Services on Demand
Journal
Article
Indicators
Related links
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.11 Tepic Jan./Dec. 2021 Epub Oct 11, 2021
https://doi.org/10.21929/abavet2021.14
Original Article
Effect of a short-term prostaglandin protocol upon synchronization and reproductive outcomes in cyclic goats
1Instituto Tecnológico de Torreón, Carretera Torreón-San Pedro de las Colonias KM 7.5, Ejido Ana, 27170, Torreón, Coahuila, México.
2Universidad Juárez del Estado de Durango, Facultad de Agricultura y Zootecnia, Venecia, Villanápoles, 35018, Gómez Palacio, Durango. México.
3Universidad Autónoma Agraria Antonio Narro, Unidad Laguna, Periférico Raúl López Sánchez, Valle Verde, 27054, Torreón, Coahuila, México.
The aim of the study was to evaluate the reproductive response of cyclic goats in northern Mexico (26° N) subjected to two administration schemes of prostaglandins (PG; 5 days vs. 10 days) to synchronize estrus response. Cyclic adult French-Alpine goats were allocated in two groups. Group G5 (n = 10), received the first PG-injection (0.2 ml; 160 μg cloprostenol) on d -5 and group G10 (n = 10) received the first PG-injection on d -10. Both groups received the second PG-injection on d 0. General reproductive outcomes after second PG-administration were similar (P>0.05) between treatments. Estrus response= 60% for both groups, estrus latency= 68h vs 52h, ovulation= 80% vs 60% (G5 vs G10, respectively; P>0.05). Since no differences occurred between groups, administration of the G5 treatment seems to be an interesting short-term synchronizing alternative protocol in that it generated important reproductive outcomes.
Keywords: French-Alpine goats; breeding season; estrus synchronization; prostaglandins; reproductive outcomes
El objetivo del presente estudio fue evaluar la respuesta reproductiva de cabras cíclicas del norte de México (26ºN) sometidas a dos protocolos de administración de prostaglandinas (PG; 5 días vs 10 días) con el fin de sincronizar la respuesta estral. Se formaron 2 grupos de hembras caprinas adultas cíclicas de la raza Alpino francés. Al Grupo G5 (n=10), se les administró la primera inyección de PG (0.2 ml; 160 μg cloprostenol) el d -5 y el grupo G10 (n=10) recibió la primera inyección de PG el d -10. Ambos grupos recibieron la segunda inyección de PG el d 0 (noviembre 1). La respuesta reproductiva general después de la segunda inyección de PG fue similar (P>0.05) entre ambos tratamientos. Respuesta estral = 60% para ambos grupos, latencia al estro = 68h vs 52h, ovulación = 80% vs 60% (G5 vs G10, respectivamente; P>0.05). Debido a que no hubo diferencias entre grupos, la administración del tratamiento G5 parece ser una alternativa interesante para la utilización de un protocolo corto ya que generó una respuesta reproductiva importante.
Palabras clave: Cabras Alpino Francés; época reproductiva; sincronización estral; prostaglandinas; respuesta reproductiva
INTRODUCTION
In small ruminants, most of the reproductive protocols to synchronize estrous cycles during the breeding season, are based on the use of exogenous hormone treatments with different doses and time regimes (Martemucci and D’Alessandro, 2011).
Prostaglandin F2α is the main luteolytic agent used to synchronize estrus during the reproductive season in these species, it is rapidly metabolized by lungs, without tissue accumulation, being an interesting alternative to the use of progestogens, eCG and hCG (Omontese et al., 2016). Nonetheless, a disadvantage of the use of prostaglandins compared to eCG or hCG is that ovulation is greatly dispersed as the response varies with the stage of the estrus cycle in which the prostaglandins are administered (Houghton et al. 1995). After the first administration of prostaglandins, short estrus cycles occur, and an important percentage of females do not respond to the second administration. In fact, by reducing the period between the first and second administrations of prostaglandins from 10 to 7 days, the occurrence of short estrus cycles is prevented and a higher reproductive response is obtained (Maia et al., 2017). It has been mentioned that, in goats, the corpus luteum is sensible to PGF2α from day 3 after the end of estrus, obtaining a high proportion of females that show estrus after the administration (Rubianes et al., 2003). As mentioned above, use of PGF2α is a clean reproductive management as it does not leave residues in the tissues (Omontese et al., 2016) also, animal welfare can be increased when reproductive strategies that make herd management faster and more efficient are implemented (Roger, 2012). Therefore, new and innovative reproductive protocols should promote animal welfare, by reducing the period of time managing the animals, reducing the use of exogenous hormones as well as by decreasing health problems in the female reproductive tract. Such strategies should diminish, in parallel, the cost of reproductive treatments as well as hand labor (Abecia et al., 2011; Gonzalez-Bulnes et al., 2011).
Based on such rationale, we hypothesized that administration of a short-term PG- synchronization protocol (5 days) to goats during the breeding season, should elicit similar results that longer PG-administration protocols (i.e. 10 or more days). Therefore, the aim of this research was to reduce treatment time, and evaluate the reproductive response of cyclic French Alpine goats in the Comarca Lagunera, Mexico (26° N).
MATERIALS AND METHODS
General
All methods and management of the experimental units used in this trial was in strict accordance with accepted guidelines for ethical use, care and welfare of animals in research at international (FASS, 2010), national (NAM, 2002) and institutional levels, with approval reference number ITT-513.2.2/1879/2014-5458-14P.
Location, animals, management and experimental groups
The trial was conducted at the Instituto Tecnologico de Torreon (ITT), located in Northern Mexico, in the Comarca Lagunera (26°23’ N, 104°47’ W and 1,100 m); the day length is 13 h 41 min in the summer solstice and 10h 19 min in the winter solstice. It was carried- out from October to November, corresponding to the goat´s natural breeding season. The health status of all the experimental units was controlled by an experienced veterinarian during the whole trial period; no health problems occurred during the trial. Besides, efforts were made to minimize any possible discomfort in the experimental animals.
Cyclic adult French-Alpine goats (n=20; 3 yrs old), were randomly allocated to two homogeneous groups (P>0.05) according to live weight (LW) and body condition score (BCS: 1= emaciated and 4= fat). Group G5 (n = 10) had 45.9 ± 1.9 kg LW and 2.4 ± 0.2 units BCS; while G10 (n = 10) and 46.8 ± 1.7 kg LW and 2.5 ± 0.1 units BCS. In order to synchronize estrus, both groups received two intravulvarly doses of a prostaglandin analog (0.2 ml; 160 μg cloprostenol). Even though the reproductive parameters of interest for the study are related to second PG-injection, and the experiment was designed to evaluate the reproductive response after the second administration, administered on the same day (day 0; Nov 1) for both groups, the reproductive parameters were measured after the first and second PG-administrations. On day -5 (Oct 27), G5 received a first PG- injection; while G10 received the first PG-injection on day -10 (Oct 22).
All the females were allocated in 6 x 6 m open pens, separated 60 m from each other. Does had ad libitum access to drinkable water and received a diet which met their nutritional requirements for maintenance, consisting of ad libitum access to alfalfa hay (17% crude protein, CP; 1.9 MCal ME/kg DM) and 100 g of commercial concentrate (14% CP, 2.7 MCal ME/kg DM), available during the whole experimental period. A schematic representation of the experimental procedure is shown in Fig. 1.
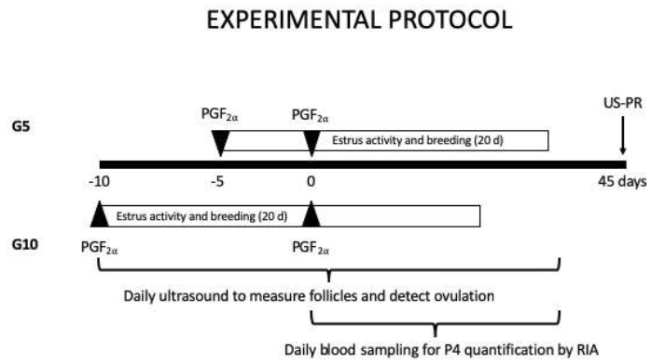
Figure 1 A schematic representation of the experimental protocol to synchronize adult cyclic French-Alpine dairy goats during the breeding season (Oct-Nov, 26° N) in northern Mexico. Both groups received two doses of prostaglandin (0.2 ml; 160 μg cloprostenol) for G5 on October 27 and G10 on October 22. Then, both groups received a second dose on November 1.
Assessment of estrous activity
Once does received the first PG-injection, estrous activity was evaluated for 20 days, twice per day (0800 & 1700 h) for 15 minutes each time, using a sexually active male provided with an apron. Does detected in standing heat were mated at least twice by a male proven for fertility and libido.
Assessment of ovarian activity and pregnancy
Goats from both groups underwent daily trans-rectal real-time, B-mode ultrasonographic scanning (Aloka SSD500 linear array; Overseas Monitor Corp. Ltd., Richmond, BC, Canada) throughout the whole research period in order to detect and measure ovarian structures. Scanning was conducted by one experimental operator with does in standing position. The transducer was inserted into the rectum until an image of ovaries was observed on the monitor. Then the transducer was rotated until both ovaries were scanned. Daily, diameter of follicles was measured (2-3, 4-5 and pre-ovulatory ≥ 6 mm), and their location on the ovaries recorded. The corpus luteum was identified on gray scale as hypoechoic area within an ovary. After both PG-administrations, ovulation was detected by measuring the preovulatory follicle with the maximal diameter (≥6 mm) and observing the morphologic changes within the ovary from follicular tissue to the formation of a corpus luteum. Pregnancy diagnosis was done at 45 days post-mating using transrectal scanning according to the procedures outlined, based on findings with the ultrasound, ovulation and number of corpus luteum percentages were calculated, (Contreras-Villarreal et al., 2016; Medan et al., 2003).
Quantification of plasma progesterone concentrations
A daily blood sampling was performed by jugular venipuncture starting on the second PG administration and lasting 10 days. Blood was centrifuged and plasma was collected in duplicated and stored at -20°C until hormonal analysis. Plasma P4 concentration was determined by radioimmuno analysis (RIA), using a commercial RIA kit (Diagnostic Products, Los Angeles, CA, USA) validated for ruminant plasma (Schneider and Hallford, 1996). The intra- and inter-assay coefficients of variation were 9.9 and 12.3% respectively. Whereas the average recovery was 94%, the sensitivity of the assay was 0.1 ng/ml.
Statistical analyses
Data on percentages of goats in estrus and pregnant were analyzed by categorical procedures using the GENMOD procedure of SAS (SAS Inst. Inc., Cary, NC, USA) with the logit link function. The only effect included in the model was treatment, with each animal considered as a single experimental unit. A one-way analysis of variance (PROC GLM) for a completely randomized design was used to test the effects of treatments upon the occurrence of estrus, ovulation, and onset of estrous, and progesterone. According to the experimental design, only plasma levels of P4 for both groups from d0 to d10 were analyzed. Statistical differences between treatments were considered significant at P<0.05.
RESULTS AND DISCUSSION
Results show that both G5 and G10 treatments generate similar reproductive responses (P>0.05) in cyclic French-Alpine goats within the breeding season. Nonetheless, after the second PG-injection, there was a trend (P=0.06) to obtain an increased pregnancy rate in G5 group females. A summary of these data is presented in Table 1.
Table 1 Reproductive response of French-Alpine goats submitted to two administrations of prostaglandins (160 μg de Cloprostenol each), with an administration interval of 5 (G5) and 10 (G10) days during the reproductive season
| 1º PG-injection | 2º PG-injection | |||||
|---|---|---|---|---|---|---|
| G5 | G10 | P value | G5 | G10 | P value | |
| Estrus (%) | 80 (8/10) | 90 (9/10) | .56 | 60 (6/10) | 60 (6/10) | 1 |
| Estrus latency (h) | 48 ± 0.0 | 79 ± 16.9 | .09 | 68 ± 13.4 | 52 ± 2.5 | .26 |
| Ovulation (%) | 100 (10/10) | 100 (10/10) | 80 (8/10) | 60 (6/10) | .36 | |
| Pregnancy rate (%) | -------- | -------- | ----- | 60 (6/10) | 20 (2/10) | .06 |
| Ovulatory follicles | 8.9 ± 0.3 (10) | 9.7 ± 0.6 (10) | .32 | 9.5 ± 0.4 (8) | 9.3 ± 0.3 (6) | .96 |
| Average of Corpus | 1.1±0.1(9) | 1.0 ± 0.0 (6) | .4 | 1.0 ± 0.0 (8) | 1.3 ± 0.3 (6) | .29 |
Concentrations of plasma progesterone after the second PG-administration for both experimental groups are shown in Fig. 2. Females from both groups were responsive to the PG-injection, registering a dramatic decline in P4 concentrations, without significant differences (P>0.05) between experimental groups from d0 to d9 of the research.
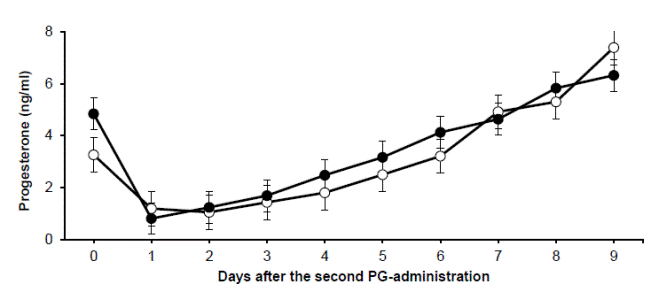
Figure 2 Plasmatic levels of progesterone for groups G5 (white circles) and G10 (black circles) after the second prostaglandin administration (d0; P > 0.05)
The observed estrus response after the second PG-injection in the G5 was similar to those reported in previous studies made at intervals from 9 to 11 days, with estrus responses from 70% to 85% (Omontese et al., 2016; Freitas et al., 2004). Besides, 80% of females ovulated after the second PG-injection, with no differences regarding the G10 group in either ovulation percentage or ovulatory follicle size and number [80 vs 60 % and 9.5 ± 0.4 (8) vs 9.3 ± 0.3 (6), respectively; P>0.05]. Such results are similar to other studies in which two PG-injections were applied at 10 to 12 days intervals (Al Yacoub et al., 2011; Kusina et al., 2001). Our findings also are in line with Martemucci and D’Alessandro (2011), who used progestogens, prostaglandins, and eCG during 5 days with 80% of ovulatory activity.
Group G5 showed a 60% pregnancy rate and 100% (6/6) fertility, suggesting that they responded to two PG-administrations with an interval of five days, which is a shorter interval than that used by Al Yacoub et al. (2011), who applied two cloprostenol administrations between 6 and 13 days of the estrous cycle, and achieved the same reproductive response with a fixed time insemination protocol. Another important aspect to be highlighted from our protocol is that the level of PG used (160 μg of cloprostenol) is lower than most of those levels used in other studies (250 μg of cloprostenol), reducing the dose around 40% (Omontese et al., 2016).
Concerning the observed reproductive outcomes from G10, the percentage of females responding to the second PG-injection can be considered low, in that 20% had a short estrous cycle (5.5 d) prior to the second PG-injection, with plasma P4 level higher than 1 ng/ml (Titi et al., 2010). Such percentage of short estrous cycles is similar to those obtained in other studies using cloprostenol as synchronizing agent, suggesting that this PG-analogue may promote a deficient growth and functionality of the luteal tissue (Vázquez et al., 2010). Also, this could be explained with the great variability found when prostaglandins are administered (Houghton et al. 1995), given that there was a greater time window from the first administration to the second, animals were in different follicular stages and this reflected in a lower pregnancy rate, as ovulations were not synchronized. In addition, the remaining 20% of those females not responding to the second PG- injection, suggests that the existing corpus luteum was not immediately suppressed after the first application, a scenario supported by the observed plasma P4 concentrations (Fig.2). Also, only 60% of ovulations occurred in G10 after the second PG-injection, an outcome that can be considered low regarding other studies that reached 90% to 100% (Al Yacoub et al., 2011; Kusina et al., 2001).
In addition, pregnancy rate and fertility of the G10 females was lower than expected (20% and 33%, respectively). In fact, from the 60% of females having standing estrus and ovulation after the second PG-administration, four of them repeated estrus between 5 to 12 days after breeding. Such response suggests that the luteogenesis process generated after the PG-treatment, aroused from low quality follicles, was unable to maintain an adequate P4 synthesis to sustain pregnancy (Al Yacoub et al., 2011). In goats, as in other mammals, the first days after ovulation are critical to enhance luteogenesis as well as to promote embryo implantation, embryogenesis and the maternal recognition of pregnancy processes (Vázquez et al., 2010).
Based on the reproductive outcomes depicted by the G5 females, our study generates information regarding the reduction in the time required to promote the onset of estrus with the use of exogenous hormones for the reproductive management of small ruminants. Besides, it is also possible to reduce the sanitary risk in the reproductive tract while also expecting a decrease in the cost of hormonal treatments by reducing the dose required (Omontese et al., 2016; Abecia et al., 2011). These results acquire particular importance when considering that, in most of the synchronizing protocols in goats and sheep, the use of prostaglandins considers a precise knowledge regarding the phase of the estrous cycle, (i.e. the luteal phase), applying an increased quantity of prostaglandins either alone or in combination with progestogens or other hormones.
CONCLUSION
Administration of a short-term prostaglandin based protocol at five days interval to synchronize estrus activity of cyclic French-Alpine goats during the breeding season, generated important reproductive outcomes when considering estrus activity, estrus latency, luteal function, and plasma progesterone levels, as well as ovulation and pregnancy rates, along with the reduction in the number of days managing the goats; the last being of physiologic importance and reproductive significance to the goat industry.
Acknowledgments:
We recognize the financial support given by the sectorial fund of the National Council of Science and Technology (CONACyT, Mexico) and the Ministry of Agriculture, Livestock, Rural Development, Fisheries and Food (SAGARPA, Mexico): 2017-4-291691.
REFERENCES
Abecia JA, Forcada F, Gonzalez-Bulnes A. 2011. Pharmaceutical control of reproduction in sheep and goats. Veterinary Clinic of North America Food Animal Practice. 27: 67-79. https://doi.org/10.1016/j.cvfa.2010.10.001 [ Links ]
Al Yacoub AN, Gauly M, Sohnrey B, Holtz W. 2011. Fixed-time deep uterine insemination in PGF2α -synchronized goats. Theriogenology. 76: 1730-1735. https://doi.org/10.1016/j.theriogenology.2011.07.005 [ Links ]
Contreras-Villarreal V, Meza-Herrera CA, Rivas-Muñoz R, Angel-Garcia O, Luna-Orozco JR, Carrillo E, Mellado M, Véliz-Deras FG. 2016. Reproductive performancee of seasonally anovular mixed-bred dairy goats induced to ovulate with a combination of progesterone and eCG or estradiol. Animal Science Journal. 87(6):750-5. https://doi.org/10.1111/asj.12493 [ Links ]
FASS, 2010. Guide for the care and use of agricultural animals in agricultural research and teaching. 3rd Edition, FASS, Champaing, IL, USA. Pp. 177. ISBN: 978-1-7362930-0-3 [ Links ]
Freitas JVF, Rondina D, Lopes Junior ES, Teixeira DIA, Paula NRO. 2004. Hormonal treatments for the synchronization of oestrus in dairy goats raised in the tropics. Reproduction Fertility and Development. 16: 415-420. https://doi.org/10.1071/RD04031 [ Links ]
Gonzalez-Bulnes A, Meza-Herrera CA, Rekik M, BenSalem H, Kridli RT. 2011. Limiting factors and strategies for improving reproductive outputs of small ruminants reared in semi-arid environments. In: Degenovine, K.M. (Ed.), Emi-arid Environments: Agriculture, Water Supply and Vegetation. Nova Science Publishers Inc ., Hauppauge, NY, USA. Pp. 41-60 (Chapter 2) ISBN: 978-1-61761-541-2. [ Links ]
Houghton JAS, Liberati N, Schrick FN, Townsend EC, Dailey RA, Inskeep EK. 1995. Day of estrus cycle affects follicular dynamics after induced luteolysis in ewes. Journal of Animal Science. 73(7):2094-2101. https://doi.org/10.2527/1995.7372094x [ Links ]
Kusina NT, Chinuwo T, Hamudikuwanda H, Ndlovu LR, Muzanenhamo S. 2001. Effect of different dietary energy level intakes on efficiency of estrus synchronization and fertility in Mashona goat does. Small Ruminant Research. 39: 283-288. https://doi.org/10.1016/S0921-4488(00)00192-9 [ Links ]
Maia Alrs, Brandao FZ, Souza-Fabjan JMG, Balaro MFA, Oliveira MEF, Facó O, Fonseca JF. 2017. Reproductive parameters of dairy goats after reveiving two doses of d-cloprostenol at different intervals. Animal Reproduction Science. 181: 16-23. https://doi.org/10.1016/j.anireprosci.2017.02.013 [ Links ]
Martemucci G, D’Alessandro AG. 2011. Induction-synchronization of oestrus and ovulation in dairy goats with different short-term treatments and fixed time intrauterine or exocervical insemination system. Animal Reproduction Science. 126: 187-194. https://doi.org/10.1016/j.anireprosci.2011.05.011 [ Links ]
Medan MS, Watanabe G, Sasaki K, Sharawy S, Groome NP, Taya K. 2003. Ovarian dynamics and their associations with peripheral concentrations of gonadotropins, ovarian steroids, and inhibin during the estrous cycle in goats. Biology of Reproduction. 69: 57-63. https://doi.org/10.1095/biolreprod.102.013334 [ Links ]
National Academy of Medicine. 2002. Guide for the care and use of laboratory animals. Co-produced by the National Academy of Medicine-Mexico and the Association for assessment and accreditation of laboratory animal care international. 1st. Edition, Harlan Mexico, DF, Mexico. ISBN-13: 978-0-309-15400-0 [ Links ]
Omontese BO, Rekwot PI, Ate IU, Ayo JO, Kawa MU, Rwuaan JS, Nwannenna AI, Mustapha RA, Bello AA. 2016. An update on oestrus synchronization of goats in Nigeria. Asian Pacific Journal of Reproduction. 5: 96-101. https://doi.org/10.1016/j.apjr.2016.01.002 [ Links ]
Roger, PA. 2012. Welfare issues in the reproductive management of small ruminants. Animal Reproduction Science. 130(3-4):141-146. https://doi.org/10.1016/j.anireprosci.2012.01.007 [ Links ]
Romano JE, Alkar A, Amstalden M. 2016. Effect of copulation on estrus duration and ovulation time in goats. Theriogenology. 85(2):330-334. https://doi.org/10.1016/j.theriogenology.2015.09.021 [ Links ]
Rubianes E, Menchaca A, Carbajal B. 2003. Response of the 1-5 day-aged ovine corpus luteum to prostaglandin F2α. Animal Reproduction Science. 78(1-2):47-55. https://doi.org/10.1016/S0378-4320(03)00046-0 [ Links ]
Schneider RJ, Hallford DM. 1996. Use of a rapid progesterone radioimmunoassy to predict pregnancy and fetal numbers in ewes. Sheep and Goat Research Journal. 12: 33-38. https://agris.fao.org/agris-search/search.do?recordID=US1997067677 [ Links ]
Titi HH, Kridli RT, Alnimer MA. 2010. Estrus synchronization in sheep and goats using combinations of GnRH, progestagen and prostaglandin F2α. Reproduction in Domestic Animals. 45: 594-599. https://doi.org/10.1111/j.1439-0531.2008.01309.x [ Links ]
Vázquez MI, Blanch MS, Alanis GA, Chaves MA, Gonzalez-Bulnes A. 2010. Effect of treatments with a prostaglandin analogue on developmental dynamics and functionality of induced corpora lutea in goats. Animal Reproduction Science. 118: 42-47. https://doi.org/10.1016/j.anireprosci.2009.05.016 [ Links ]
Received: June 27, 2020; Accepted: March 02, 2021











 text in
text in 



