Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.11 Tepic Jan./Dec. 2021 Epub May 21, 2021
https://doi.org/10.21929/abavet2021.1
Clinical case
Secondary infection by Leucobacter chromiiresistens in a dog with malignant axillary schwannoma type III
1Área Académica de Medicina Veterinaria y Zootecnia, Instituto de Ciencias Agropecuaria, Universidad Autónoma del Estado de Hidalgo. México.
2Instituto de Investigaciones en Ciencias Veterinarias, Universidad Autónoma de Baja California, Mexicali, Baja California, México.
3Facultad de Medicina Veterinaria y Zootecnia, Universidad Autónoma del Estado de México. México.
4Facultad de Ciencias Veterinarias, Universidad Austral de Chile, Valdivia, Chile.
The present report describes a schwannoma type III found in a 12-year-old male SharPei dog, this neoplasm was excised and analyzed using histopathological and immunohistochemistry examinations. This schwannoma was classified as high malignancy and presented metastasis to the axillary node. A secondary bacterial septicemia was identified with routine and molecular techniques. The Inmunohistochemical diagnosis of the mass, corroborated the presumptive diagnosis, which suggested a Schwannoma as well as its malignancy degree. In addition, Leucobacter chromiiresistens was isolated, a specie of which to date there are no reports as a pathogen in animals or humans.
Keywords: Schwannoma; SharPei; Leucobacter chromiiresistens
El presente reporte describe un schwannoma tipo III encontrado en un macho SharPei de 12 años, la neoplasia fue retirada y analizada por histopatología e inmunohistoquímica. El schwannoma fue clasificado con alta malignidad y con metástasis al linfonodo axilar. Con técnicas de rutina y moleculares se identificó una septicemia bacteriana secundaria. El diagnostico inmunohistoquímico confirmó el diagnóstico presuntivo de schwannoma, así como su grado de malignidad. Adicionalmente se aisló e identificó Leucobacter chromiiresistens, especie de la cual a la fecha no hay reportes como patógeno de animales o humanos.
Palabras clave: Schwannoma; SharPei; Leucobacter chromiiresistens
INTRODUCTION
Cutaneous tumors of neural origin are uncommon in domestic animals but are likely under-diagnosed because of their histologic similarity to other more common tumors of the skin. The name peripheral nerve sheath tumor (PNTs) is a broad term proposed to include all tumors arising from peripheral nerves (neurofibromas/neurofibrosarcomas, neurinomas, neurilemmomas, and schwannomas); however, because most tumors are composed of Schwann cells, the term schwannoma is appropriate for the majority of the tumors (Maxie et al., 2016).
The schwannoma (neurilemmoma) is a tumor of myelinated nerves that originates in the sheath of neurolemmocytes (Schwann cells), and presents a benign or malignant appearance. Neurilemmomas have been reported in animals including dogs, approximate incidence of these tumors in dogs was 3.8 in 100,000 animals and are most commonly observed in the cranial nerves, brachial plexus nerves, and in the seventh cervical nerves causing spinal cord compression (Ramírez et al., 2000; Baka et al., 2017). The schwannoma in dogs are reported in uveal, orbital, perianal, thoracic wall, among others nevertheless are usually subcutaneous, the neurilemmomas in the skin from dogs the usual location is the limbs and back (Maxie et al., 2016; Gaitero et al., 2008). The animals with tumors are prone to various metabolic, physiological, and secondary infections, including those caused by bacteria (Azap et al., 2012). The present report describes a schwannoma type III in a dog and with secondary infection caused by Leucobacter chromiiresistens.
CASE HISTORY
A Shar-Pei dog, male with 12-year-old, visited the Veterinary Hospital of the Autonomous University of Hidalgo State, to assess a new tissue growth in the right axillary region. The general physical examination revealed grade II periodontal disease, body condition 4/9, and presence of a newly formed tissue mass located subcutaneously in the right axillary region. The mass (15 cm long, 5 cm wide, 3 cm deep) was multi-lobed in appearance, non-painful, firm, slightly displaceable, and showed alopecia.
Hemogram and biochemical profiles were performed, no abnormalities were reported in the hemogram, but the biochemical profile showed marginal hyperproteinemia associated with inflammatory reaction. A blood sample was collected with BD Vacutainer® system in a heparin tube for blood culture, according to the methodology described in the Manual of Products and Laboratory Procedures (Zimbro et al., 2009).
Nodulectomy was performed one week after the physical examination, which involved an incision in the skin in the axillary region of the brachial plexus area. A newly formed tissue was observed that was highly vascularized, poorly delimited, non-encapsulated, and asymmetric, and involved the musculocutaneous nerve and possibly the radial nerve. Since the neoplasm was found to significantly infiltrate the underlying muscle tissue, partial resection of the deep pectoral muscle had to be performed. Furthermore, an enlarged and firm regional lymph node was observed. The excised regional tumor and lymph node mass were fixed in 4% formalin for histopathological and immunohistochemically studies (Prophet et al., 1995).
In the tumoral mass, numerous spindle-shaped neoplastic cells were observed, which were arranged in short intertwined bundles (Verocay’s bodies), supported and delimited by fibrovascular stroma (Figure 1, A). In other parts, there were extensive areas of granular eosinophilic material and few fusiform cells (histological pattern Antoni A and Antoni B) (Musha et al., 2018; Salazar et al., 2016).
The cells were fusiform, with poorly defined cell borders, moderate loose cytoplasm, central oval nucleus with sharp edges, granular thick chromatin, and moderate anisocytosis, anisokaryosis, and kariomegaly (Figure 1, B), and fourty three mitotic figures was counted in 2.37 mm2 in the periphery of the tumor in the area with the most mitotic activity. Extensive areas of coagulative necrosis, hemorrhage, and lymphocyte and neutrophil clusters were observed between the cells. The lymphatic vessels were seen distended by clusters of fusiform neoplastic cells. These findings were indicative of a malignant neurilemmoma (schwannoma) grade III (high degree of malignancy) (Ahmadi et al., 2012; Sharif et al., 2017).
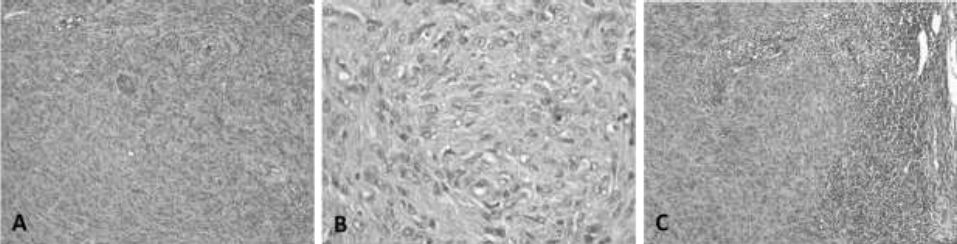
General appearanceof tumor:
A. The neoplastic cells, spindle-shaped, are arranged in short bundles (Verocay´s bodies), oriented in the three planes of space and supported by a fibrovascular stroma, relatively scarce.
B. 400X. Details of tumor cellularity. The cells are fusiform, with poorly defined cell borders, central oval nucleus, with thick granular chromatin and moderate anisocytosis, anisokaryosis and kariomegaly. C. 100X. Metastasis to regional lymphonode: The neoplastic tissue occupies the cortex and partially the medulla of lymphonode.
Figure 1 Neurilemmoma. Canine, Shar-pei, male, 4 years. H-E. A. 100X.
In the regional lymph node, there was a depletion of lymphoid cells, observing 60% of lymphoblasts, 15% mature lymphocytes, 15% plasmatic cells, and 10% of macrophages. The medullary sinuses revealed dense clusters of fusiform cells that replaced a large part of the parenchyma of the lymph node, with a very thin cortex and without the characteristic presence of lymphoid nodules (Figure 1, B). These findings corresponded to lymphatic permeation and metastasis to axillary nodes (Ahmadi et al., 2012).
To determine the tissue origin of the cells, an indirect immunohistochemistry was performed using the streptavidin-biotin-peroxidase technique with polyclonal antibodies, which tested positive for la glial fibrillary acidic protein (Figure 2, A), S-100 protein (Figure 2, B), and vimentin (Figure 2, C).
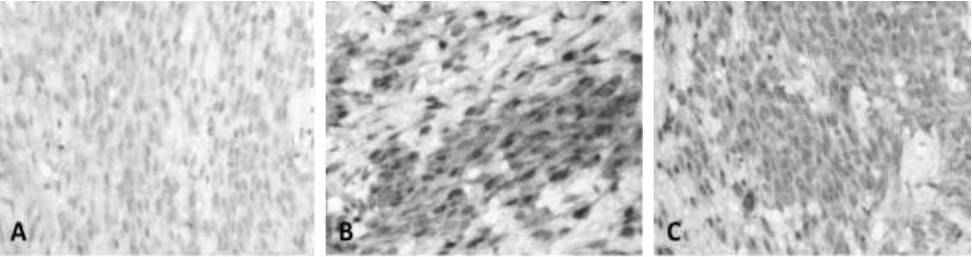
A. Fibrillar Acid Protein Glial, in a discrete but uniform pattern in most neoplastic cells.
B. Protein S-100, very notorious marking in groups of tumor cells
C. Vimentin, uniform labeling in cell groups of the neurilemoma.
Figure 2 Neurilemmoma. Canine, Shar-pei, male, 4 years. Immunolabeling with HRP. 400X.
In the processing of sample by bacteriological techniques, growth of yellow-pigmented colonies was observed with presence of Gram positive bacilli. The isolated strain were identified by amplification, sequencing, and comparing of the 16S rRNA gene (Zaragoza et al., 2017). The results of molecular analysis showed that the strain it had a similarity of 99.4% with Leucobacter chromiiresistens (Table 1 and Figure 3).
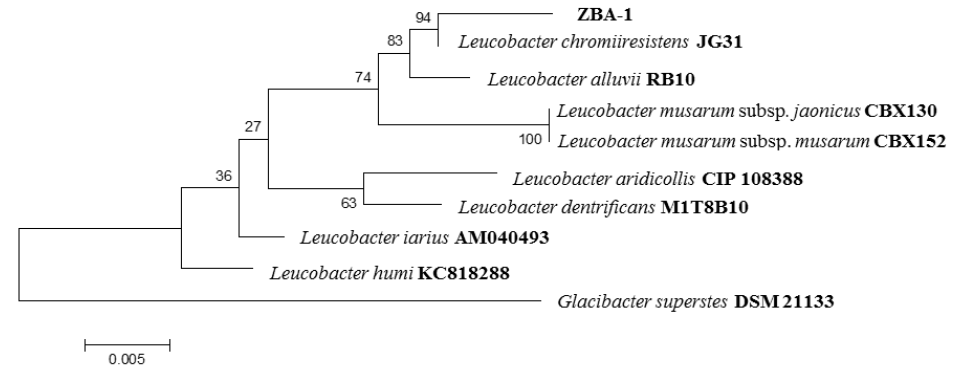
Figure 3 Phylogenetic tree constructed by comparing the 16S rRNA gene sequence from the strain isolated and reference strains.
Table 1 Comparison of 16S rRNA gene sequences of the strains isolated with those deposited in GenBank, using BLAST and EzTaxon
| Strain | Size (Bp) | Similar species | Similarity Blast (%) | Similarity Eztaxon (%) |
|---|---|---|---|---|
| ZBA-1 | 1400 | Leucobacter chromiiresistens | 99 | 99.4 |
| Leucobacter alluvii | 98 | 98.7 | ||
| Leucobacter musarum subsp. japonicus | 98 | 98.0 | ||
| Leucobacter musarum subsp. musarum | 98 | 98.0 | ||
| Leucobacter humi | 97 | 97.7 | ||
| Leucobacter iarius | 97 | 97.4 |
Bp: base pair
DISCUSSION
The Schwannomas can have different locations and presentations, in this study is reported an axillary schwannoma type III with high malignancy with metastasis to the axillary node, in a12-year-old male Shar-Pei. Axillar Schwannomas are not common in dogs. Nevertheless, in a study performed by Gaitero et al., in 2008 there were reported two canine PNSTs cases in the right axilla region, both of them in mixed breed dogs one of the tumors had a 3x2 cm dimension, similar to the reported Schwannoma in the present case (5x3 cm), these authors mention that there isnt´t breed predilection for PNSTs either is mentioned that affected animals were predominantely old dogs similar to this case.
In the literature it is reported that approximately 42% of dogs diagnosed with schwannomas have been euthanized as in the present case (Jones et al., 1975), after the surgical procedure, the patient was sent home with analgesia management that included meloxicam 0.1 mg/kg, tramadol 3 mg/kg, cephalexin 30 mg/kg, and omeprazole 1 mg/kg, and was advised rest and use of the Elizabethan collar. However, after 7 days, the patient presented with generalized weakness, pale mucous membranes, tachycardia, weak pulse, and loss of 2 kg weight. Due to the gradual deterioration of the patient, family members decide to opt for the euthanasia procedure, without authorization for necropsy.
The results of molecular analysis of bacteria determined that the strain it has a similarity of 99.4% with Leucobacter chromiiresistens, a Gram-positive, irregular rod-shaped, non- motile and yellow-pigmented bacterium, this species to date has no reports of infection in humans and animals (Sturm et al., 2011). In accordance with the literature, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, and Bacteroides spp are most commonly associated with secondary infections in patients with cancer (Lopardo et al., 2014). In this regard, the Leucobacter genus is not common in secondary infections in patients with cancer, but there are reports of infection by this genus, mainly in patients with terminal cancer (Adderson et al., 2008). The injuries caused by the secondary infection by Leucobacter chromiiresistens were unidentified, because the necropsy was not authorized.
CONCLUSION
The Inmunohistochemical diagnosis of the mass, corroborated the presumptive diagnosis, which suggested a schwannoma as well as its malignancy degree. Additionally, Leucobacter chromiiresistens was isolated, a specie of which to date there are no reports as a pathogen in humans or animals.
ACKNOWLEDGMENTS
The authors would like to acknowledge assistance from the Secretary of Research of Autonomous University of Hidalgo State and a special acknowledge to Dr. Julio C. Osorio Baños┼ for all his contributions in this case report.
REFERENCES
Adderson EE, Boudreaux JW, Hayden RT. 2008. Infections Caused by Coryneform Bacteria in Pediatric Oncology Patients. The Pediatric Infectious Disease Journal. 27(2):136-41. https://doi.org/10.1097/INF.0b013e31814fab12 [ Links ]
Ahmadi N, Oryan A, Ghane M, Daneshbod Y. 2012. Cutaneous schwannoma in a cow. Brazilian Journal of Veterinary Pathology. 5(2):81-85. http://bjvp.org.br/wp-content/uploads/2015/07/DOWNLOAD-FULL-ARTICLE-18-20881_2012_8_18_23_56.pdf [ Links ]
Azap A, Bozkurt GY, Yüksel MK, Kutlu H, Topçuoğlu P, Aypak A. 2012. Secondary infections in cancer patients with febrile neutropenia. Turkish Journal of Hematology. 29 (3):254-258. https://www.journalagent.com/tjh/pdfs/TJH_29_3_254_258.pdf [ Links ]
Baka O, Psalla D, Soubasis N, Polizopoulou Z. 2017. Cranial vena cava syndrome in a Dog with a brachial plexus malignant peripheral nerve sheath tumour. Australian Veterinary Practitioner. 47(4):113-116. https://www.researchgate.net/publication/330412296_Cranial_Vena_Cava_syndrome_in_a_dog_with_a_brachial_plexus_malignant_peripheral_nerve_sheath_tumour [ Links ]
Gaitero L, Anor S, Fondevila D, Pumarola M. 2008. Canine cutaneous spindle cell tumours with features of peripheral nerve sheath tumours: a histopathological and immunohistochemical study. Journal of Comparative Pathology. 139(1):16-23. https://doi.org/10.1016/j.jcpa.2008.03.003 [ Links ]
Jones BR, Williams OJ. 1975. Malignant schwannoma of the brachial plexus in a dog. Australian Veterinary Journal. 51(1):40-42. https://doi.org/10.1111/j.1751-0813.1975.tb14496.x [ Links ]
Lopardo HA. 2014. Infecciones por bacterias poco comunes y oncogénesis bacteriana. Revista Argentina de Microbiología. 46(1):1-6. https://doi.org/10.1016/S0325-7541(14)70066-5 [ Links ]
Maxie G. 2016. Nervous system en: Jubb, Kennedy & Palmer's. Pathology of Domestic Animals. St. Louis, Missouri EEUU: Elsevier Health Sciences. Pp. 404-405. ISBN: 978-0-7020-5317-7 [ Links ]
Musha A, Ogawa M, Yokoo S. 2018. Granular cell tumors of the tongue: fibroma or schwannoma. Head & Face Medicine. 14(1):1-7. https://doi.org/10.1186/s13005-017-0158-9. [ Links ]
Prophet EB, Mills B. Métodos Histotecnológicos. 1995. Instituto de Patología de las Fuerzas Armadas de los Estados Unidos de América (AFIP). Washington, D.C. United State of America. [ Links ]
Ramírez DM, Calzada LA, Colín FR. 2000. Schwannoma en la región pélvica de un perro de raza Doberman. Veterinaria México OA. 31(2):169-72. https://www.redalyc.org/pdf/423/42331215.pdf [ Links ]
Salazar MF, Tena Suck ML, Rembao Bojorquez D, Salinas Lara C. 2016. Intraventricular Neurilemmoma (Schwannoma): Shall GFAP Immunostaining Be Regarded as a Histogenetical Tag or as a Mere Histomimetical Trait. Case Reports in Pathology. 2016:6-12. http://dx.doi.org/10.1155/2016/2494175 [ Links ]
Sharif M, Mohamed A, Reinacher M. 2017 Malignant renal schwannoma in a cat. Open Veterinary Journal. 7(3):214-20. http://dx.doi.org/10.4314/ovj.v7i3.3 [ Links ]
Sturm G, Jacobs J, Spröer C, Schumann P, Gescher J. 2011. Leucobacter chromiiresistens sp. nov., a chromate-resistant strain. International Journal of Systematic and Evolutionary Microbiology. 61(4):956-60. https://doi.org/10.1099/ijs.0.022780-0 [ Links ]
Zaragoza BA, Rivero PN, Valladares CB, Isaac OK, Moreno PP, Sandoval TH. 2017. Molecular Identification of Mycobacterium Species of Public Health and Veterinary Importance from Cattle in the South State of México. Canadian Journal of Infectious Diseases and Medical Microbiology. 2017:1-7. https://doi.org/10.1155/2017/6094587 [ Links ]
Zimbro MB, Power DA, Miller SM, Wilson GE, Johnson JA. 2009. Manual of Microbiological Culture Media. Second Edition. Maryland United State of America. ISBN 0-9727207-1-5. https://www.trios.cz/wp-content/uploads/sites/149/2016/08/DIFCO-A-BBL-MANUAL-2.pdf [ Links ]
Received: August 29, 2020; Accepted: December 03, 2020; Published: January 04, 2021











 text in
text in 



