Introduction
The definitive treatment of intracranial aneurysms – both ruptured and unruptured – is aimed at obliterating their lumen without interfering with normal adjacent circulation, thus reducing or even eliminating the risk of further rupture. To achieve this, the standard treatment modalities are microsurgical clipping and endovascular therapy1. Even though technologic advances in both treatment modalities have improved clinical outcomes in the past years2, an attempt to prove superiority of one modality over another remains a challenge that appears unattainable due to the wide heterogeneity of the affected population.
Aneurysm location and its anatomical configuration may act in favor of one treatment modality over another. Several studies focused on middle cerebral artery (MCA) aneurysms have offered evidence to support microsurgical clipping for aneurysms in this location1,3-5. This can have a considerable impact since MCA aneurysms account for roughly one-third of all intracranial aneurysms and are usually underrepresented in major trials6. In this study, we offer a comparison of clinical and radiological outcomes between both treatment modalities focused exclusively on saccular MCA aneurysms and offer a treatment algorithm based on available evidence.
Materials and methods
We performed a retrospective comparative study including all patients with saccular MCA aneurysms treated at our canter from 2015 to 2019. Patients were excluded if they did not have a complete 12-month follow-up, or if they harbored other aneurysms treated by any modality. Patients with multiple aneurysms were included only if definitive treatment was exclusively aimed at one or more MCA aneurysms in a single treatment session. The treatment modality was agreed on by the surgical and the endovascular teams on a case-by-case basis. Microsurgical clipping was preferred in patients with complex aneurysms and in those with a clear indication for surgical management due to other associated conditions (e.g. intracranial hematoma, hydrocephalus, and symptomatic aneurysms). Endovascular therapy, on the other hand, was favored in older patients harboring unruptured asymptomatic aneurysms with favorable anatomic features for embolization (small, non-complex aneurysms with a dome-to-neck ratio > 2). Complex aneurysms were defined as those with > 25 mm of maximum diameter, those previously treated by any modality, vascular branches arising from the aneurysm dome, or an irregular morphology (non-spherical, asymmetric, or multilobulated domes). When both modalities were considered to be equally appropriate, other factors such as age, occlusion durability, and patient's preference were individually examined and discussed to reach an agreement. Both the surgical and the endovascular teams were permanently available at our center during the study. The hospital institutional review board approved the study; our institution's ethics committee did not require patient consent for retrospective analysis of patients' records and image studies.
Demographic and clinical variables of the patients at the time of diagnosis were collected, including sex, age, clinical presentation, and initial clinical status as assessed by the Hunt and Hess grading scale in case of subarachnoid hemorrhage (SAH)7. We recorded the radiological grade in each case as assessed by the modified Fisher scale8, as well as the presence of a Sylvian or intracerebral hematoma requiring surgical evacuation. Intracranial aneurysms were diagnosed by digital subtraction angiography (DSA), thin-sliced computed tomography angiography (CTA), or magnetic resonance angiography (MRA). Aneurysm morphological variables were collected, including location, concomitant aneurysms, affected side, neck width, dome width, dome height, dome-to-neck ratio, and complexity.
Patients were divided into two groups according to the performed treatment, either microsurgical clipping or endovascular therapy using detachable coils or stent-assisted coiling, as deemed appropriate by the treating interventionist. Clinical outcomes included mortality, functional status as assessed by the modified Rankin scale (mRS)9, rebleeding events, ischemic changes on follow-up imaging, and repeat intervention through any treatment modality. The occurrence of radiological vasospasm and delayed cerebral ischemia was also recorded. Radiological outcome was the rate of residual aneurysms on follow-up studies. Radiological follow-up was performed by either DSA, CTA, or MRA, but suspected residual aneurysms were confirmed with DSA in every case and defined as any degree of contrast filling within the neck or dome of the aneurysm. Procedure-related complications were classified as intraoperative mortality, intraoperative aneurysm rupture, clip- or coil-associated vessel occlusion (perforating or distal branches), and surgical site infection. A comparative analysis was performed between both treatment groups, comparing baseline variables and outcomes at the end of a 12-month follow-up period. In addition, we performed a subgroup analysis in patients who presented with SAH. Outcomes were also compared according to clinical presentation (SAH vs. unruptured aneurysms).
Frequencies of categorical variables, as well as mean and standard deviation of continuous variables, are reported. Statistical analysis was performed with SPSS software (version 23.0; IBM Corp., Armonk, NY). We used Student's t-test for normally distributed and Mann–Whitney U-test for non-parametric continuous variables and Chi-squared test for categorical variables. p < 0.05 was considered statistically significant.
Results
In the studied period, 77 patients harboring 95 MCA aneurysms were included in the analysis. Out of the 77 patients included in the study, 50 (65%) underwent microsurgical clipping and 27 (35%) were treated by endovascular therapy. Table 1 depicts clinical and demographic variables of the patients in each treatment group as well as morphological variables of the treated aneurysms. A higher portion of patients who presented with SAH and a lower portion of patients with unruptured aneurysms were treated with microsurgical clipping. Twelve patients (16%) harbored an intracranial hematoma that was considered amenable for surgical evacuation and were exclusively treated by surgery. There were significantly more complex aneurysms treated by surgical clipping than by endovascular therapy. There were no other significant differences in the remaining variables between both groups.
Table 1 Clinical, demographic, and morphological variables of MCA aneurysms divided by treatment group
| Variable | Clipping (n = 50) | Endovascular (n = 27) | OR (CI 95) | p | Total (n = 77) |
|---|---|---|---|---|---|
| Female, sex | 35 (70%) | 16 (59.3%) | 1.604 (0.6 4.26) | 0.342 | 51 (66.2%) |
| Mean age, years (SD) | 51.5 ± 14.2 | 51.1 ± 15.4 | - | 0.920 | 51.38 ± 14.5 |
| Diagnosis | 6.56 (2.24 19.15) | < 0.001 | |||
| SAH | 42 (84%) | 12 (44.4%) | 54 (70.1%) | ||
| Unruptured | 8 (16%) | 15 (55.6%) | 23 (29.9%) | ||
| HH (n = 54)a | - | 0.095 | |||
| I | 9 (21.4%) | 0 | 9 (16.7%) | ||
| II | 12 (28.6%) | 6 (50%) | 18 (33.3%) | ||
| III | 11 (26.2%) | 2 (16.7%) | 13 (24.1%) | ||
| IV | 10 (23.8%) | 4 (33.3%) | 14 (25.9%) | ||
| V | 0 | 0 | 0 | ||
| mFisher (n = 54)a | - | 0.292 | |||
| 0 | 3 (7.1%) | 0 | 3 (5.6%) | ||
| 1 | 6 (14.3%) | 2 (16.7%) | 8 (14.8%) | ||
| 2 | 7 (16.7%) | 5 (41.7%) | 12 (22.2%) | ||
| 3 | 17 (40.5%) | 4 (33.3%) | 21 (38.9%) | ||
| 4 | 9 (21.4%) | 1 (8.3%) | 10 (18.5%) | ||
| Hematoma | 12 (24%) | 0 | 17.86 (1.01 314.5) | 0.001 | 12 (15.6%) |
| Affected side (n = 95)b | 0.84 (0.36 1.99) | 0.696 | |||
| Right | 35 (56.5%) | 20 (60.6%) | 55 (57.9%) | ||
| Left | 27 (43.5%) | 13 (39.4%) | 40 (42.1%) | ||
| Multiple aneurysms | 23 (46%) | 14 (51.9%) | 0.791 (0.31 2.02) | 0.624 | 37 (48.1%) |
| Location (n = 95)b | - | 0.797 | |||
| Bifurcation | 41 (66.1%) | 25 (75.8%) | 66 (69.5%) | ||
| M1 | 11 (17.7%) | 4 (12.1%) | 15 (15.8%) | ||
| M2 | 8 (12.9%) | 3 (9.1%) | 11 (11.6%) | ||
| M3 | 2 (3.2%) | 1 (3%) | 3 (3.2%) | ||
| Complex (n = 95)b | 13 (21%) | 1 (3%) | 8.49 (1.05 68.1) | 0.009 | 14 (14.7%) |
| Neck width (mm ± SD) | 2.83 ± 1.1 | 3.5 ± 2.1 | - | 0.189 | 3.07 ± 1.5 |
| Dome height (mm ± SD) | 5.9 ± 4.3 | 6.1 ± 5.3 | - | 0.970 | 6.01 ± 4.6 |
| Dome width (mm ± SD) | 4.7 ± 3.2 | 5.6 ± 4.2 | - | 0.218 | 5.01 ± 3.6 |
| Dome-to-neck ratio | 2.0 ± 0.9 | 1.7 ± 0.9 | - | 0.135 | 1.9 ± 0.9 |
aOnly patients who presented with subarachnoid hemorrhage are included
bThe side, location, and complexity of the treated lesions are reported by total aneurysms. In bold: statistically significant.
CI 95: 95% confidence interval; HH: Hunt and Hess grading scale; MCA: middle cerebral artery; mFisher: modified Fisher scale; OR: odds ratio; SAH: subarachnoid hemorrhage; SD: standard deviation.
Outcomes reported by treatment group are depicted in table 2. There were 3 deaths (3.9%) recorded at the end of the follow-up period, all of them belonging to the microsurgical group and to patients presenting with SAH. Causes of death were a rebleeding episode in one patient, one case of hospital-acquired pneumonia, and a spontaneous intracerebral hematoma secondary to hypertension in another patient, distant and unrelated to the treated aneurysm. At the end of follow-up, 56 patients (73%) had a good functional status (mRS 0-2), with 35 (45.5%) being completely asymptomatic and independent for activities of daily living (mRS 0). There were no differences in clinical outcomes at the end of follow-up between both treatment groups. Residual aneurysms were observed less frequently in the microsurgical treatment group (OR = 0.09; 0.03-0.27, p < 0.001). The observed differences were maintained after discarding neck residuals and comparing only dome residuals (OR = 0.05; 0.01-0.43, p < 0.001).
Table 2 Patient-related and aneurysm-related outcomes at 12-month follow-up by treatment group
| Outcome | Clipping (n = 50) | Endovascular (n = 27) | OR (CI 95) | p | Total (n = 77) |
|---|---|---|---|---|---|
| Mortality | 3 (6%) | 0 | 4.05 (0.2-81.4) | 0.103 | 3 (3.9%) |
| Ischemic changes | 14 (28%) | 5 (18.5%) | 1.71 (0.54-5.41) | 0.357 | 19 (24.7%) |
| Vasospasm | 18 (36%) | 4 (14.8%) | 3.23 (0.97-10.8) | 0.050 | 22 (28.6%) |
| DCI | 14 (28%) | 4 (14.8%) | 2.24 (0.66-1.64) | 0.192 | 18 (23.4%) |
| mRS | - | 0.365 | |||
| 0 | 21 (42%) | 14 (51.9%) | 35 (45.5%) | ||
| 1 | 9 (18%) | 6 (22.2%) | 15 (19.5%) | ||
| 2 | 5 (10%) | 1 (3.7%) | 6 (7.8%) | ||
| 3 | 2 (4%) | 3 (11.1%) | 5 (6.5%) | ||
| 4 | 5 (10%) | 2 (7.4%) | 7 (9.1%) | ||
| 5 | 5 (10%) | 1 (3.7%) | 6 (7.8%) | ||
| mRS 0-2 | 35 (70%) | 21 (77.8%) | 0.66 (0.22-1.98) | 0.465 | 56 (72.7%) |
| Rebleeding (n = 95)a | 3 (4.8%) | 2 (6.1%) | 0.79 (0.13-5.0) | 0.801 | 5 (6.5%) |
| Residual (n = 95)a | 7 (11.3%) | 19 (57.6%) | 0.094 (0.03-0.267) | < 0.001 | 26 (27.4%) |
| Dome residual (n = 95)a | 1 (1.6%) | 8 (24.2%) | 0.051 (0.006-0.43) | < 0.001 | 9 (9.5%) |
| Retreatment (n = 95)a | 4 (6.5%) | 6 (18.2%) | 0.310 (0.08-1.19) | 0.084 | 10 (10.5%) |
aReported by total aneurysms. In bold: statistically significant.
CI 95: 95% confidence interval; DCI: delayed cerebral ischemia; OR: odds ratio; mRS: modified Rankin scale.
In the subgroup analysis of patients presenting with SAH (n = 54), there were no significant differences in clinical, demographic, or morphological variables between treatment groups. Moreover, there were no differences in functional outcome, ischemic events, and repeat intervention rates between both groups. However, 9.5% of aneurysms treated by microsurgical clipping and 58.3% of those treated by endovascular therapy displayed a residual lesion (OR = 0.08; 0.02-0.35, p = 0.001).
As expected, patients presenting with SAH were more frequently associated to ischemic changes (OR = 4.82; 1.01-22.9, p = 0.034), radiological vasospasm (OR = 32.5; 1.88-563.8, p < 0.001), DCI (OR = 23.8; 1.37-414.5, p = 0.002), and a poor functional status (OR = 12.99; 1.61-111.1, p = 0.003) than those who were treated for an unruptured aneurysm. However, the rate of residual aneurysms did not differ between clinical presentation groups (OR = 0.45; 0.17-1.15, p = 0.092).
Procedure-related complications occurred in three cases after microsurgical clipping (6%) and in three cases after endovascular therapy (11.1%). Complications associated with microsurgery were two cases of perforating vessel occlusion (both occurring in complex aneurysms) and one case of intraoperative aneurysm rupture, presenting also a surgical site infection. In endovascular procedures, two cases presented distal vessel occlusion (one of them being a complex aneurysm) and one case presented intraprocedural rupture. There were no statistically significant differences in mortality or in procedure-related complications between both groups.
Discussion
Due to the wide heterogeneity of intracranial aneurysms, it would be unreasonable to establish strictly defined criteria for treatment selection without a proper case-by-case analysis. In this study, even though treatment modality was initially suggested based on certain anatomical and clinical considerations, it is difficult to weigh a specific feature over the rest of them since many of those supporting both options often coexist in the same patient. This might explain the apparent equivalence of baseline variables between both groups except for those that strongly favored surgical treatment (complex aneurysms and associated intracranial hematoma). The significant proportion of patients with these features might partly account for the imbalance of total cases in each treatment group. In other centers, different external factors could influence treatment decision, such as institutional policies, surgeon's expertise, resource availability, and costs. In this regard, even though associated costs might be similar between both treatment modalities10,11, it could still remain a significant concern in some centers where endovascular procedures are not readily accessible and may ultimately shift the balance toward surgical clipping.
Taking all these considerations in mind, it would be inappropriate to reduce the management of patients with MCA aneurysms to predetermined strict treatment policies (e.g., “clip-first” or “coil-first” policies). Consequently, we herein propose a simple treatment algorithm regarding the optimal treatment of patients with aneurysms in this location (Fig. 1), addressing the complex interaction between these factors in a stepwise fashion. It is not by any means intended to dictate the best treatment modality for every patient harboring an MCA aneurysm but rather to provide initial guidance as to whether clinical equipoise is properly considered to be present to further discuss both treatment options.
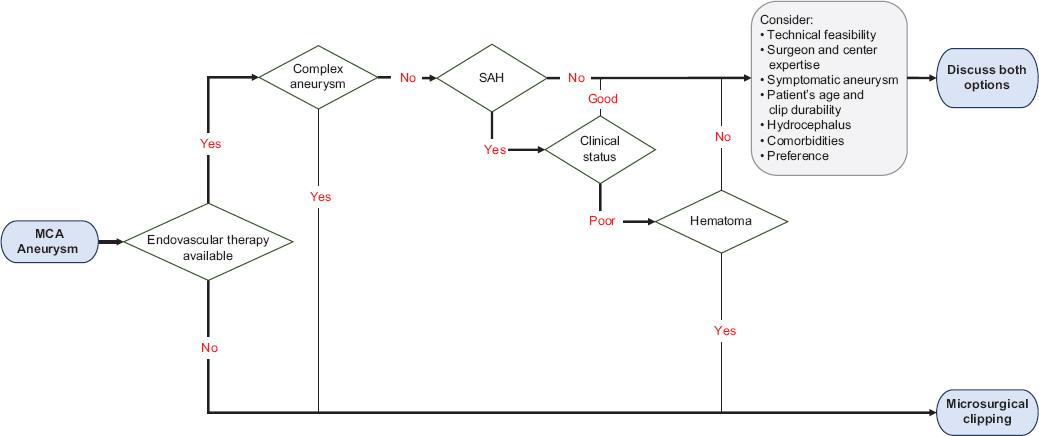
Figure 1 Proposed treatment algorithm for patients with MCA aneurysms. Certain factors may strongly favor microsurgical clipping, such as resource availability, complex aneurysms, or the presence of intracerebral or Sylvian hematomas amenable to surgical evacuation. Nevertheless, all other aspects should be considered before discussing both treatment options. MCA: middle cerebral artery; SAH: subarachnoid hemorrhage.
Clinical outcome
In the studied population, a good functional status (mRS ≤ 2) at 1 year was observed in 72.7% of patients, but this number has to be interpreted considering the higher proportion of patients with SAH included. If we consider only unruptured aneurysms, a good functional status is observed in 95.7% of patients, which is significantly different than for SAH patients (63%, p = 0.003), and similar to the previous studies done in other high-volume neurosurgical centers5,6,12-14. Although functional status at the end of follow-up was similar between both treatment groups, the significantly higher proportion of patients who presented with SAH in the microsurgical group might lead to a biased interpretation of these results due to the inherently worse prognosis of this clinical condition.
Post-treatment ischemic changes were observed in 19 patients (24.7%), with no significant difference between both treatment groups. It is worth noting that the high rate of ischemic lesions should be viewed in the context of a higher proportion of patients presenting with SAH, since ischemic changes were observed more frequently in this subset of patients (OR = 4.82, p = 0.034), which could mostly be attributed to the occurrence of vasospasm (41% of patients presenting with SAH). Otherwise, the rate of ischemic changes in unruptured aneurysms resembled those reported in the previous studies15-17.
There were three deaths at the end of follow-up, accounting for a mortality rate of 3.9% of the studied population. It is worth mentioning that at the present time, it could be too early to observe a difference in mortality – if any – attributable to rebleeding episodes, which would be expected in a long-term follow-up depending on residual aneurysms, as suggested by the previous studies18,19.
Radiological outcome
Although clinical outcomes were similar between both groups, radiological outcome (i.e., the presence or absence of residual aneurysms on follow-up DSA) significantly favored microsurgical clipping (OR = 0.09, p < 0.001), especially when comparing only dome residuals (OR = 0.05, p < 0.001). This difference may increase over time as the previous studies have suggested18-20, which could potentially affect clinical outcomes in a long-term follow-up. It is worth noting that a dome residual in a coiled aneurysm does not necessarily represent a higher risk of aneurysm growth than a neck residual, since other factors may influence clot formation and aneurysm occlusion, such as contrast filling within the coil mass21. Bearing this in mind, the difference of “high-risk” residual aneurysms between groups could be different than originally reported.
While other studies report complete and near-complete occlusion rates as a single successful outcome, we decided to classify any degree of contrast filling as a residual aneurysm due to the potential growth these seemingly small lesions might have over time20-23. This definition may include small neck remnants which would otherwise be considered as adequately occluded in other studies and might account for the apparently high rate of residual aneurysms in our study. Accordingly, when considering only dome residuals, the rate of residual aneurysms decreased to 9.5%, similar to other high-volume centers24.
Complications
Procedure-related complications were reported in 7.8% of cases, with no significant difference between treatment groups. It should be noted that three out of four cases of procedural vessel occlusion could be attributed to complex aneurysms, which have been associated with more procedural complications3. Overall, the low frequency of procedure-related complications is in line with other high-volume centers and could support both treatment modalities as relatively safe options for aneurysm occlusion, and thus should not be used as a defining parameter to choose one treatment over another12,25.
There are several high-quality studies that have analyzed and compared outcomes between both treatment modalities3,12,19,26,27. One of the most prominent worldwide is the International Subarachnoid Aneurysm Trial (ISAT)27, where patients with ruptured intracranial aneurysms considered suitable for both therapeutic options were randomized into either microsurgical clipping or endovascular coiling. They report a higher probability of independent survival at 1 year in patients treated with endovascular coiling13, a tendency that remains present in the long-term follow-up18. This difference in 1-year outcome in favor of endovascular therapy is supported by another high-quality study28, although it is not persistent during long-term follow-up19. However, these studies also report a higher risk of residual aneurysms19 and rebleeding events13,18,29 in patients treated by endovascular therapy. Despite its high impact in the medical community, the ISAT study has raised some concerns and has to be interpreted with caution, especially due to the low proportion of aneurysms included, selection of a particular subset of patients (e.g., good clinical status, selected location, and size of aneurysms), sample bias due to a higher pre-treatment mortality in the surgical group, and different expertise among several neurosurgical centers, questioning the applicability of these results to the rest of the affected population. In particular, a judicious projection of these results would be valid only within the same subset of aneurysms (i.e., those suitable for both treatment modalities) and should not be used as rationale for a pre-specified treatment policy for all aneurysms. Moreover, in the ISAT study, MCA aneurysms were underrepresented, accounting for 14% of the included patients27, making it inappropriate to confidently apply their results to our patients.
Our study, on the other hand, analyzes outcomes exclusively for MCA aneurysms treated in a high-volume center, effectively reducing heterogeneity regarding aneurysm location and surgeon's expertise. Given existing evidence1, a prospective randomized trial similar to the ISAT study is unlikely to be carried out for only MCA aneurysms. However, our findings contribute to the previous studies focused on this population5,24,30,31, which may aid in identifying those cases in which both modalities can be considered equally suitable for MCA aneurysms, potentially guiding future prospective studies.
Advances in endovascular therapy have widened the range of lesions suitable for this treatment, with favorable outcomes reported in patients with MCA aneurysms4,24,32. In the near future, comparative studies will likely include other novel endovascular techniques to evaluate the impact of new technologies in outcomes after endovascular procedures. However, these devices are not routinely available worldwide, and their adequate occlusion rate still remains a significant concern in other studies33.
Considering its retrospective nature, since patients were divided according to the received treatment, this study does not pretend to prove superiority of one treatment modality over another in every patient with an MCA aneurysm. There are some circumstances in which one modality is undoubtedly indicated due to certain anatomical or clinical features that might favor such treatment but may also directly affect outcome (e.g., clinical condition and presence of an intracranial hematoma). Therefore, the apparent equivalence of both treatment modalities regarding clinical outcome might be due to an adequate patient selection rather than an intrinsic advantage of one technique over another. For this reason, the results of this analysis must be interpreted as a depiction of clinical practice attending to established treatment criteria and recommendations, and not as a guideline to determine the best treatment modality for any patient with an MCA aneurysm. Ultimately, treatment decision will depend on several coexisting and often discordant factors, but ideally it will not influence clinical outcome by itself.
Conclusions
It is clear that both microsurgical clipping and endovascular therapy play an important role in the treatment of patients with intracranial aneurysms, and MCA aneurysms are not the exception. In these patients, even though clinical outcomes at 1 year are similar between both treatment modalities, microsurgical clipping is associated with a significantly lower risk of residual aneurysms.
The heterogeneous nature of this disease makes any attempt to establish a standard treatment modality for all intracranial aneurysms futile, impractical, and unethical. In every patient harboring an intracranial aneurysm, multiple patient-related and aneurysm-related factors have to be taken into consideration for an adequate treatment strategy. For this reason, the available evidence must be applied on an individualized basis in a shared decision-making process between neurosurgeons, interventionists, and patients, taking into account the local expertise and resources.
Portions of this work were submitted in abstract form to be presented as e-poster at the 2021 AANS Annual Scientific Meeting, on August 21-25, 2021.











 text new page (beta)
text new page (beta)


