Services on Demand
Journal
Article
Indicators
Related links
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.11 Tepic Jan./Dec. 2021 Epub Apr 04, 2022
https://doi.org/10.21929/abavet2021.41
Original Article
Environmental factors associated with the prevalence of Haemonchus spp in lambs from the central zone of Sinaloa
1Facultad de Medicina Veterinaria y Zootecnia, Universidad Autónoma de Sinaloa.
2Instituto de Investigaciones en Ciencias Veterinarias, Universidad Autónoma de Baja California
Sheep are a species exploited in different areas of production. They are prone to different pathogens, highlighting parasites such as Haemonchus contortus. The prevailing climate and husbandry management practices are considered the main factors driving the spatial and temporal distribution of the nematode. Its distribution is worldwide, causing economic losses due to morbidity and mortality, and prevalence studies have been reported in different countries such as India, Spain, Nigeria, and Mexico; therefore, the aim of this study was to determine the edaphoclimatic factors of different zones from Culiacan municipality and the production system that influence the prevalence of Haemonchus spp. in lambs. The research was carried out in Culiacan municipality, Sinaloa, Mexico, over a period of one year. It was an observational study, which included 23 sheep production units distributed in 10 districts of the municipality, with a total of 1520 samples of feces from animals under 3 months of age. Feces were processed individually by flotation technique. The overall prevalence was 13.42 %, and the autumn season (OR 2.38 (1.69-3.34) P<0.001), valley zone (OR 2.70 (1.21-6.02); P<0.016) and extensive system (OR 4.81 (3.38-6.85); P<0.0001) were risk factors associated with the presence of the nematode in lambs, so they should be considered for the establishment of preventive measures and control of parasitosis.
Keywords: Haemonchus; prevalence; sheep; risk factor; gastrointestinal nematode
Los ovinos son una especie explotada en diferentes ámbitos de la producción. Estos son propensos a diferentes patógenos, destacando parásitos como Haemonchus contortus. El clima predominante y las prácticas de manejo en la crianza se consideran los principales factores que impulsan la distribución espacial y temporal del nematodo. Su distribución es mundial, ocasiona pérdidas económicas por morbilidad y mortalidad, se han reportado estudios de prevalencia en diferentes países como en India, España, Nigeria, México; por ello, el objetivo de este trabajo fue determinar los factores edafoclimáticos de las distintas zonas del municipio de Culiacán y sistema de producción que influyen en la prevalencia de Haemonchus spp en corderos. La investigación se realizó en el municipio de Culiacán, Sinaloa, México, en un periodo de un año, fue un estudio observacional, para el cual se incluyeron 23 unidades de producción ovina distribuidas en 10 sindicaturas del municipio, se realizó un muestreo por época del año colectando un total de 1520 muestras de heces procedentes de animales menores a 3 meses de edad. Las heces se procesaron individualmente por técnica de flotación. La prevalencia general fue de 13.42 %, y la época de otoño (OR 2.38 (1.69-3.34) P<0.001), zona de valle (OR 2.70 (1.21-6.02); P<0.016) y sistema extensivo (OR 4.81 (3.38-6.85); P<0.0001) resultaron factores de riesgo asociados a la presencia del nematodo en los corderos, por lo que deben considerarse para el establecimiento de medidas preventivas y de control de la parasitosis.
Palabras clave: Haemonchus; prevalencia; ovinos; factor de riesgo; nematodo gastrointestinal
INTRODUCTION
Gastrointestinal nematode infections affect the health of small ruminants compromising their production and reproduction, more frequently in young developing animals, causing low weight gain and growth retardation, making them one of the main causes of economic losses (González et al., 2011; Asmare et al., 2016; Kuma et al., 2019), mainly in the costs incurred in treatment and control (Tramboo et al., 2015). Sheep are generally more prone to gastrointestinal parasitism, due to their feeding on larvae 3 contaminated pastures (Tariq et al., 2008). Among gastrointestinal parasites, Haemonchus is the species with the highest economic importance (Rinaldi et al., 2015). This nematode is located in the abomasum, feeds on the blood of sheep and goats, can be found in other ruminants such as cattle (Getachew et al., 2007), it is among the most pathogenic in sheep (Besier et al., 2016), its extensive geographical distribution and resistance against anthelmintic control measures makes it a threat to the sustainability of sheep farming (Saccareau et al., 2017). The rainy season favors its frequency, with animals grazing during the early morning hours (Mederos et al., 2010), the prevailing climate (temperature, rain and humidity) and management practices in husbandry are considered the main factors driving its distribution (Rinaldi et al., 2015); its distribution is heterogeneous and depends on variables that differ from one area to another, even from one farm to another, such as management, prevention and control (Musella et al., 2011). On the other hand, studies on the prevalence in grazing animals is high in areas with tropical climates in both hemispheres (O´connor et al., 2006), young animals and pregnant females are more susceptible to helminths as opposed to adult animals due to their nutritional status and low level of immunity (Vieira et al., 2014). The prevalence of Haemonchus has been reported worldwide, in India Tramboo et al. (2015) out of a total of 1200 animals sampled 55 % were positive to the nematode; in Mexico, 32 % were found positive out of 219 sampled from grazing sheep (Hernández et al., 2007); in the Sinaloa region when analyzing 120 sheep from an extensive production system a frequency of 17.5 % was reported (Gaxiola et al., 2010). Therefore, the aim of this study was to determine the edaphoclimatic factors of different zones from Culiacán municipality and the production system that influence the prevalence of Haemonchus spp. in lambs in the central zone of Sinaloa.
MATERIAL AND MHETHODS
Study area
The study was carried out in Culiacán municipality, Sinaloa, Mexico (24º 46' 13'' NL and 107º 21' 14'' WL). The region is characterized by a BS1 (h') w(w)(e) climate, defined as semi-dry, very warm, with rainfall in summer, according to the Köppen classification and modified by García (2004); with an average annual temperature of 25. 9 ºC, with an average maximum of 30.4 ºC in June and July, and an average minimum of 20.6 ºC in January; the average relative humidity is 68%, with a maximum of 81% in September and a minimum of 51% in April; the average annual rainfall is 688.5 mm (CIAPAN, 2002).
Type of study and sample size
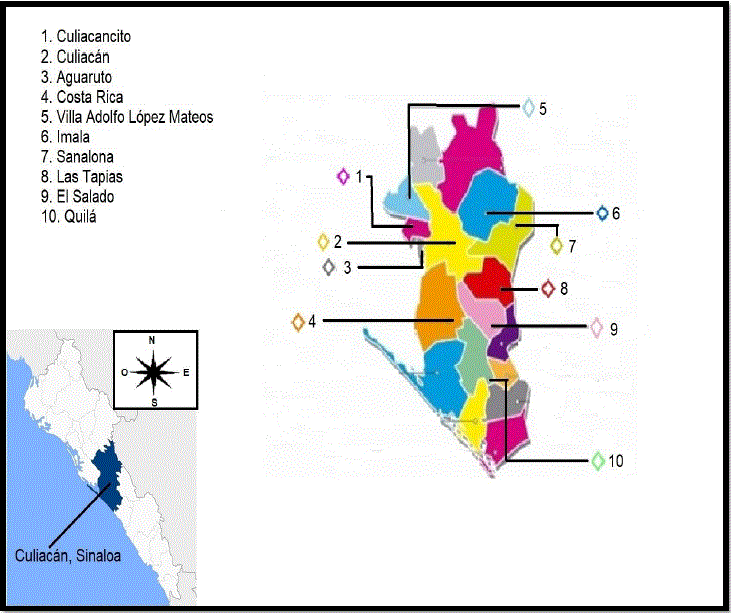
This is an observational, cross-sectional, descriptive study. Twenty-three extensive, semi- intensive and intensive ranches were sampled. In the municipality of Culiacán, 125 Sheep Production Units (SPU) are registered (SIAP, 2013), so the sample represented 18.4% of the SPUs. Ten of the 18 districts of Culiacán municipality, Sinaloa were considered (Figure. 1), the selection of the production units was made by convenience, based on the cooperation of the owner and ease of access. The sample size was determined with the following formula:
Where: n is the sample size, Z is 1.96 for 95 % confidence, p is the expected frequency of the factor to be studied, q is 1-p, B is precision or admitted error (Jaramillo & Martínez, 2010). The calculated sample size was 380 feces samples in each season (summer, fall, winter and spring) and because the number of lambs in the production unit was unknown at the time of the visit, the number of adults was considered (Table 1), and a number of lambs representing at least 10% of the adults in each production unit was sampled, which were randomly selected to complete the calculated sample size. Sampling was conducted by time of year and a total of 1520 samples were obtained from lambs less than ninety days (d) of age.
Table 1 Location of sheep production unit by district and population of adult sheep
| District | *SPU | Adult sheep | Lambs sampled |
|---|---|---|---|
| Villa Adolfo L.M | Agrícola Limón La Hacienda La granjita Alboradas |
80 150 120 180 |
10 20 17 18 |
| El Salado | El alacrán | 70 | 11 |
| Costa Rica | El trabajo Agrícola Sanfer Agrícola Tabachines |
20 150 180 |
5 15 18 |
| Quilá | Naranjos La Loma |
70 30 |
12 5 |
| Aguaruto | Fetasas Agrícola del Río Los Cabritos |
200 45 200 |
22 5 20 |
| Culiacancito | San Sebastián Agrícola Quiroz Los Otates |
250 250 180 |
25 25 20 |
| Las Tapias | Santa María | 130 | 13 |
| Sanalona | Baldomero | 20 | 5 |
| Imala | Guayacanes | 200 | 24 |
| Culiacán | Guásima Agrícola Mojolo Ganadera Verdugo Campo Morelia |
230 300 80 180 |
23 32 15 20 |
*SPU= Sheep Production Units
Sample collection and laboratory analysis
During each visit to the production units, a form was filled out to record information on the following factors: time of year (summer, fall, winter or spring), location (highlands or valley), production system (intensive, semi-intensive, extensive). The lambs were chosen randomly. Feces were taken directly from the rectum with a latex glove, individually identified, and refrigerated in containers at 4 ºC with ice and refrigerants for transfer to the Parasitology laboratory of the Faculty of Veterinary Medicine and Animal Husbandry for processing and analysis.
The diagnosis of Haemonchus spp was performed by coproparasitoscopic analysis using the qualitative Faust flotation technique (Zajac & Conboy, 2011), being a useful and widely used means in preliminary studies to determine what types of parasites are present in fecal samples (Medeiros et al., 2018), for parasite detection, optical microscopy with 10x and 40x objectives was used, eggs were identified based on their characteristic morphology, dark brown blastomeres and size described by Ljungström et al., 2018.
Statistical analysis
Lambs were considered positive with at least one Haemonchus spp egg; prevalence was estimated as the number of positive sheep among the total sheep sampled according to category.
The results of microscopic observation (positive or negative) were summarized in contingency tables by factor and analyzed for association between the result and the factor, using the Chi-squared test. Statistical difference was considered statistical difference with a P≤0.05.
For factors with more than two categories, the results were dichotomized. Next, to determine the risk factors for positive results, multivariate logistic regression analysis was applied. The general model was:
Where: π (x), the value of π can vary as the value of x changes, and we want to describe its dependence; the values of xi = (x1,...,xp) are the predictor variables p, xi represents the vector of independent variables; exp is the base of the natural logarithms 2.71828; α is the value of the intercept; βi are the values of the regression coefficients. For this analysis, the LOGISTIC procedure (SAS, 2001) with the backward option was used to estimate the degree of association [odds ratio (OR)] and confidence intervals. The alpha level to consider association between the factor with the positive outcome, and to estimate the risk factor was P ≤ 0.05.
RESULTS AND DISCUSSION
From a total of 1520 samples analyzed, 204 were positive for Haemonchus spp representing a prevalence of 13.42 % in the annual period analyzed; the method used to detect the nematode was the flotation technique, a test mainly used in stool examination in animal diagnosis, it concentrates eggs, parasite oocysts and separates the debris present in the sample (Zajac & Conboy, 2011; Rinaldi et al. 2011; Medeiros et al., 2018), one of the main advantages of using this test is that it has a high egg recovery rate (Medeiros et al., 2018), causes less damage to cysts and eggs (Zajac & Conboy, 2011), which allows proper morphological identification facilitating the characteristic structure observation, egg dimensions to be identified (Indre et al., 2010; Mahmood et al., 2019), the technique prevents flotation of trematode eggs and it is not as specific to determine the species of parasites observed (Zajac & Conboy, 2011; Ljungström et al., 2018), therefore results using this technique report only the Haemonchus genus. Factors studied are presented in Table 2, Chi-Square tests indicated that the three factors were significant (P<0.05), year time, production zone, production system; similarly for the analysis of risk factors were significant (P≤0.05) in the multivariate logistic regression model (Table 3). The results by year time showed a higher prevalence in autumn with 20.53 %, no difference was observed between winter and spring and the lowest prevalence was found in summer with 7.89 % (P<0.0001).
Table 2 Risk factors associated with the presence of Haemonchus spp. eggs in feces of lambs in SPU located in the municipality of Culiacán, Sinaloa, Mexico
| Risk factor | N | Positive samples | Percentage | P1 |
|---|---|---|---|---|
| Year season | 0.0001 | |||
| Summer | 380 | 30 | 7.89c | |
| Autumn | 380 | 78 | 20.53a | |
| Winter | 380 | 50 | 13.16b | |
| Spring | 380 | 46 | 12.11bc | |
| Production zone | 0.0001 | |||
| High | 183 | 7 | 3.83b | |
| Valleys | 1337 | 197 | 14.73a | |
| Production system | 0.0001 | |||
| Extensive | 437 | 112 | 25.63a | |
| Semi-intensive | 248 | 50 | 20.16a | |
| Intensive | 835 | 42 | 5.03b |
1Probability values of the Chi-square test. abc Different literals in the percentages of positive samples in each risk factor indicate statistical difference (P ≤ 0.01).
Table 3 Odds ratios for risk factors associated with the presence of Haemonchus spp. eggs in lamb feces in SPU located in the municipality of Culiacán, Sinaloa, Mexico
| Risk factor | Odd ratio | CI 95 % | P Value |
|---|---|---|---|
| Year season S-W-S Otoño | Reference 2.38 | 1.69-3.34 | 0.001 |
| Production season High Valleys | Reference 2.70 | 1.21-6.02 | 0.016 |
| Production system: Intensive-Semi Extensive | Reference 4.81 | 3.38-6.85 | 0.0001 |
S=Summer, W=Winter, S=Spring; CI = Confidence interval; P = Probability.
The results of the study showed that when comparing the different seasons of the year, there is a 2.38 times higher risk of presenting Haemonchus spp. in autumn than in the rest of seasons (P<0.001). The results of the present investigation in relation to the summer season with 7.89 % are close to those described in England with 10.5 % (Broughan & Wall., 2007), the low prevalence in summer can be attributed to the high temperatures that occur at this time in the area described, which is why the larvae decrease their activity, due to the negative phototropism to intense light (Soca et al., 2005), in another study related to year times in India reported the presence of the order Strongylida with 63.2 % in summer and 58.4 % in autumn, 52.77 % in winter and 61.3 % in spring (Tramboo et al., 2015), denoting difference with the current work, since in autumn there was a prevalence of 20.52 % being higher than that of summer with 7.89 %, this can be interpreted by the maximum temperatures that occur in summer in the study area, in addition to the increased humidity in the pastures in the autumn season that favor larval migration by positive hydrotropism (Soca et al., 2005), regarding winter and spring, differences were also found between the two studies, since in India the prevalence increased from winter to spring, this is attributed to the precipitation that favors the humidity of the pastures for the presence of the parasite. Contrary to the present result, there was no significant difference from winter to spring in relation to the nematode presence, this is interpreted to the state of hypobiosis in which the helminth enters, in an unfavorable period nutritionally, so it tends to lower its metabolism and activity (Soca et al., 2005); in a study conducted in Iran (Moghaddar, 2008), sampled lambs under five months, during the four seasons of the year, it was reported the presence of nematodes 25.9, 22.3, 50 and 53.1 % for autumn, winter, spring and summer, respectively. The differences that stand out among the studies is the report of nematodes in general and therefore a higher percentage of positive lambs, another factor that indicates a difference between the prevalence between seasons is precipitation. In the case of Iran, there is more rainfall in March and April, so there are more nematodes in spring and summer, and in the study region of Culiacán, rainfall occurs in August and September, favoring the conditions for Haemonchus in autumn, coinciding with a study conducted in the same area with the protozoan Cryptosporidium spp. in lambs, showing 2. 2 times more risk of presenting the parasite in autumn than in summer (Castro et al., 2017), although they are taxonomically classified in different phylum the climatic conditions at this time favor both.
When analyzing the geographic zone of the region, the results indicate a higher prevalence for the valley with 14.73 % as opposed to the highland zone with 3.83 % (P<0.0001), and 2.70 times higher risk of presenting the nematode in lambs in the valley zone (P<0.016). 016), presenting similarity to what was found in Ethiopia the difference that was reported in general were gastrointestinal nematodes, in valley production zone was higher than the high zone, with 95 and 68.6 % respectively and coincide in terms of higher risk of presenting Haemonchus in the valley production zone (Asmare et al., 2016), likewise in Switzerland in high zones a low presence of the parasite was reported in comparison with medium and low zones in Italy and Ireland (Rinaldi et al., 2015), the water source is one of the key characteristics for the survival and dissemination of nematodes such as Haemonchus (Musella et al., 2011; Rinaldi et al., 2015), which occurred more in valley areas mainly because of dams, these areas are lower areas that can carry contaminants through water tributaries, including parasite eggs from production units or wild animals that live in higher areas, favoring the dissemination of these when this water is used for irrigation, animal consumption, among other activities of common use. Gastroenteric parasites and especially Haemonchus have managed to adapt to different ecosystems, their ability to adapt and survive different environments allow the infection of new hosts (Munguía et al., 2018).
According to the production system the presence of Haemonchus spp was found 25.63% in extensive production system, 20.13 % in semi-intensive system and 5.03% in intensive system (P<0.0001) and when analyzing the association of parasitosis this was 4.81 times more risk of occurrence in lambs under an extensive production system (P<0.0001). The results differ from those performed by Zapata et al. (2016) and Herrera et al. (2013) in which no statistical difference was found among the three production systems analyzed. The conditions of the present study in animals under extensive systems are not systematized, they did not manage a deworming schedule, which agrees with Mederos et al. (2010) who indicate that the highest levels of gastrointestinal parasites occur in production units where there is no routine deworming management; in addition to the type of grazing-based feeding facilitates the ingestion of the parasite larvae present in the vegetation, and allows transmission as the animals themselves contaminate the grazing area (Belina et al., 2017; Akyüz et al., 2019); on the other hand, in the semi- intensive and intensive systems, deworming management was programmed and feeding was operated with diets prepared mainly in the intensive system which favored a lower percentage of the parasite presence in these systems due to the positive impact given by better quality diets on health; Cériac et al. (2019) and Naeem et al. (2021) point out that quality nutrition supplemented with high protein, amino acids stimulates the expression of host resistance and resilience, stimulates immunity, decreases parasite proliferation.
CONCLUSION
The autumn season, valley area and extensive production system are the edaphoclimatic factors associated with the prevalence of Haemonchus spp (13.42%) in lambs in Culiacán municipality, Sinaloa; therefore, these aspects should be taken into account to develop strategies for the prevention and control of parasitosis. H. contortus is recognized as one of the main parasites affecting sheep, which makes it necessary to consider this agent as a possible cause of productive disorders even in early stages of the animals' age, as well as a possible infection source from young animals to the rest of the flock.
ACKNOWLEDGMENTS
To the Association of Sheep and Goat Breeders Culiacán AC. and the National Council of Science and Technology (CONACyT) Mexico, for the economic support for the training of human resources in this project.
REFERENCES
Akyüz M, Kirman R. Yaya S, Gülbeyen H, Güven E. 2019. Endoparasites Determined by Fecal Examination in Sheep in Erzurum Province. Turkiye Parazitol Derg. 43(4): 187-193. https://doi.org/10.4274/tpd.galenos.2019.6512 [ Links ]
Asmare K, Sheferaw D, Aragaw K, Abera M, Sibhat B, Haile A, Kiara H, Szonyi B, Skjerve E, Wieland B. 2016. Gastrointestinal nematode infection in small ruminants in Ethiopia: A systematic review and meta-analysis. Acta Tropica. 160: 68-77. ISSN: 0001-706X. https://doi.org/10.1016/j.actatropica.2016.04.016 [ Links ]
Belina D, Giri A, Hailu S, Eshetu A. 2017. Gastrointestinal Nematodes in Ruminants: The Parasite Burden, Associated Risk Factors and Anthelmintic Utilization Practices in Selected Districts of East and Western Hararghe, Ethiopia. Journal of Veterinary Science and Technology. 8: 433. https://doi.org/10.4262/2157-7579.1000433 [ Links ]
Besier RB, Kahn LP, Sargison ND, Van-Wyk JA. 2016. The Pathophysiology, Ecology and Epidemiology of Haemonchus contortus Infection in Small Ruminants. Haemonchus contortus and Haemonchosis - Past, Present and Future. Trends. 93: 95-143. ISSN: 0065- 308X. https://doi.org/10.1016/bs.apar.2016.02.022 [ Links ]
Broughan JM, Wall R. 2007. Faecal soiling and gastrointestinal helminth infection in lambs. International journal for parasitology. 37(11): 1255-68. ISSN: 0020-7519. https://doi.org/10.1016/j.ijpara.2007.03.009 [ Links ]
Castro DCN, Castro DCN, Enríquez VI, Portillo LJJ, Gaxiola CSM. 2017. Prevalencia y factores de riesgo asociados a Cryptosporidium spp en corderos de pelo del municipio de Culiacán, Sinaloa, México. Revista Científica de la Facultad de Ciencias Veterinarias de la Universidad de Zulia. 27(4): 211-220. ISSN: 2477-944X. https://www.redalyc.org/jatsRepo/959/95953011003/html/index.html [ Links ]
Cériac S, Archimède H, Feuillet D, Félicité Y, Giorgi M, Bambou JC. 2019. Supplementation with rumen-protected proteins induces resistance to Haemonchus contortus in goats. Scientific Reports. 9(1): 1237. https://doi.org/10.1038/s41598-018-37800-3 [ Links ]
CIAPAN (Centro de Investigaciones Agrícolas del Pacífico Norte). 2002. Guía para la asistencia técnica del Valle de Culiacán. Instituto Nacional de Investigaciones Agrícolas, Forestales y Pecuarias. Culiacán, Sinaloa, México. Pp. 92. [ Links ]
Garcia E. 2004. Modificaciones al sistema de clasificación climática de Köppen (para adaptarla a las condiciones climáticas de la República Mexicana). QuintaEdición. Instituto de Geografía. Universidad Nacional Autónoma de México. Pp. 144. ISBN 970- 32-1010-4. [ Links ]
Gaxiola CSM, Castro DCN, Borbolla IJE, Cárcamo ANM, Cota GSC, Villalba RJ E, Gaxiola MJ, Barraza TCL, Pérez CJA, Martínez T, Sosa GC, Meza TMA, Mimiaga LG, Rodríguez GMA. 2010. Frecuencia de parásitos gastrointestinales en ovinos del municipio de Culiacán, Sinaloa, México. 6° Seminario Internacional en Reproducción Animal y Producción de Leche y Carne. 2° Seminario Internacional de Avances en Producción Animal. Mazatlán, Sinaloa, México, 11 y 12 de marzo. Pp. 295. [ Links ]
Getachew T, Dorchies P, Jacquiet P. 2007. Trends and challenges in the effective and sustainable control of Haemonchus contortus infection in sheep. Review. Parasite-Journal De La Societe Francaise De Parasitologie. 14: 3-14. ISSN: 1252-607X. https://doi.org/10.1051/parasite/2007141003 [ Links ]
González GR, Córdova PC, Torres HT, Mendoza GP, Arece GJ. 2011. Prevalencia de parásitos gastrointestinales en ovinos sacrificados en un rastro de Tabasco, México. Veterinaria México. 42: 125-135. ISSN: 0301-5092. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0301-50922011000200003 [ Links ]
Hernández S, Segura I, Olivares PJ, Almazán MT. 2007. Prevalencia de nematodos gastrointestinales en ovinos en pastoreo en la parte alta del MPIO. de Cuetzala del Progreso, Guerrero-México. Redvet. 8(9): 1-7. ISSN: 1695-7504. https://www.researchgate.net/publication/26475878_Prevalencia_de_nematodos_gastrointestinales_en_ovinos_en_pastoreo_en_la_parte_alta_del_MPIO_De_Cuetzala_del_Pr ogreso_Guerrero-Mexico [ Links ]
Herrera OL, Ríos OL, Zapata SR. 2013. Frecuencia de la infección por nemátodos gastrointestinales en ovinos y caprinos de cinco municipios de Antioquia. Revista MVZ Córdoba. 18(3): 3851-3860. ISSN: 0122-0268. https://doi.org/10.21897/rmvz.157 [ Links ]
Indre D, Dărăbuş GH, Oprescu I, Morariu S, Mederle N, Ilie MS, Imre M. 2010. Morphometrical studies on some eggs of gastrointestinal nematodes from sheep. Lucrări Ştiinłifice Medicină Veterinară. 1: 30-35. https://www.usab-tm.ro/vol10MV/5_vol10.pdf [ Links ]
Jaramillo C, Martínez JJ. 2010. Epidemiologia veterinaria. España: Editorial el Manual Moderno. Pp 110-125. ISBN: 978-607-448-038-2. https://www.manualmoderno.com/apoyos_electronicos/9786074480382/jaramillo_main_ p.php [ Links ]
Kuma B, Abebe R, Mekbib B, Sheferaw D, Abera M. 2019. Prevalence and intensity of gastrointestinal nematodes infection in sheep and goats in semi-intensively managed farm, South Ethiopia. Journal of Veterinary Medicine and Animal Health. 11(1): 1-5. ISSN: 2141-2529. https://doi.org/10.5897/JVMAH2018.0705 [ Links ]
Ljungström S, Melville L., Skuce PJ, Höglund J. 2018. Comparison of Four Diagnostic Methods for Detection and Relative Quantification of Haemonchus contortus Eggs in Feces Samples. Frontiers in veterinary science. 4: 239-239. ISSN: 2297-1769. https://doi.org/10.3389/fvets.2017.00239 [ Links ]
Mahmood OI, Muhsin SN, Hussein M. 2019. Morphological Diagnosis for Some Eggs of Gastrointestinal Nematodes from Sheep. Tikrit Journal for Agricultural Sciences. 19(3): 6-9. ISSN: 2664-0597. https://www.researchgate.net/publication/343738495_Morphological_Diagnosis_for_Some_Eggs_of_Gastrointestinal_Nematodes_from_Sheep [ Links ]
Medeiros KL, Lucio FA, Bowman DD. 2018. Evaluation of Parasite Egg and Cyst Recovery Using Devices Designed for Centrifugal or Stationary Flotation. J Am Anim Hosp Assoc. 54(1):36-45. https://doi.org/10.5326/JAAHA-MS-6549 [ Links ]
Mederos A, Fernandez S, Vanleeuwen J, Peregrine AS, Kelton D, Menzies P, LeBoeuf A, Martin R. 2010. Prevalence and distribution of gastrointestinal nematodes on 32 organic and conventional commercial sheep farms in Ontario and Quebec, Canada (2006- 2008). Veterinary parasitology. 170: 244-52. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2010.02.018 [ Links ]
Moghaddar N. 2008. Seasonal variation in the incidence of gastro-intestinal nematodes in sheep in Iran. Journal of Applied Animal Research. 34: 153-155. ISSN: 0974-1844. https://doi.org/10.1080/09712119.2008.9706961 [ Links ]
Munguía XJ, Navarro GR, Hernández CJ, Molina BR, Cedillo CJ, Granados RJ. 2018. Parásitos gastroentéricos, población Haemonchus contortus en caprinos en clima semiárido de Bacum, Sonora, México. Abanico Veterinario. 8(3): 42-50. ISSN 2448-6132. http://dx.doi.org/10.21929/abavet2018.83.2 [ Links ]
Musella V, Catelan D, Rinaldi L, Lagazio C, Cringoli G, Biggeri A. 2011. Covariate selection in multivariate spatial analysis of ovine parasitic infection. Preventive veterinary medicine. 99: 69-77. ISSN: 1873-1716. https://doi.org/10.1016/j.prevetmed.2010.11.012 [ Links ]
Naeem M, Iqbal Z., Roohi N. 2020. Ovine haemonchosis: a review. Tropical Animal Health and Production. 53(1): 19. https://doi.org/10.1007/s11250-020-02439-8 [ Links ]
O'connor LJ, Walkden BSW, Kahn LP. 2006. Ecology of the free-living stages of major trichostrongylid parasites of sheep. Veterinary Parasitology. 142: 1-15. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2006.08.035 [ Links ]
Rinaldi L, Coles GC, Maurelli MP, Musella V, Cringoli G. 2011. Calibration and diagnostic accuracy of simple flotation, McMaster and FLOTAC for parasite egg counts in sheep. Vet Parasitol. 177(3-4): 345-52. https://doi.org/10.1016/j.vetpar.2010.12.010 [ Links ]
Rinaldi L, Catelan D, Musella V, Cecconi L, Hertzberg H, Torgerson PR, Mavrot F, De Waal T, Selemetas N, Coll T, Bosco A, Biggeri A, Cringoli G. 2015. Haemonchus contortus: spatial risk distribution for infection in sheep in Europe. Geospatial Health. 9(2): 325-331. ISSN: 1970-7096. https://doi.org/10.4081/gh.2015.355 [ Links ]
Saccareau M, Salle G, Robert-Granie C, Duchemin T, Jacquiet P, Blanchard A, Cabaret J, Moreno CR. 2017. Meta-analysis of the parasitic phase traits of Haemonchus contortus infection in sheep. Parasites & Vectors. (10): 201. ISSN: 1756-3305. https://doi.org/10.1186/S13071-017-2131-7 [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera). 2013. Población ganadera ovina. Culiacán, Sinaloa, México. https://nube.siap.gob.mx/cierre_pecuario/ [ Links ]
Soca M, Roque E, Soca M. 2005. Epizootiología de los nematodos gastrointestinales de los bovinos jóvenes. Pastos y Forrajes. 28: 175-185. ISSN: 0864-0394. https://www.redalyc.org/pdf/2691/269121675001.pdf [ Links ]
Tariq KA, Chishti MZ, Ahmad F, Shawl AS. 2008. Epidemiology of gastrointestinal nematodes of sheep managed under traditional husbandry system in Kashmir valley. Veterinary parasitology. (158): 138-143. ISSN: 0304-4017. https://doi.org/10.1016/j.vetpar.2008.06.013 [ Links ]
Tramboo SR, Shahardar RA, Allaie IM, Wani ZA, Bushra MS. 2015. Prevalence of gastrointestinal helminth infections in ovine population of Kashmir Valley. Veterinary world. 8(10): 1199-1204. ISSN: 0972-8988. https://doi.org/10.14202/vetworld.2015.1199-1204 [ Links ]
Vieira VD, Vilela VL, Feitosa TF, Athayde AC, Azevedo SS, Souto DV, Silveira GL, Melo LR. 2014. Sheep gastrointestinal helminthiasis in the Sertão region of Paraíba State, Northeastern Brazil: prevalence and risk factors. Brazilian journal of veterinary parasitology. 23(4): 488-494. ISSN: 0103-846X. https://doi.org/10.1590/s1984-29612014089 [ Links ]
Zajac AM, Conboy GA. 2011. Veterinary clinical parasitology. Iowa, USA: Editorial John Wiley & Sons, Inc. Pp. 354. ISBN: 978-0-8138-2053-8. https://www.wiley.com/en-ad/Veterinary+Clinical+Parasitology%2C+8th+Edition-p-9780813820538 [ Links ]
Zapata SR, Velásquez VR, Herrera OLV, Ríos OL, Polanco EDN. 2016. Prevalencia de nematodos gastrointestinales en sistemas de producción ovina y caprina bajo confinamiento, semiconfinamiento y pastoreo en municipios de Antioquia, Colombia. Revista de Investigaciones Veterinarias del Perú. 27(2): 344-354. ISSN: 1609-9117. https://doi.org/10.15381/rivep.v27i2.11647 [ Links ]
Received: July 26, 2021; Accepted: November 08, 2021











 text in
text in