Services on Demand
Journal
Article
Indicators
Related links
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.11 Tepic Jan./Dec. 2021 Epub May 21, 2021
https://doi.org/10.21929/abavet2021.3
Original Article
Plant extracts evaluation for the control of Oesophagostomum dentatum in hairless Mexican pigs
1Departamento de Veterinaria y Zootecnia, Universidad de Guanajuato. Carretera Irapuato-Silao km 9, CP 36500 Irapuato, Guanajuato, México.
2Centro de Ciencias Agropecuarias, Universidad Autónoma de Aguascalientes. Av Universidad 940, col. Ciudad Universitaria, CP 20131, Aguascalientes, Aguascalientes. México.
3Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara. Camino Ramón Padilla Sánchez 2100 Nextipac, 45200 Zapopan, Jalisco, México,
4Centro de Ciencias Económico Administrativas, Universidad Autónoma de Aguascalientes Av Universidad 940, col. Ciudad Universitaria, CP 20131, Aguascalientes, Aguascalientes. México.
In Mexico, the Oesophagostomum dentatum is considered one of the main gastrointestinal endoparasites affecting the most important breed in rural pig farming, the hairless pig [Cerdo Pelon Mexicano (CPM)]. In this research, it was evaluated the in vitro biological efficacy of vegetable extracts of ginger, mint, thyme and oregano, with the aim of finding new natural alternatives for the control of Oesophagostomum dentatum; being compared its efficiency with ivermectin (IVM) and dimethylsulfoxide (DMSO). During the investigation, 380 samples of excrements (extracted directly from the anus) of CPM were collected, with an average weight (±SD) 40±5 kg per animal. These samples were analyzed by means of McMaster technique, achieving to identify Oesophagostomum dentatum eggs. The evaluation of the treatments' effectiveness was carried out in cell culture microplates incubated for 48 h at 25±1 ºC using different doses of the vegetable extracts and comparing their control effectiveness with IVM and DMSO. Obtaining that the biological effectiveness of the ginger extract (3%) is similar to that of the IVM (1%), achieving the elimination and immobilization of the Oesophagostomum dentatum larva in 62%. While the extracts of oregano, thyme and mint presented a percentage of biological effectiveness of less than 20%.
Keywords: parasites; gastrointestinal; nematodes; Sus scrofas domesticus; ginger
En México, el Oesophagostomum dentatum es considerado uno de los principales endoparásitos gastrointestinales que afecta a la raza de mayor importancia en la porcicultura rural [Cerdo Pelón Mexicano (CPM)]. En esta investigación se evaluó la eficacia biológica in vitro de extractos vegetales de jengibre, hierbabuena, tomillo y orégano, con el objetivo de encontrar nuevas alternativas de carácter natural para el control de Oesophagostomum dentatum; siendo comparada su eficiencia con ivermectina (IVM) y dimetilsulfóxido (DMSO). Durante la investigación, se colectaron 380 muestras de excretas (extraídas directamente del ano) de CPM, con un peso promedio (±DE) 40±5 kg por animal. Dichas muestras fueron analizadas mediante la técnica de McMaster, logrando identificar huevos de Oesophagostomum dentatum. La evaluación de la eficacia de los tratamientos se realizó en microplacas de cultivo celular incubadas durante 48 h a 25±1 ºC utilizando diferentes dosis de los extractos vegetales y comparando su eficacia de control con IVM y DMSO. Obteniendo que la efectividad biológica del extracto de jengibre (3%) es similar al de la IVM (1%), logrando la eliminación e inmovilización de la larva de Oesophagostomum dentatum en un 62%. Mientras que los extractos de orégano, tomillo y hierbabuena presentaron un porcentaje de efectividad biológica menor al 20%.
Palabras claves: parásitos; nematodos; gastrointestinales; Sus scrofas domesticus; jengibre
INTRODUCTION
During the last years, in Mexico, pork production has generated more than 350,000 direct jobs and 1.7 million indirect jobs, causing an exponential growth of 10.79%, as a consequence of production increases and an improvement in prices in the market for consumption of this meat (Rebollar et al., 2016). One of the most important activities in the country is rural pig farming, being the Mexican Hairless pig (CPM) one of its main protagonists, since it has been characterized mainly by its rusticity and varied diet (Lemus and Ly, 2010). This type of production is affected by the presence of parasites that limit the productive potential of pigs, causing loss of appetite and immune response; consequently, a decrease in live weights and alterations in food conversion rates (Louie et al., 2007).
It is important to highlight that the prevalence of parasitosis depends exclusively on the management system, the sanitation and hygiene conditions and on different types of variables, such as climate, temperature and humidity, which influence the life cycles of parasites (Frontera et al., 2009); where one of the parasites with the highest prevalence in pig production is Oesophagostomum dentatum (Cordero et al., 2000). Currently the control methods that have been chosen for this type of parasitosis have been less and less effective, because these nematodes have had a rapid evolution and development of resistance against the chemicals used for their control, which represents a risk for human health (Taylor et al., 2009a). Currently, three large families of antiparasitics are frequently used by pig farmers, macrocyclic lactones (IVM, moxidectin, doramectin), imidazoles, tetrahydropyrimidine (levamisole, moratel) and benzimidazoles (fenbendazole, oxfendazole and albendazole), depending on the areas in which production is developed (Encalada et al., 2014). The abuse of these chemicals has caused a problem of resistance to antiparasitics (Kaplan and Vidyashankar, 2012). Furthermore, their misuse can cause them to enter the environment as an equal compound (unchanged) or as a metabolite; to later be transported and distributed in water, sediments, soil and flora (Horvat et al., 2012), causing considerable alterations in the ecosystem.
For this reason there is a growing interest in exploring natural alternatives, with properties capable of acting as bacteriostats, bactericides and antiparasitics (Aguilera, 2012). Plants, as part of their metabolism, synthesize different components called secondary metabolites (Dávila et al., 2017). Various investigations carried out have shown a great diversity of plants that possess these metabolites capable of inhibiting the growth and development of pathogens (Rizo et al., 2017).
The objective of this study was to evaluate the biological efficacy of different plant extracts, such as: ginger (Zingiber officinale), oregano (Origanum vulgare), thyme (Thymus) and peppermint (Mentha spicata); previously reported for their active compounds capable of acting as bactericides; comparing them with commercial products that are currently used for the control of Oesophagostomum dentatum present in CPM.
MATERIAL AND METHODS
Study area: The study was carried out at the Center for the Conservation of the Mexican Hairless Pig and at the Laboratory of Parasitology and Biological Control, of the Division of Life Sciences of the University of Guanajuato. The samples were collected from CPM, with average weights 40 ± 5 kg, originating from rural areas of the municipality of Huehuetla, Hidalgo and Zacapoaxtla, Puebla, Mexico. A total of 380 samples taken from the rectum were collected and placed in properly identified polyethylene bags (Aguilar et al, 2016). These were transferred to the laboratory, where they were stored at a temperature of 4 ± 1ºC until their processing, which was not greater than 48 h.
Vegetative material: Fresh leaves of mint (Mentha spicata), thyme (Thymus) and oregano (Origanum vulgare) (approximately 1kg per sample) were collected in the Zacapoaxtla municipality, Puebla, located at an altitude of 1825 m. a.s. l., as well as ginger bulbs (Zingiber officinale) (approximately 2 kg) in Huehuetla municipality, Hidalgo, located at an altitude of 520 m. a. s. l. The material was stored and transferred under refrigeration at 4 °C in a portable mini-refrigerator (Chefman/RJ48-BLACK; Cooling & Heating Company, United States), to avoid changes in its composition (Salem et al., 2006). Later they were subjected to a drying process under the shade for a week, and finally both the leaves and bulbs were crushed in a semi-industrial mill to a size of approximately 1 mm.
Obtaining the hydroalcoholic extract (HA): 100 g were used for each sample and they were subjected to a maceration process with a mixture of water and methanol (70:30 v/v) for 24 h, then the solution was filtered through different filters, using (gauze and filter paper) to obtain an extract free of impurities. Once the extract was obtained, it was frozen at -42 °C and finally the lyophilization process was carried out (lyophilizer 7670520; LABCONCO, Kansas City, United States). The lyophilized extract was frozen for later use (Salem et al., 2006).
Biological material: the parasitological diagnosis was made using the anaerobic egg storage technique, described by (Coles et al., 2006) modified; which consists of processing the samples using the sedimentation technique found in the fecal content and allowing the parasite eggs to concentrate at the bottom of the falcon tube. Thus, 30 mL of water and 4 g of feces were placed in each falcon tube, the sample was homogenized, pouring it into a sieve and centrifuged for 5 min at 300 rpm. Subsequently, the flotation technique was performed with 30 mL of glucose solution with a density of 1: 200 (Cringoli et al., 2004); achieving in this way that the parasite eggs at the bottom of the falcon tube float due to the density of the solution; the mixture was again centrifuged for 5 min at 300 rpm. Finally, the sedimentation technique was performed again with 30 mL of distilled water to concentrate the eggs at the bottom of the tube, centrifuging at 300 rpm and establishing the concentration of eggs per mL of sterilized distilled water.
McMaster´s technique: The McMaster coproparasitoscopic technique was performed, using a saturated Sheather solution with a specific gravity of 1,200 to estimate the amount of eggs per g of excrement, the appropriate gravity being to carry out the technique (Cringoli et al., 2004). 2 g of excrement and 28 mL of saturated solution were used; the sample was homogenized and placed in the McMaster chamber (MM-OP; PROLAB, Jalisco, Mexico), using a pipette with gauze as a filter, avoiding obstruction when reviewing the sample. The necessary volume was placed to perform the reading on one side of the chamber, letting it rest for 2 min before being observed under the microscope and carrying out the egg count (Rodríguez et al., 2016).
To estimate the number of eggs per g of excrement, the total volume obtained from mixing the excrement and the 30 mL solution was adjusted; considering that each compartment of the chamber measures 1 cm2 with a height of 0.15 cm, therefore the reading of both compartments is 0.30 mL of the total initial volume of 30 mL (Rodríguez et al., 2016). The concentration of eggs per g of feces of the parasite found was obtained by means of the McMaster formula (Bowman, 2013).
The parasite eggs were identified with the morphological keys (Coffin, 1952), and the larval diagnosis was made with the morphological keys of Van Wyk et al., (2004), being able to observe the cranial limb of the larvae, the terminal appendages and the morphology of the tail.
Experimental design: the experiment was established under a completely randomized design of 19 treatments of 20 repetitions each, having a total of 380 experimental units. Each experimental unit consisted of 1200 µL (1100 µL of ginger extract plus 100 µL of solution with 70 nematode larvae). Each experimental unit consisted of a total of 100 µL of live nematodes (with an average of 70 larvae).
Inhibition test of larval migration: a larval culture was carried out to bring the nematodes to the third stage of the larva. For this, the necessary conditions were provided for the hatching of the egg, making modifications to the technique of McArthur et al., (2015). Excrement was placed in perforated plastic containers, in order to provide an aerobic environment, sterile sawdust was added and the excrement was homogenized by adding sterile distilled water. The mixture was covered at 23-25 ºC for 10 d, with a daily visual evaluation in case of requiring the addition of moisture and oxygenating the samples, stirring the culture with the help of a spatula. Once the incubation time had concluded, the culture was placed in a Baermann funnel; in this way the larvae of Oesphagostomum dentatum were separated from the fecal content. After one day in the funnel, the liquid was obtained and the larvae were concentrated by centrifugation, to later carry out their counting and dilutions, being identified with the morphological keys described by Quiroz (2011).
Dose and number of applications: Evaluations were made with doses of ginger extract (Zingiber officinale) at 0.1%, 0.3%, 0.5%, 0.7%, 0.9%, 1%, 3% (Gawel et al., 2003); oregano (Origanum vulgare) at 1%, 3% and 5%, as mentioned by Borbolla and Velásquez, 2004 (citado en Guerra et al., 2008); thyme (Thymus vulgaris) at 1%, 3% and 5% (Ramos and Hernández, 2018); peppermint (Mentha spicata) at 1%, 3% and 5% (Lagarto et al, 1997); comparing the effect of each one with the water control, DMSO 5% (Rendal et al., 2004) and IVM at 1% (Chávez et al., 2006).
For the dilution of the nematodes, a pipette graduated in 100 µL was used, with which the sample collected from the funnel was extracted, deposited in each well of the cell microplate of 1200 µL volume, a sample of 100 µL of nematodes; repeating the same procedure for each experimental unit. For the experimental units of the extracts of the organic treatments to be evaluated, a 5% extract stock solution was used (equivalent to 2 mL of extract + 38 mL of distilled water = 40 mL of solution); thus calculating the equivalents to each percentage as mentioned above for each experimental unit according to its inclusion percentage.
For the dilution of each treatment dose, the necessary amount of distilled water was deposited to complete the 1200 µL volume, in each experimental unit. The Control treatment was added with 100 µL of nematodes and a total of 1100 µL of distilled water. During the experiment, a single application of the antiparasitics was carried out, and with the help of a manual counter, the live and dead nematodes of each experimental unit were counted through a microscope with a 10x and 40x objective.
Statistical analysis: For the evaluation of each antiparasitic, its effectiveness was determined, comparing it with the control group (Barrere et al., 2013). Mortality percentages were adjusted with the Abbott formula (Abbott,1987), and an analysis of variance of the different treatments was performed and a comparison of means was made using a Tukey test at 95% confidence, with the statistical package Statgraphics 9.0 (Cosialls et al., 2000).
RESULTS AND DISCUSSION
The percentage of biological effectiveness of the different antiparasitics evaluated was as follows: first evaluation (0 h), it was 0% in each treatment; this for being the first count after the application. For the second evaluation (24 h after the application of the treatments), no significant differences were shown in the mortality of nematodes between the evaluated treatments. In the third evaluation (48 h after the application of the treatments), it was observed that the percentage of biological effectiveness of the different treatments increased. However, IVM (1%) and ginger extract (Zingiber officinale) (3%) were the ones that showed the greatest effectiveness for the control of Oesophagostomum dentatum (Table 1).
Table 1 Percentage of biological effectiveness of the dewormers and plant extracts evaluated
| Dewormers | % Inclusion | Evaluation 1 (0 hours) | Evaluation 2 (24 hours) | Evaluation 3 (48 hours) |
|---|---|---|---|---|
| % | ||||
| T1: Control H2O | --- | 0 | 0 | 0 |
| T2: Ivermectin | 1 | 0 | 32.56 | 62.72 |
| T3: DMSO | 5 | 0 | 0 | 10.02 |
| T4: Ginger | 0.1 | 0 | 1.19 | 2.08 |
| T5: Ginger | 0.3 | 0 | 2.37 | 5.19 |
| T6: Ginger | 0.5 | 0 | 6.15 | 11.46 |
| T7: Ginger | 0.7 | 0 | 12.74 | 24.27 |
| T8: Ginger | 0.9 | 0 | 19.54 | 36.09 |
| T9: Ginger | 1 | 0 | 22.69 | 45.36 |
| T10: Ginger | 3 | 0 | 32.54 | 62.93 |
| T11: Peppermint | 1 | 0 | 1.67 | 3.04 |
| T12: Peppermint | 3 | 0 | 2.54 | 5.98 |
| T13: Peppermint | 5 | 0 | 4.66 | 9.53 |
| T14: Thyme | 1 | 0 | 1.49 | 2.49 |
| T15: Thyme | 3 | 0 | 2.71 | 5.80 |
| T16: Thyme | 5 | 0 | 3.85 | 8.03 |
| T17: Oregano | 1 | 0 | 3.29 | 6.48 |
| T18: Oregano | 3 | 0 | 5.48 | 11.18 |
| T19: Oregano | 5 | 0 | 8.80 | 17.02 |
Figure 1 shows that the biological effectiveness of ginger extract (Zingiber officinale) (3%) is similar to that of IVM (1%), in 62%; This extract has an exponential growth of 26.76% on average as its concentration percentage increases; while the extracts of oregano (Origanum vulgare), thyme (Thymus) and mint (Mentha spicata), maintain a percentage of biological effectiveness of less than 20%; being these little effective in the mortality of the evaluated nematode. As reported by a study by Taylor et al. (2009 a) a multiple resistance of gastrointestinal nematodes to the main families of antiparasitics (benimidazoles, imidazoles and microcyclic lactose) has been verified. Geurden et al. (2015), reported that IVM has been one of the most used dewormers in the last 40 years, due to the resistance that parasites develop in different sites in Germany, France, England and Italy. 40 Animal Production Units (753 animals) were studied, keeping a record of the eggs observed in the excrement and observing a decrease in the effectiveness of IVM and moxidectin in the 8 Animal Production Units.
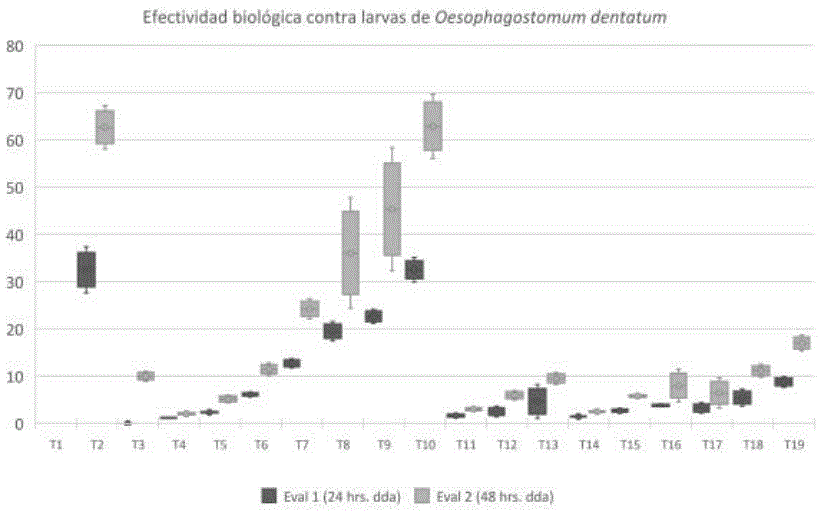
daa: days after application
Figure 1 Percentage of biological effectiveness of each dewormer used on the larvae of Oesophagostomum dentatum during the evaluation 1 (to 24 h after applying the treatment) and in the evaluation 2 (to 48 h after applying the treatment)
In Mexico, Alonso et al., (2015) evaluated in 21 Animal Production Units in Veracruz state, during the period of January 2012-April 2013; among which only 2 Animal Production Units have parasites susceptible to IVM, being the other 15 those that present resistance; Furthermore, they were able to identify through questionnaires that this problem originates mainly because an inadequate deworming practice is carried out; being one of the main problems in the state of Guanajuato, since the producers indicate deworming without adequate control, which generated that only 2 Animal Production Units present parasitosis susceptible to IVM. Paraud et al., (2016) have verified in France, that the latter can not only modify the effectiveness of IVM, but also the existence of a cross resistance is possible, reporting a resistance to macrocyclic lactones in sheep, as they demonstrated the first multiple resistance of gastrointestinal nematodes against the same family of antiparasitic; This could be a problem in the present study, because 60% of the Animal Production Units evaluated presented resistance to IVM, and cross resistance in France was observed in farms suspected of resistance.
Paradoxically, research carried out in Mexico by Rizo (2017), shows the great diversity of plants that present metabolites capable of inhibiting the growth and development of pathogens (Phytophthora ssp., Colletotrichum gloeosporioides, Moniliophthora roreri); One of these plants evaluated has been the extract of ginger (Zingiber officinale). Studies reported by Lin et al. (2010), have verified the effect of ginger (Zingiber officinale) as a dewormer with respect to mortality and reduced mobility in the larvae of Anisakis simplex, a species of gastrointestinal nematode present in marine mammals, fish, crustaceans and humans.
The effectiveness of the extract of ginger (Zingiber officinale) at 3% to eliminate and immobilize the larvae of Oesophagostomum dentatum, is similar in an efficiency to 62% to that of IVM1% at 48 h, proving in this study that the biological effectiveness of the Ginger extract (Zingiber officinale) has a growth with respect to the dose response curve of this extract. Research has been conducted in Japan on the anthelmintic activity of compounds isolated from ginger root (Zingiber officinale), syhogaol, shogaol, and gingerol. They have shown that the above compounds kill and reduce mobility in Anisakis simplex larvae, a species of gastrointestinal nematode present in marine mammals, fish, crustaceans and humans between 24 and 72 h (Lin et al., 2010). In turn, Acuña and Torres, (2010) in a study conducted on the medicinal properties of ginger (Zingiber officinale), have reported Gingerol as the most studied active component for its various pharmacological effects, among the most prominent anti-inflammatory and anthelmintic.
According to these results, verifying the effectiveness of ginger (Zingiber officinale), to eliminate and immobilize the larvae of Oesophagostomum dentatum, an adequate parameterization has been made to the Gompertz mathematical model to estimate the lethal dose50 (LD50) of the ginger extract; as shown in table 2, taking the following values for the parameterization, according to the Solver application of the Microsoft Office 2017 Excel program (Correa, 2004).
y= a* exp(-b* exp (-c*t))
a= 62.40846543
b= 6.979583949
c= 2.910435786
y= 62.40846543 * exp (-6.979583949* exp(-2.910435786 *t))
Table 2 Parametrization of the Gompertz model to determine DL50 of ginger
| % Inclusion | % BE | Gompertz | SS | |
|---|---|---|---|---|
| GINGER (Zingiber officinale) | 0.1 | 2.07868 | 0.338436046 | 3.028449019 |
| 0.3 | 5.19798 | 3.382901301 | 3.294510684 | |
| 0.5 | 11.4595 | 12.24378442 | 0.615102049 | |
| 0.7 | 24.2727 | 25.12103484 | 0.719671995 | |
| 0.9 | 36.0928 | 37.53441718 | 2.078260097 | |
| 1 | 45.3562 | 42.67651026 | 7.180737091 | |
| 2 | 60 | 61.13021894 | 1.277394859 | |
| 3 | 62.9262 | 62.33817928 | 0.345768365 |
BE: % Biological effectiveness. SS: Sum of squares
The results adjusted to the Gompertz parametric model have determined that the LD50 of ginger (Zingiber officinale) is obtained with an inclusion of 1.1858% of this extract; thus being able to combat 50% of the larvae of Oesophagostomum dentatum present in the race of CPM evaluated. Various studies carried out to estimate the LD50 have verified that the Gompertz mathematical model is one of the most accurate, without presenting a large margin of error, compared to other models such as polynomial, logistic and linear regression (Molina and Melo, 2010).
With respect to the other vegetable extracts evaluated, the results obtained by the Statgraphics 9.0 software have not been relevant, since its percentage of effectiveness does not exceed 18%.
CONCLUSIONS
The biological effectiveness of ginger extract (Zingiber officinale) (3%) is similar to that of ivermectin (1%) in 62% of the samples evaluated. Therefore, the effectiveness of the extract to eliminate and immobilize the larvae of Oesophagostomum dentatum is demonstrated, with a lethal dose50 is 1.1858% estimated by the mathematical model of Gompertz. Although the vegetable extracts of oregano (Origanum vulgare), thyme (Thymus) and peppermint (Mentha spicata) were evaluated, although they had reports of being previously evaluated as antiparasitic of gastrointestinal nematodes and reports of antibacterials, they do not present a relevant percentage of biological effectiveness ( less than 18%), against Oesophagostomum dentatum larvae.
It is necessary to continue with the evaluations to be able to verify in vivo the effects of ginger (Zingiber officinale), as an antiparasitic in the productions of backyard pigs against the larvae of Oesophagostomum dentatum, and thus be able to compare its dose and profitability per kilogram of the animal, with regarding ivermectin.
ACKNOWLEDGEMENT
We are grateful to the National Council of Science and Technology (CONACyT), for the funding granted for the master's studies of the second author.
REFERENCES
Abbott WS. 1987. A method of computing effectiveness of an insecticide. Journal of the American Mosquito Control Association. 3(2) 302-303. ISSN: 8756-971X https://www.biodiversitylibrary.org/content/part/JAMCA/JAMCA_V03_N2_P302-303.pdf [ Links ]
Acuña O, Torres A. 2010. Aprovechamiento de las propiedades funcionales del jengibre (Zingiber officinale) en la elaboración de condimento en polvo, infusión filtrante y aromatizante para quema directa. Revista Politécnica. 29(1) 60-69 ISSN: 1390-0129 https://bibdigital.epn.edu.ec/bitstream/15000/4343/1/RP-No.29%288%29.pdf [ Links ]
Aguilar LA, Florian CJ. 2016. Diagnóstico situacional de los parásitos gastroentéricos en la crianza artesanal de cerdos (Sus scrofa doméstica) de traspatio en la zona urbana del Municipio de Santo Tomas Departamento de Chontales. Tesis de licenciatura. Universidad Nacional Agraria. Pp. 5-6. https://repositorio.una.edu.ni/3421/ [ Links ]
Aguilera GS. 2012. Validación de seis fitofármacos: ajenjo, nogal, pasionaria, salvia, sábila y jengibre en pacientes adultos de diecinueve a setenta años de edad en Quito, junio-agosto de 2010. Tesis de licenciatura. Pontificia Universidad Católica del Ecuador. http://repositorio.puce.edu.ec/bitstream/handle/22000/5252/T-PUCE-478.pdf?sequence=1&isAllowed=y [ Links ]
Alonso DM, Arnaud OR, Becerra NR, Torres AJ, Rodríguez VR, Quiroz RR. 2015. Frequency of cattle farms with ivermectin resistant gastrointestinal nematodes in sheep in Veracruz, México. Veterinary Parasitology. 212(3-4), 439-443. ISSN: 0304-4017 https://www.sciencedirect.com/science/article/abs/pii/S0304401715003520 [ Links ]
Barrere F, Shakya KP, Menzies PI, Peregrine AS, Prichard RK. 2013. Assessment of benzimidazole resistance in Haemonchus contortus in sheep flocks in Ontario, Canada: Comparison of detection methods for drug resistence. Veterinary Parasitology. 198(1): 159-165. ISSN: 0304-4017. https://www.sciencedirect.com/science/article/abs/pii/S0304401713004457 [ Links ]
Bowman DD. 2013. Georgis´ Parasitology for Veterinarians. 10a ed. Philadelphia, USA. Editorial Elsevier. Pp. 496. ISBN. 9781455740062 https://www.elsevier.com/books/georgis-parasitology-for-veterinarians/bowman/978-1-4557-4006-2 [ Links ]
Chávez RM, Reveles HR, Saldivar ES, Muñoz EJ, Morales VM, Moreno GM. 2006. Evaluación del albendazol, ivermectina y nitazoxanida en infeccion causada por Trichinella spiralis en modelo suino. Archivos Venezolanos de Farmacología y Terapéutica. 25:8-84. ISSN 0798-0264. http://ve.scielo.org/scielo.php?pid=S0798-02642006000200008&script=sci_arttext [ Links ]
Coffin DL. 1952. Laboratorio clínico en medicina veterinaria. 3a edición. Boston, Massachusetts. Pp.355 [ Links ]
Coles GC, Jackson F, Pomroy WE, Prichard RK, Samson HG, Silvestre A, Taylor MA, Vercruysse J. 2006. The detection of anthelmintic resistance in nematodes of veterinary importance. Veterinary Parasitology. 136:167-185. ISSN: 0304-4017 https://www.sciencedirect.com/science/article/abs/pii/S0304401705005649 [ Links ]
Cordero CM, Rojo F, Martínez A, Sánchez M, Hernández S, Navarrete I. 2000. Parasitología Veterinaria. España: Ed. McGraw-Hill. Pp. 935 ISBN: 84-486-0236-6. https://dialnet.unirioja.es/servlet/libro?codigo=489596 [ Links ]
Correa HJ. 2004. RUMENAL: procedimiento para estimar los parámetros de cinética ruminal mediante la función Solver de Microsoft Excel®. Revista Colombiana de Ciencias Pecuarias. 17(3): 250-254. ISSN: 2256-2958. https://revistas.udea.edu.co/index.php/rccp/article/view/323947/20781127 [ Links ]
Cosialls IL, Solanas PA, Núñez PM, Jiménez FM, Miralles PD, Serra DG. 2000. Estadística aplicada con SPSS y Statgraphics. España. Edicions Universitat Barcelona. Pp. 119. ISBN: 84-8338-214-8. https://books.google.com.mx/books?hl=es&lr=&id=odNxBeRLXUEC&oi=fnd&pg=PA5&dq=Statgraphics+9.0&ots=5hbaIg50jt&sig=U74bUpOfwfagLOIGifWiQ2DFQxc&redir_esc=y#v=onepage&q=Statgraphics%209.0&f=false [ Links ]
Cringoli G, Rinaldi L, Veneziano V, Capelli G, Scala A. 2004. The influence of flotation solution, simple dilution and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Veterinary Parasitology. 123:121-131. ISSN: 0304-4017 https://www.sciencedirect.com/science/article/abs/pii/S0304401704002377 [ Links ]
Dávila JG, González SI, Báez OL, Gaona, ÁE, Zaragoza SE. 2017. Optimización de la destilación de Origanum Vulgare L, con efecto antifúngico en Moniliophthora roreri. Espacio I+D, Innovación Más Desarrollo. 6(14):138-151. ISSN: 2007-6703. https://doi.org/10.31644/IMASD.142017.a07 [ Links ]
Encalada ML, Tuyub SH, Ramírez VG, Mendoza GP, Aguilar ML, López AM. 2014. Phenotypic and genotypic characterization of Haemonchus spp. And other gastrintestinal nematodes resistant to benzimidazole in infected calves from the tropical regions of Campeche State, México. Veterinary Parasitology. 205: 246-254. ISSN: 0304-4017 https://www.sciencedirect.com/science/article/abs/pii/S0304401714003793 [ Links ]
Frontera E, Pérez M, Alcaide M, Reina D. 2009. Patología parasitaria porcina: en imágenes. España: Ed. Servet. Pp. 268. ISBN: 84-92569-12-3. https://dialnet.unirioja.es/servlet/libro?codigo=490758 [ Links ]
Gawel A, Kotonski B, Madej JA, Mazurkiewicz M. 2003. Effect of silimarin on chicken and turkey broilers' rearing and the production índices of reproduction hen flocks. Medycyna Weterynaryjna. 59:517-520. ISSN: 0025-8628. https://eurekamag.com/research/034/804/034804936.php [ Links ]
Geurden T, Chartier C, Franke J, Di RA, Traversa D, Von SG, Demeler J, Vanimisetti HB, Bartram DJ, Denwood MJ. 2015. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Journal Parasitology Drugs Drug Resist. 5(3):163-171. ISSN: 2211-3207 https://www.sciencedirect.com/science/article/pii/S2211320715300087 [ Links ]
Guerra C, Galán JA, Méndez J, Perea EM. 2008. Evaluación del efecto del extracto de orégano (Oreganum vulgare) sobre algunos parámetros productivos de cerdos destetados. Tumbaga. 1:6-29. ISSN Online: 2216-118x. https://dialnet.unirioja.es/servlet/articulo?codigo=3993531 [ Links ]
Horvat AJ, Petrovic M, Babic S, Pavlovic DM, Asperger D, Pelko S, Mance AD, Kastelan MM. 2012. Analysis, occurrence and fate of anthelmintics and their transformation products in the environment. Trends Analyt. Chem. 31: 61-84. ISSN:0165-9936 https://www.sciencedirect.com/science/article/abs/pii/S0165993611002937 [ Links ]
Kaplan RM, Vidyashankar AN. 2012. An inconvenient truth: global worming and anthelmintic resistance. Veterinary Parasitology. 186:70-78. ISSN: 0304-401 https://www.sciencedirect.com/science/article/abs/pii/S0304401711007801 [ Links ]
Lagarto PA, Tillán CJ, Cabrera GY. 1997. Toxicidad aguda oral del extracto fluido de Mentha spicata L. (hierbabuena). Revista Cubana de Plantas Medicinales. 2:6-8. ISSN: 1028-4796. http://scielo.sld.cu/scielo.php?pid=S102847961997000200002&script=sci_arttext&tlng=pt [ Links ]
Lemus C, Ly CC. 2010. Estudio de sustentabilidad de cerdos mexicanos pelones y cuinos. La iniciativa Nayarita. Revista computarizada de producción porcina. 17(2): 89-98. ISSN:1026-9053 http://www.iip.co.cu/RCPP/172/172_10artCLemus.pdf [ Links ]
Lin RJ, Chen CY, Lee JD, Lu CM, Chug LY, Yen CM. 2010. Larvicidal constituents of Zingiber officinale (ginder) against Anisakis simplex. Repositorio Institucional Universidad Médica de Kaohsiung. http://ir.lib.kmu.edu.tw/handle/310902000/18749 [ Links ]
Louie K, Vlassoff A, Mackay AD. 2007. Gastrointestinal nematode parasites of sheep: A dynamic model for their effecton liveweight gain. International Journal Parasitol. 37:233-241. ISSN: 0020-7519. https://www.sciencedirect.com/science/article/abs/pii/S0020751906003511 [ Links ]
McArthur CL, Handel IG, Robinson A, Hodgkinson JE, Bronsvoort BM, Burden F, Kaplan RM, Matthews JB. 2015. Development of the larval migration inhibition test for comparative analysis of ivermectin sensitivity in vyathostomin populations. Veterinary Parasitology. 212: 292-298. ISSN: 0304-4017. https://www.sciencedirect.com/science/article/abs/pii/S0304401715003003 [ Links ]
Molina VF, Melo MS. 2010. Importance of the statistical method applied to calculate the EC50 and EC95 of some isothiocyanates evaluated against Rhizoctonia solani Kuhn. Agronomía Colombiana. 28(2):235-244. ISSN 0120-9965 http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-99652010000200013 [ Links ]
Munguía XJ, Valenzuela MW, Leyva CJ, Morales PM, Figueroa CJ. 2013. Potencial de orégano como alternativa natural para controlar Haemonchus contortus en ovino de pelo. Revista Lationoameriacana de Recursos Naturales. 9 (1): 150-154. ISSN: 2594-0384. http://revista.itson.edu.mx/index.php/rlrn/article/view/222/157 [ Links ]
Paraud C, Marcotty T, Lespine A, Sutra JF, Pors I, Devos I. 2016. Cross-resistance to moxidectin and ivermectin on a meat sheep farm in France. Veterinary Parasitology. 226.88-92. ISSN: 0304-4017. https://www.sciencedirect.com/science/article/abs/pii/S0304401716302527 [ Links ]
Quiroz RH, Figueroa CJ, Ibarra VF y López AM. 2011. Epidemiología de enfermedades parasitarias en animales domésticos. México. 1ª edición. Compact disc. Pp. 643. ISBN:978-607-00-4015-3. https://www.academia.edu/31033300/Epidemiologia_de_enfermedades_parasitarias_en_Animales_Domesticos [ Links ]
Ramos PA, Hernández SR. 2018. Extractos comerciales de tomillo (Thymus vulgaris) y de algarrobo (Ceratonia siliqua) en la dieta de lechones destetados. Revista Científica. 8:25-38. ISSN: 2221-5921. http://revistas.unprg.edu.pe/openjournal/index.php/revistacientifica/article/view/249 [ Links ]
Rebollar RA, Rebollar RS, Gómez TG, Hernández MJ, González RF. 2016. Crecimiento y especialización regional del sector pecuario en México, 1994 a 2013. Revista Mexicana de Ciencias Pecuarias. 7(3): 391-403. ISSN: 2428-6698 http://www.scielo.org.mx/scielo.php?pid=S2007-11242016000300391&script=sci_arttext [ Links ]
Rendal VM, Rodríguez CM, Fernández MR, Sánchez IJ, Segura IR, Veiga BA, Andión NC. 2004. Efecto del almacenamiento en fase gaseosa sobre la viabilidad celular, la apoptosis y la actividad funcional en aortas de cerdo criopreservadas. Estudio preliminar. Angiología. 56:107-121. ISSN: 0003-3170. https://www.sciencedirect.com/science/article/abs/pii/S0003317004748555 [ Links ]
Rizo MM, Vuelta LD, Lorenzo GA. 2017. Agricultura, desarrollo sostenible, medioambiente, saber campesino y universidad. Ciencia en su PC. (2):106-120. ISSN: 1027-2887. https://www.redalyc.org/pdf/1813/181351615008.pdf [ Links ]
Rodríguez VR, Apanaskevich DA, Ojeda CM, Trinidad MI, Reyes NE, Esteve GM, de León AP. 2016. Ticks collected from humans, domestic animals, and wildlife in Yucatan, Mexico. Veterinary Parasitology. 215:106-113. ISSN: 0304-4017. https://www.sciencedirect.com/science/article/abs/pii/S0304401715300777 [ Links ]
Salem AZ, Salem MZ, El-Adawy MM, Robinson PH. 2006. Nutritive evaluations of some browse tree foliages during the dry season: secondary compounds, feed intake and in vivo digestibility in sheep and goats. Animal Feed Science and Technology. 127:251-267. ISSN: 377-8401. https://doi.org/10.1016/j.anifeedsci.2005.09.005 [ Links ]
Taylor MA, Learmount J, Lunn E, Morgan C, Craign BH. 2009. Multiple resistence to anthelmintics in sheep and comparison of methods used for their detection. Small Ruminant Research. 86:67-70. ISSN: 0921-4488. https://doi.org/10.1016/j.smallrumres.2009.09.020 [ Links ]
Van WJ, Cabaret J, Michael LM. 2004. Morphological identification of nematode larvae of small ruminants and cattle simplified. Veterinary parasitology. 119:277-306. ISSN: 0304-4017. https://www.sciencedirect.com/science/article/abs/pii/S0304401703004734 [ Links ]
Received: September 03, 2020; Accepted: December 21, 2020; Published: January 17, 2021











 text in
text in 



