to pulmonary edema. In elderly patients with co-existing left ventricular restriction, increased mean atrial pressures (> 10 mm Hg) and ASD, pre-conditioning treatment with intravenous dopamine, milrinone, and furosemide for 48–72 hr before definitive ASD closure with an Amplatzer septal occluder has been shown to prevent congestive heart failure after PTC.24
TEE during device positioning, deployment, and release
Device preparation for delivery is an important process of PTC and requires a meticulous approach on behalf of the interventional cardiologist (Figure 13).

In order to ensure stability during device delivery, the interventional cardiologist will position a supportive guidewire, through the ASD and left atrium, most often into the left upper pulmonary vein (LUPV). The echocardiographer should follow the guidewire at 40-60° in the mid-esophageal view, and confirm its correct positioning from the RA, through the ASD and left atrium into the LUPV, where it should be left to give support and stability to the delivery sheath (Figure 14). Given the fragility of the left atrial appendage, it is essential to avoid entering this thin-walled structure with catheters or the stiff guidewire, because this could cause perforation and lead to pericardial effusion.

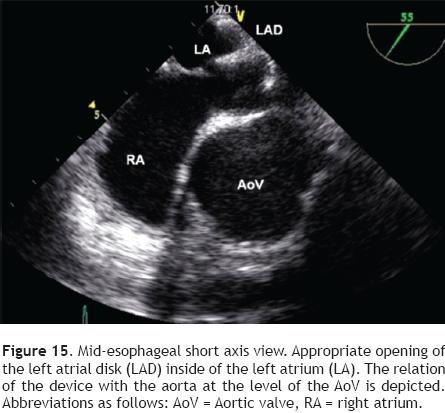
After having loaded the device in the delivery sheath, its insertion must be performed under TEE guidance. It is important to ensure that the tip of the delivery sheath is located in the left atrium, before deploying the left atrial disk of the closure device, in order to avoid deployment in the LUPV, the left ventricle or the left atrial appendage as this could cause deformation of the device, device entrapment or perforation of the atrial wall. Once the correct distal sheath position and the partially opened left disc position are confirmed by TEE, the left disk can be completely deployed (Figure 15). The device is then pulled back under TEE guidance toward the IAS so that the lower portion of the device catches the Ao or, in its absence, it encroaches the base of the aortic root. Thereafter the device is pulled toward the RA, so that its superior portion catches the superior aspect of the ASD (Figure 16). When resistance of the septum is encountered and TEE confirms good apposition of the LA disk with the rims of the ASD, the right atrial disk of the prosthesis is opened inside the RA, allowing the prosthesis to grasp the rims of the ASD between its two disks (Figure 17).


Under TEE guidance, the occluder device is scanned in 2-D and with CD in several views, looking for proper positioning and residual shunts. The echocardiographer must confirm that both disks are fattened with good apposition, and assess residual shunting. It is not uncommon to have discrete residual central or peri-prosthetic shunts, which usually will disappear after endothelialization of the occluder device (Figure 18).

When the Ao is absent, a typical "Y" pattern of the device being sandwiched around the AA should be seen (Figure 19). Failure to achieve this "Y" pattern of both disks requires device repositioning before release because this could lead to laceration of the aortic wall.

When a large Eustachian valve (EV) or Chiari network is present, it should be mentioned to the operator because it can cause device entrapment during deployment of the right atrial disk. This serious complication can be prevented by pushing back the structure using a second catheter.
Nearby structures might be compromised after positioning of the occluder device. In defects with a deficient IVC rim, infow from the IVC or the CS might be obstructed. Mitral valve leafets might be encroached by the occluder device, producing mitral regurgitation in a defect with a defcient AV rim and, infow from the SVC and RUPV might be compromised in a defect with a defcient SVC rim.
The Minnesota maneuver or wiggle is performed prior to release, to ensure stability of the occluder device.25 This maneuver consists in a gentle pushing and pulling (to and fro) motion of the device, to ensure that the device does not prolapse into the left atrium and that, conversely, the device does not prolapse into the RA (Figure 20). After this maneuver, the device is released. It is not uncommon to observe a change of position of the device en bloc with the inter-atrial septum, as tension is relaxed (Figure 21).


Immediate post procedural evaluation
A thorough evaluation for presence of residual shunts is performed for future correlation. The device and adjacent structures are evaluated8 to rule out device14 mal-positioning, interference with aortic, mitral, or tricuspid valvular function, caval, CS, or pulmonary venous return obstruction, and pericardial effusion.
Follow up
The presence of residual shunts should be reassessed; this could be achieved with contrast echocardiography with agitated normal saline, which opacifies the right sided cardiac chambers and may demonstrate the un-opacified jet of the left to right shunt. The potential of paradoxical embolus may be assessed by increasing right sided pressures with the Valsalva maneuver.13,26
The results of ASD closure with PTC for secundum ASD have been reported; approximately 7% of patients have a residual shunt immediately after device release. In the long term, a 97% complete shunt closure rate should be expected.27 The remaining residual shunts are usually not hemodynamically significant.
Transcatheter ASD closure is followed by near normalization of heart structure and function. The reversal of RV volume overload has been shown as early as 3 weeks post procedure in children and 9 months in adults,28 also systolic pulmonary artery pressure dropped to near normal levels during the following few months.
Abnormal septal motion of the inter-ventricular septum is expected to normalize shortly after the procedure. The degree of right bundle branch block (RBBB) diminished after PTC with an overall improvement in 61% of patients, whereas a RBBB pattern became more marked in 2.5% of the patients.15
It is important to be aware of the potential long term complications such as encroachment of mitral or aortic valve leafets, impairment of fow from the pulmonary veins, reactive or hemorrhagic pericarditis, and migration or dislodgement of the device. The use of aspirin 48 hours prior the procedure and for at least six months after the procedure is recommended, as well as antibiotic prophylaxis7 for six months after the procedure.
Follow up should include transthoracic echocardiography (TTE) the day following device deployment. Long-term follow up should be performed with TTE at three, six and 12 months after the procedure and when clinically indicated thereafter. In many centers a TEE is performed if there is clinical suspicion of a complication or if the TTE is inconclusive.
Conclusions
Percutaneous closure of significant shunting associated with secundum ASD represents an attractive less-invasive alternative therapy to surgery and is being increasingly performed worldwide. TEE is the ideal imaging and assessment tool to evaluate and guide procedures and determine immediate procedural success, while ruling out complications. Familiarization with TEE in this context is essential for the echocardiographer involved in the modern care of patients with ASD.16
Acknowledgements
To Catheryne Roy from the MHI Audiovisual Department for the designs and drawings in this manuscript.
References
1. Hoffman JI, Christianson R. Congenital heart disease in a cohort of 19,502 births with long-term follow-up. Am J Cardiol 1978;42:641-647. [ Links ]
2. Dickinson DF, Arnold R, Wilkinson JL. Congenital heart disease among 160 480 liveborn children in Liverpool 1960 to 1969. Implications for surgical treatment. Br Heart J 1981;46:55-62. [ Links ]
3. Silvestry FE, Kerber RE, Brook MM, et al. Echocardiography-guided interventions. J Am Soc Echocardiogr 2009;22:213-231. [ Links ]
4. Zabal C, Chio F, Amplatz K, et al. Percutaneous closure of an interatrial communication with the Amplatzer device. The first case in Mexico. Arch Inst Cardiol Mex 1998;68:147-152. [ Links ]
5. Boccalandro F, Baptista E, Muench A, et al. Comparison of intracardiac echocardiography versus transesophageal echocardiography guidance for percutaneous transcatheter closure of atrial septal defect. Am J Cardiol 2004;93:437-440. [ Links ]
6. Silversides CK, Dore A, Poirier N, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: shunt lesions. Can J Cardiol 2010;26:e70-79. [ Links ]
7. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:e1-121. [ Links ]
8. Cooke JC, Gelman JS, Harper RW. Echocardiologists' role in the deployment of the Amplatzer atrial septal occluder device in adults. J Am Soc Echocardiogr 2001;14:588-594. [ Links ]
9. Suarez De Lezo J, Medina A, Pan M, et al. Transcatheter occlusion of complex atrial septal defects. Catheter Cardiovasc Interv 2000;51:33-41. [ Links ]
10. Franke A, Kuhl H P, Rulands D, et al. Quantitative analysis of the morphology of secundum-type atrial septal defects and their dynamic change using transesophageal three-dimensional echocardiography. Circulation 1997;96:II-323-327. [ Links ]
11. Podnar T, Martanovic P, Gavora P, et al. Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv 2001;53:386-391. [ Links ]
12. Shanewise JS, Cheung AT, Aronson S, et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg 1999;89:870-884. [ Links ]
13. Denault AY. Transesophageal echocardiography multimedia manual: a perioperative transdisciplinary approach. New York. Taylor & Francis. 2005. XXVI, 581. [ Links ]
14. Mathewson JW, Bichell D, Rothman A, et al. Absent posteroinferior and anterosuperior atrial septal defect rims: Factors affecting nonsurgical closure of large secundum defects using the Amplatzer occluder. J Am Soc Echocardiogr 2004;17:62-69. [ Links ]
15. Remadevi KS, Francis E, Kumar RK. Catheter closure of atrial septal defects with deficient inferior vena cava rim under transesophageal echo guidance. Catheter Cardiovasc Interv 2009;73:90-96. [ Links ]
16. Cao Q, Radtke W, Berger F, et al. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transoesophageal echocardiography. Eur Heart J 2000;21:941-947. [ Links ]
17. Hanley PC, Tajik AJ, Hynes JK, et al. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: report of 80 consecutive cases. J Am Coll Cardiol 1985;6:1370-1382. [ Links ]
18. Agmon Y, Khandheria BK, Meissner I, et al. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation 1999;99:1942-1944. [ Links ]
19. Harper RW, Mottram PM, McGaw DJ. Closure of secundum atrial septal defects with the Amplatzer septal occluder device: techniques and problems. Catheter Cardiovasc Interv 2002;57:508-524. [ Links ]
20. Zhu W, Cao QL, Rhodes J, et al. Measurement of atrial septal defect size: a comparative study between three-dimensional transesophageal echocardiography and the standard balloon sizing methods. Pediatr Cardiol 2000;21:465-469. [ Links ]
21. Staniloae CS, El-Khally Z, Ibrahim R, et al. Percutaneous closure of secundum atrial septal defect in adults a single center experience with the amplatzer septal occluder. J Invasive Cardiol 2003;15:393-397. [ Links ]
22. Demkow M, Ruzyllo W, Konka M, et al. Transvenous closure of moderate and large secundum atrial septal defects in adults using the Amplatzer septal occluder. Catheter Cardiovasc Interv 2001;52:188-193. [ Links ]
23. Ewert P, Berger F, Nagdyman N, et al. Masked left ventricular restriction in elderly patients with atrial septal defects: a contraindication for closure? Catheter Cardiovasc Interv 2001;52:177-180. [ Links ]
24. Schubert S, Peters B, Abdul-Khaliq H, et al. Left ventricular conditioning in the elderly patient to prevent congestive heart failure after transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv 2005;64:333-337. [ Links ]
25. Masura J, Gavora P, Formanek A, et al. Transcatheter closure of secundum atrial septal defects using the new self-centering amplatzer septal occluder: initial human experience. Cathet Cardiovasc Diagn 1997;42:388-393. [ Links ]
26. Johansson MC, Eriksson P, Guron CW, et al. Pitfalls in diagnosing PFO: characteristics of false-negative contrast injections during transesophageal echocardiography in patients with patent foramen ovales. J Am Soc Echocardiogr 2010;23:1136-1142. [ Links ]
27. Knepp MD, Rocchini A P, Lloyd TR, et al. Long-term follow up of secundum atrial septal defect closure with the amplatzer septal occluder. Congenit Heart Dis 2010;5:32-37. [ Links ]
28. Mainzer G, Braver Y, Khoury A, et al. Morphologic, mechanical, conductive, and hemodynamic changes following transcatheter closure of atrial septal defect. Congenit Heart Dis 2010;5:25-31. [ Links ]














