Facilitation is recognized as a positive plant-plant interaction that allows plant establishment in areas with harsh environments (Callaway 1995). In these environments, nurse plants, generally trees and shrubs, create benign microhabitats under their canopies that enhance the recruitment and survival of facilitated plants during their more susceptible life phases (Sosa & Fleming 2002). For this reason, the facilitation interaction has a major influence on community structuring, their dynamics, and diversity, making nurse plants key players in water-limited ecosystems (Filazzola & Lortie 2014, Molina-Montenegro et al. 2016). The mechanisms to explain this crucial relationship are multiple, with biotic factors being less studied (Bashan et al. 2000, Valiente-Banuet & Verdú 2007).
It is currently recognized that the rhizosphere of certain nurse species harbors a greater abundance and diversity of beneficial microorganisms compared to bare soil (Bashan et al. 2000, Molina-Montenegro et al. 2015, 2016). Furthermore, the association of plants with microorganisms, such as nitrogen-fixing bacteria and arbuscular mycorrhizal fungi, represents another positive aspect of the facilitation phenomena (Carrillo-García et al. 1999; Filazzola & Lortie 2014). The arbuscular mycorrhizal association (AM) stands out for its abundance and ecological importance (Lambers & Oliveira 2019). This is derived from the benefits and services they provide to plants and ecosystems, in addition to being a key mechanism in the facilitation of the nurse effect (Filazzola & Lortie 2014, Molina-Montenegro et al. 2015). In this association, fungi provide plants with nutrients, mainly phosphorus and nitrogen, and a greater availability of water in exchange for carbohydrates and a place to conduct their life cycle (Lambers & Oliveira 2019). However, the indirect facilitating effect mediated by microorganisms, such as arbuscular mycorrhizal fungi (AMF), between nurses and their facilitated plants has not been clearly established (Bashan et al. 2000) and has received less attention (Molina-Montenegro et al. 2015).
Plants growing in arid zones constantly face abiotic stress such as drought, associated with sporadic and unpredictable precipitation, extreme temperatures, high solar radiation, and nutrient deficits (Apple 2010). Nurse plants ameliorate the environment under their canopies, creating benign microhabitat conditions that increase the recruitment and survival of other plant species (Nobel 1989, Valiente-Banuet & Ezcurra 1991, Méndez et al. 2006). In deserts, the survival and establishment of many cactus species take place under nurse plants, where their small seeds and seedlings are protected from both biotic and abiotic stress (Valiente-Banuet & Ezcurra 1991, Godínez-Álvarez et al. 2003, Suzán-Azpiri & Sosa 2006). It is also recognized that the recruitment, survival, and growth of many species of columnar cacti from the Sonoran Desert, such as Carnegiea gigantea (Engelm.) Britton y Rose (saguaro), Pachycereus schottii (Engelm.) Britton & Rose, (senita), Pachycereus pringlei (S. Watson) Britton & Rose (cardón), and Stenocereus thurberi (Engelm.) Buxb. (organ pipe cactus), occur mainly under these nurse plants, with Olneya tesota A. Gray (ironwood; Fabaceae), being one of the two most important nurse plant for this region (Suzán et al. 1996, Suzán-Azpiri & Sosa 2006). Under nurse species, including O. tesota, the microenvironment is more benign, with greater soil fertility associated with the accumulation of nutrients such as organic matter, nitrogen, and potassium (Suzán et al. 1996, Carrillo-García et al. 2000). The higher fertility and better soil properties under nurse plants have been linked to the abundance of AMF, owing to their role in soil formation and stabilization, their capacity to acquire and exchange nitrogen (N), phosphorus (P), and water, thereby enhancing the performance of the facilitated species (Carrillo-García et al. 1999, Montesinos-Navarro et al. 2016, Sortibrán et al. 2018).
Despite its importance, the interactions between columnar cacti and AMF associated with plant facilitation are still poorly known. In this study, we took a descriptive approach to this issue by evaluating the mycorrhizal status and evaluating soil properties of the rhizosphere of four columnar cacti (saguaro, senita, cardon, and organ pipe cactus) growing both under and outside the nurse plant canopy (O. tesota). These columnar cacti are some of the most abundant and iconic species of the Sonoran Desert (Dimmitt 2015). Therefore, a study evaluating their association with AMF might highlight the role of AMF in plant facilitation. We hypothesized that there would be a greater number of AMF structures and mycorrhization of roots in the facilitated plants under the nurse, compared to the same species growing outside the nurse. This expectation stems from the fundamental role of AMF in the nurse-facilitated species relationship between ironwood and cacti, given their modifying role in the soil properties.
Materials and methods
Study area and species. The study was conducted in the Central Gulf Coast subdivision of the Sonoran Desert, situated along the road connecting Hermosillo and Bahía de Kino in the municipality of Hermosillo, Sonora, México (28° 52’ 27.7’’ N; 111° 57’ 32.4’’ W). The climate is characterized by hot temperatures, with an average monthly maximum of 34.1 °C during August, an average minimum of 6.6 °C during January, and a mean annual temperature of 20.5 °C. The average annual rainfall is 124 mm, concentrated in July, August, and September (Servicio Meteorológico Nacional, SMN 2019); whereas relative humidity varies from 43.9 % during March to 78.1 % during August (Red de Estaciones Meteoreológicas Automáticas de Sonora, REMAS 2023). The vegetation comprises Sonoran Desert scrub, characterized by legume trees and columnar cacti (Shreve & Wiggins 1964).
We investigated four columnar cacti species: Carnegiea gigantea (saguaro), Pachycereus schottii (senita), Pachycereus pringlei (cardon), and Stenocereus thurberi (organ pipe cactus). This group of species reach heights exceeding 3 m. C. gigantea and P. pringlei are arborescent, and both produce white nocturnal flowers pollinated mainly by bats (Turner et al. 1995). P. schottii and S. thurberi are shrubby species with several vertical stems originating from their base. From March to August, P. schottii produce pink to lavender-colored flowers pollinated by moths (Fleming & Holland 1998). Fruits with numerous small seeds are produced at the end of the dry season, and germination occurs with the onset of summer rains. Olneya tesota (ironwood) is a tree that can reach up to 11 m in height. Belonging to the family Fabaceae, it features compound leaves. Its brief flowering period occurs in May, with pale lavender-colored flowers (Dimmitt 2015) (Figure 1). This species is distributed throughout the Sonoran Desert, almost completely confined to it, and seeds cannot be established in places where temperatures fall below -7 °C. Ironwood replaces palo verde and mesquite as the primary nurse in the Central Gulf Coast subdivision and parts of the Lower Colorado River Valley subdivision of the Sonoran Desert (Dimmitt 2015, Figure 1).
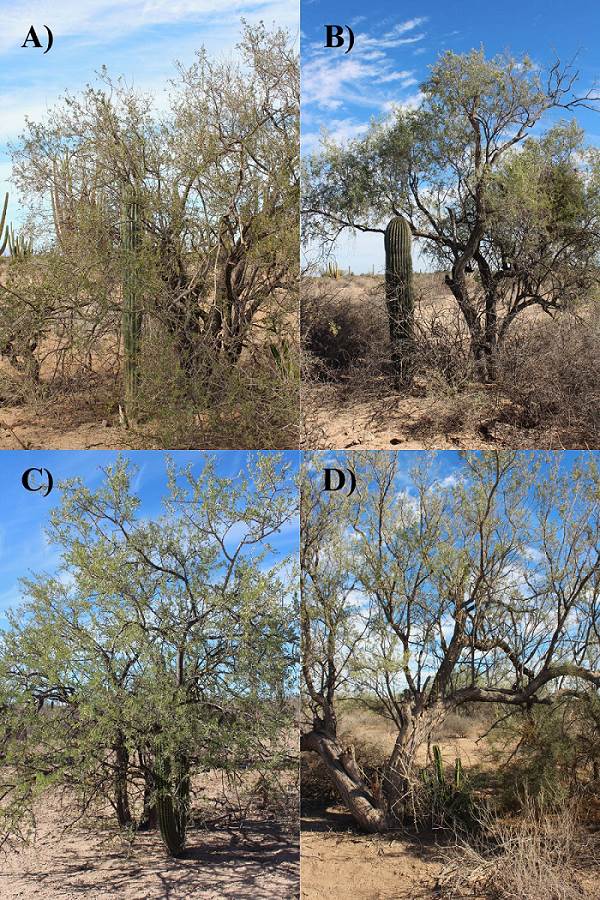
Figure 1 Pre-reproductive individuals of the columnar cacti associated with the nurse plant, Olneya tesota, in the study area. A) Pachycereus pringlei, B) Carnegiea gigantea, C) Stenocereus thurberi, D) Pachycereus schottii.
Sampling. Six individuals of comparable size were selected from each cactus species, with three associated with O. tesota as a nurse plant, and three growing outside the canopy in bare areas. The criteria to select individuals were comparable size within species, no damage signals presented, no reproductive structures present and the individuals must be completely under the canopy of nurse plant or completely out of the influence of some canopy. In October 2020, samples of fine roots (n = 24) and rhizosphere soil (n = 24) from a depth of 0-15 cm were collected from each individual. The roots were carefully stored in plastic bags and subsequently processed for staining. Soil samples were also collected and stored in plastic bags at room temperature, awaiting processing for soil spore determination and chemical analysis.
Mycorrhizal status determination. In the collected roots, the following variables were assessed: the percentage of mycorrhizal colonization (MC), visual density (VD), percentages of hairy roots (HR), and the number of spores 100 g-1 of dry soil. Initially, roots were stained with the blue dye of trypan, utilizing the modification by Álvarez-Sánchez & Monroy Ata (2008), based on the method proposed by Phillips & Hayman in 1970. Root bleaching was performed with 10 % KOH at room temperature for 24 hours, followed by acidification with 10 % HCl for 10 minutes, and staining with 0.05 % trypan blue in lactoglycerol.
The percentages of MC, VD and HR were determined using the modification by Herrera-Peraza et al. (2004) of the gridline intersection method proposed by Giovannetti & Mosse (1980). Following this method, roots were randomly scattered in a Petri dish reticulated to 1 cm, and the number of roots intercepting the grid lines was counted. They were then visually classified for MC and HR simultaneously, based on the schemes proposed by Herrera-Peraza et al. (2004). VD was calculated as the percentage of the root occupied by the mycorrhizal fungi. The number of spores in the soil was determined by counting those present in two soil fractions resulting from wet sieving and decanting technique (Gerdemann & Nicolson 1963) with meshes of 125 and 38 μm. To extract spores from the soil, an aliquot of each soil fraction was centrifuged for five minutes in a water and 2M sucrose gradient at 1,500 rpm. The interface was extracted and distributed over a Doncaster plate. Subsequently, the number of spores along the circumference of the plate was counted under a stereoscopic microscope (Herrera-Peraza et al. 2004).
Chemical analysis of soil. Soil samples of the same individuals where roots were collected for a total of 6 per species, three from cacti individuals below the canopy and three from cacti outside the canopy, were sampled for a total of 24 samples. Soil samples were dispatched to the Laboratory of Terrestrial Biogeochemistry and Chemistry at the Institute of Ecology, UNAM, for the chemical determination of pH, organic matter, total N, nitrate, ammonium, total and assimilable phosphorous. Organic C was analyzed in an automated C-analyzer (Schimadazu 5005A) by grinding a 5 g dried subsample (100-mesh screen). The concentration of total N and total P was determined by acid digestion (Anderson & Ingram 1993) using an NP analyzer (Technicon Autoanalyzer III). Soil available orthophosphate (PO4 3-) was extracted using the Olsen & Sommers (1982) method. Additionally, ammonium (NH4) and nitrate (NO3) levels were determined using KCL 2N (TIS 1977).
Statistical analysis. We tested whether there were significant differences in mycorrhizal variables between roots collected under the nurse O. tesota and in bare soil, and whether there were significant differences among species, using generalized linear models (GLM). Mycorrhizal colonization (MC), percentage of visual density (VD) and percentage of hairy roots (HR) were declared with normal distribution whereas the number of spores 100 g-1 of soil was declared with a Poisson distribution. Similarly, chemical soil variables were analyzed using GLM, testing whether there are significant differences between soil samples collected under O. tesota compared to bare soil as well as significant differences among species. All statistical analyses were performed using JMP version 11 (SAS Institute Inc., Cary, NC). From now on, we use soil and bare area interchangeably.
Results
Mycorrhizal status. All studied columnar cacti were colonized by AMF. Significant differences were recorded for most mycorrhizal variables below the nurse plant compared to bare areas (Figure 2, Table 1). For mycorrhizal colonization (MC), significant greater values were recorded in roots from cacti associated with O. tesota, compared to those in bare areas (Figure 2A). However, no significant differences were observed for MC among the columnar cacti (Table 1). Similarly, for visual density (VD), significant higher values were observed in roots from cacti within the canopy of O. tesota, compared to those in bare areas (Figure 2B, Table 1), and no significant differences were recorded among species (Table 1). In contrast to MC and VD, no significant differences were observed for the percentage of hairy roots (HR) in cacti associated with O. tesota and bare areas (Figure 2C, Table 1) and in this case, significant differences were observed among species of cacti for HR (Figure 3A, Table 1). Finally, for the number of spores 100 g-1 of dry soil, highly significant differences were recorded for soil samples within the canopy of O. tesota compared to bare areas (Figure 2D, Table 1). For the number of spores 100 g-1 of dry soil, we also observed significant differences among species of columnar cacti (Figure 3B, Table 1).
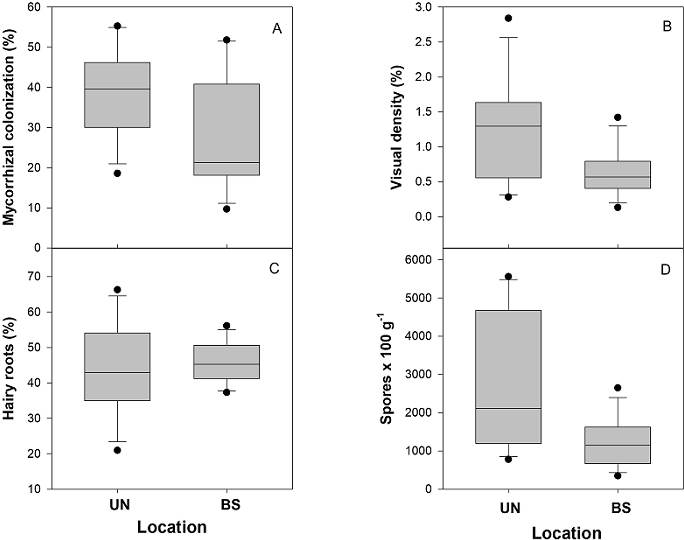
Figure 2 Box plots of mycorrhizal variables for the four species of columnar cacti growing associated with Olneya tesota UN and bare soil BS. Percentage of mycorrhizal colonization under the nurse plant and in bare soil. (A); Percentage of visual density under the nurse plant and in bare soil. (B); Percentage of hairy roots under the nurse plant and in bare soil. (C); Number of spores per 100 g of dry soil under the nurse plant and in bare soil. (D).
Table 1 Mean, standard deviation and statistical test using generalized linear models of each mycorrhizal variable testing the effect of location (under the nurse Olneya tesota and in bare soil) and species of columnar cacti.
| Mycorrhizal variable | Mean and standard deviation | Location (under nurse vs bare soil) | Species of columnar cacti | |
|---|---|---|---|---|
| Under nurse | Bare soil | |||
| MC (%) | 38.93 ± 10.85 | 28.22 ± 14.44 | χ2 = 5.19, DF = 1, P = 0.02 | χ2 = 5.58, DF = 3, P = 0.13 |
| C. gigantea | 39.26 ± 18.46 | 24.45 ± 7.52 | ||
| P. pringlei | 36.07 ± 6.16 | 16.52 ± 5.99 | ||
| P. schottii | 39.49 ± 6.69 | 44.11 ± 6.77 | ||
| S. thurberi | 40.9 ± 14.33 | 27.81 ± 20.1 | ||
| VD (%) | 1.21 ± 0.74 | 0.64 ± 0.34 | χ2 = 5.80, DF = 1, P = 0.01 | χ2 = 0.28, DF = 3, P = 0.96 |
| C. gigantea | 1.50 ± 1.28 | 0.52 ± 0.15 | ||
| P. pringlei | 1.27 ± 0.69 | 0.42 ± 0.25 | ||
| P. schottii | 0.93 ± 0.38 | 0.86 ± 0.14 | ||
| S. thurberi | 1.13 ± 0.68 | 0.74 ± 0.59 | ||
| HR (%) | 43.99 ± 13.46 | 46.12 ± 5.74 | χ2 = 0.39, DF = 1, P = 0.52 | χ2 = 8.86, DF = 3, P = 0.03 |
| C. gigantea | 33.65 ± 15.38 | 45.23 ± 9.47 | ||
| P. pringlei | 45.8 ± 7.28 | 43.09 ± 5.89 | ||
| P. schottii | 60.54 ± 5.72 | 47.62 ± 4.11 | ||
| S. thurberi | 35.97 ± 1.35 | 48.52 ± 3.54 | ||
| No. spores/100 g soil | 2,748.33 ± 1,768.38 | 1,222.5 ± 630.25 | χ2 = 7,220.06, DF = 1, P < 0.0001 | χ2 = 3,214.61, DF = 3, P < 0.0001 |
| C. gigantea | 3,826.7 ± 2,452.94 | 1,310 ± 1,160.47 | ||
| P. pringlei | 2,220 ± 988.38 | 776.67 ± 430.04 | ||
| P. schottii | 3,363.33 ± 2,331.14 | 1,483.33 ± 381.36 | ||
| S. thurberi | 1,583.33 ± 72.34 | 1,320 ± 285.83 | ||
MC = Percentage of mycorrhizal colonization. VD = Percentage of visual density. PR = Percentage of hairy roots.
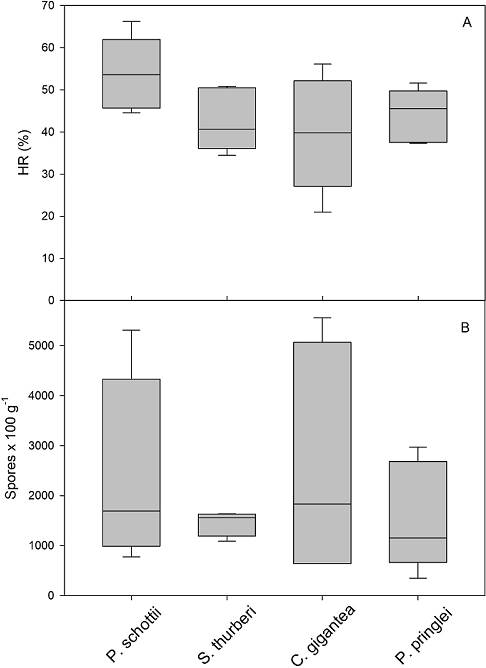
Figure 3 Box plots of the percentage of hairy roots (A) and number of spores per 100 g of dry soil (B), among the four species of columnar cacti in the study area.
Soil properties. Several chemical properties showed greater values under the canopy of O. tesota compared to bare soil, while others were similar between the nurse and bare soil (Figure 4, Table 2). pH was similar under the canopy compared to bare soil (Table 2); whereas organic matter was significantly higher in soils associated with O. tesota, compared to bare soil (Table 2, Figure 4). In contrast, no significant differences for pH and organic matter were recorded among species of cacti (Table 2). For ammonium and total nitrogen, no significant differences were recorded between soils under the nurse plant and bare soil, and neither among species (Table 2). In contrast, clear significant differences were observed for nitrate in soil samples under O. tesota compared to bare soil (Table 2, Figure 4). For all forms of soil nitrogen (ammonium, nitrate and total), no significant differences were observed among species (Table 2). Finally, for total and assimilable phosphorous, clear significant differences were recorded between soil samples under O. tesota and bare soil (Table 2, Figure 4) but no differences were observed among species (Table 2).
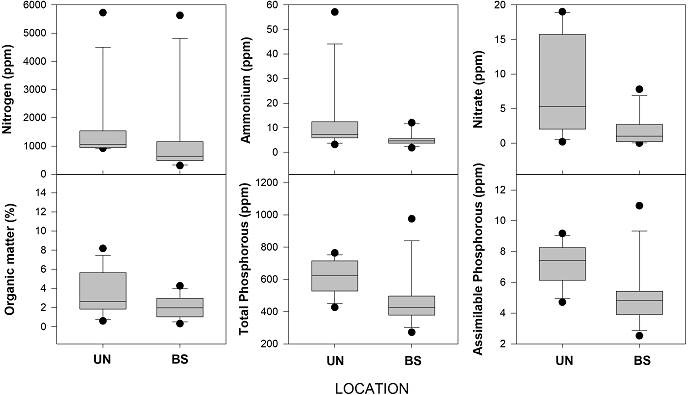
Figure 4 Box plots of chemical soil properties from samples taken under the nurse plant and in bare soil. Soil properties: Nitrogen (ppm), ammonium (ppm), nitrate (ppm), organic matter (%), total phosphorous (ppm) and assimilable phosphorous (ppm).
Table 2 Mean, standard deviation and statistical test using generalized linear models of each nutrient variable testing the effect of location (under the nurse Olneya tesota and in bare soil) and species of columnar cacti.
| Nutrient variable | Mean and standard deviation | Location (under nurse vs bare soil) | Species of columnar cacti | |
|---|---|---|---|---|
| Under nurse | Bare soil | |||
| pH | 8.17 ± 0.26 | 8.11 ± 0.58 | χ2 = 0.14, DF = 1, P = 0.70 | χ2 = 3.80, DF = 3, P = 0.28 |
| C. gigantea | 8.17 ± 0.21 | 8.36 ± 0.02 | ||
| P. pringlei | 8.01 ± 0.16 | 8.36 ± 0.06 | ||
| P. schottii | 8.16 ± 0.22 | 7.55 ± 1.08 | ||
| S. thurberi | 8.33 ± 0.42 | 8.15 ± 0.09 | ||
| Organic matter (%) | 3.46 ± 2.30 | 2.03 ± 1.14 | χ2 = 4.53, DF = 1, P = 0.03 | χ2 = 5.00, DF = 3, P = 0.17 |
| C. gigantea | 5.48 ± 2.79 | 2.32 ± 1.70 | ||
| P. pringlei | 3.15 ± 2.13 | 0.76 ± 0.38 | ||
| P. schottii | 3.00 ± 2.58 | 2.87 ± 0.58 | ||
| S. thurberi | 2.23 ± 1.17 | 2.19 ± 0.40 | ||
| Nitrogen (ppm) | 1,539.83 ± 1,342.81 | 1,248.25 ± 1,543.41 | χ2 = 0.30, DF = 1, P = 0.57 | χ2 = 3.60, DF = 3, P = 0.30 |
| C. gigantea | 2,769 ± 2,575.24 | 1,493.33 ± 1,308.86 | ||
| P. pringlei | 1,252 ± 3,74.84 | 467.67 ± 73.04 | ||
| P. schottii | 1,184.67 ± 218.73 | 735.33 ± 104 | ||
| S. thurberi | 953.67 ± 51.54 | 2,296.67 ± 2,885.28 | ||
| Ammonium (ppm) | 12.20 ± 14.51 | 5.39 ± 2.93 | χ2 = 2.83, DF = 1, P = 0.09 | χ2 = 1.96, DF = 3, P = 0.57 |
| C. gigantea | 9.13 ± 5.21 | 7.10 ± 4.26 | ||
| P. pringlei | 23.52 ± 29.10 | 3.58 ± 1.91 | ||
| P. schottii | 7.57 ± 1.92 | 6.65 ± 3.32 | ||
| S. thurberi | 8.58 ± 4.69 | 4.23 ± 0.81 | ||
| Nitrate (ppm) | 7.85 ± 6.94 | 1.90 ± 2.31 | χ2 = 8.90, DF = 1, P = 0.002 | χ2 = 5.28, DF = 3, P = 0.15 |
| C. gigantea | 4.10 ± 2.53 | 0.68 ± 0.49 | ||
| P. pringlei | 6.85 ± 10.54 | 0.73 ± 0.78 | ||
| P. schottii | 11.55 ± 5.51 | 5.13 ± 2.50 | ||
| S. thurberi | 8.88 ± 8.50 | 1.05 ± 1.10 | ||
| Total phosphorous | 622.58 ± 105.33 | 463.15 ± 174.84 | χ2 = 7.38, DF = 1, P = 0.006 | χ2 = 1.91, DF = 3, P = 0.59 |
| C. gigantea | 593.63 ± 168.68 | 409.64 ± 61.08 | ||
| P. pringlei | 652.74 ± 118.40 | 441.96 ± 57.93 | ||
| P. schottii | 616.03 ± 100.32 | 589.67 ± 334.68 | ||
| S. thurberi | 627.93 ± 75.87 | 411.32 ± 126.60 | ||
| Assimilable phosphorous | 7.27 ± 1.35 | 5.02 ± 2.08 | χ2 = 9.32, DF = 1, P = 0.002 | 2 = 1.15, DF = 3, P = 0.76 |
| C. gigantea | 6.80 ± 2.05 | 4.60 ± 0.87 | ||
| P. pringlei | 7.79 ± 1.70 | 4.65 ± 0.79 | ||
| P. schottii | 7.01 ± 1.31 | 6.37 ± 3.99 | ||
| S. thurberi | 7.49 ± 0.55 | 4.44 ± 1.65 | ||
Discussion
In this study, we found greater abundance of AMF structures in roots of columnar cacti growing under the canopy of O. tesota compared to bare areas outside the nurse species. Greater mycorrhization (MC and VD) was recorded in roots of cacti under the nurse plant, as well as greater abundance of AMF spores in their rhizosphere. Furthermore, several soil parameters of the rhizosphere under the nurse, showed greater values compared to bare soil, containing more organic matter, nitrate, total and assimilable phosphorus available for the plant, which can favor the establishment and growth of the facilitated species. Together, these results suggest that arbuscular mycorrhizal fungi may play a key role in the process of facilitation of cacti under nurse plants.
As predicted, MC in roots was greater under the canopy of the nurse plant compared with bare areas, whereas no differences were recorded among species. There are not many studies reporting the mycorrhizal status of columnar cacti in arid zones. In a study in the Sonoran Desert, in Baja California Sur, Carrillo-García et al. (1999) reported similar differences in MC between roots under the canopy of O. tesota and Prosopis articulata S. Watson (Fabaceae) and bare areas, for S. thurberi, L. schottii and P. pringlei. These authors found less than 10 % MC for these columnar cacti species under nurse canopies; whereas values between 36-40 % were found in this study, probably reflecting differences in annual rainfall, which was 30 % lower in our study site. Low values of MC in cacti species (less than 50 %) have been also associated with the greater production of “rain roots” in cacti (Nobel 1988, Carrillo-García et al. 1999). These roots are specific features of cacti living in extremely dry soils, as they allow them to increase the uptake of water and nutrients, as a rapid response to soil water pulses (Nobel 1989). Mean annual rainfall in our study site is low (124 mm per year), and no significant differences were detected for hairy roots (HR) between cacti under the canopy and in bare areas. However, cacti from bare areas also show high values of MC. Therefore, more studies are needed to elucidate whether cacti in this region use both rain roots and AMF to rapidly get water and nutrients.
Other studies in arid and semiarid zones reported MC in roots of several cacti species but not in the context of facilitation under nurse plants. García-Sánchez et al. (2007), reported spore density and the percentage of MC in 30 different plant species, including six cacti (Echinocereus cinerascens (DC.) Haage, Ferocactus latispinus (Haw.) Britton & Rose, Mammillaria elongata DC., Mammillaria sempervivi DC., and Opuntia streptacantha Lem., Stenocactus sp.), from four areas of thorn scrub at the Mezquital Valley, Hidalgo state, in Mexico. For cacti, they found between 0-46 % of MC, values that are like the ones recorded in this study. In successional sites of a tropical dry forest in México, MC in roots of several cacti (Opuntia karwinskiana Salm-Dyck, Opuntia puberula Hort.Vindob. ex Pfeiff., Pilosocereus collinsii (Britton & Rose) Byles & G.D. Rowley and Pachycereus pecten-aboriginum (Engelm. ex S.Watson) Britton & Rose, were higher (45-89 %) than the values found in this study, probably associated with greater annual rainfall in the dry forest (Guadarrama et al. 2014).
Visual density (VD) is an estimate of the percentage of the root cortical tissue that is occupied by the AMF. This parameter was higher in cacti growing under the nurse canopy, indicating that the AMF cover greater space in roots of plants growing under the nurse plant than those growing in bare areas. However, this difference does not imply that the association is less functional in cacti growing in bare areas, because the net nutrient exchange between plants and AMF is not proportional to variables such as VD and MC (Herrera-Peraza et al. 2004). Columnar cacti with less VD may survive much less in bare areas than under nurse plants (Turner et al. 1966). However, we cannot discard the possibility that some of the sampled cacti in bare areas were associated in the early seedling phase with nurse plants that have died in recent years.
In all studied columnar cacti, a greater number of spores was recorded in the rhizosphere under the O. tesota canopy, compared to bare areas. This finding may reflect that more appropriate environmental conditions under nurse plants and better physicochemical soil properties, such as a greater level of organic matter and nutrients, induce greater sporulation. The high number of spores was recorded at the end of the rainy season and the beginning of the dry season, when soil was sampled. At this time of the year most plants decrease its metabolic activity due to the lower water availability (Smith & Read 2008), limiting the contribution of photosynthates to the AMF, which induces their sporulation. This way, AMF ensures viable propagules until the next rainy season, when plants activate their metabolic activity again (Aguilera-Gómez et al. 2007, Aguilar-Fernández et al. 2009). Spore numbers reported in this study are higher than those reported for other arid regions in México (Carrillo-García et al. 1999, Camargo-Ricalde et al. 2003, Gutiérrez-Ruacho et al. 2018). Several factors may influence the numbers of spores including rainfall, the fungi species, soil properties and temperature among others (Ochoa-Meza et al. 2009).
Differences in soil quality under nurse canopies and bare soil has led to the hypothesis that nurse plants form “resource islands”: soil structure, stability and chemical composition are of better quality compared with bare soil outside the nurse (Carrillo-García et al. 2000, Mihoč et al. 2016). In this study, soil under O. tesota had greater OM, compared to bare areas. The percentage of OM may be influenced by the canopy size of the nurse plant, the amount of leaf litter produced by the nurse and the different facilitated species, as well as the decomposition rate, which is also influenced by the abundance of OM decomposing microorganisms (Rodríguez-Echeverria et al. 2016, Salazar et al. 2019, Ding & Eldrige 2021). O. tesota is a tree that may reach a mean crown diameter of 9 m in mature individuals (Turner et al. 1995), producing a high amount of leaf litter. Thus, the canopy of O. tesota may exert an important influence on the greater OM found under its canopy in this study.
The positive influence of nurse plants on soil properties has been described as one of the primary mechanisms involved in the facilitation process (Mihoč et al. 2016). In this study, soil under O. tesota had greater concentration of one form of nitrogen (nitrate) and total and assimilable phosphorous. Differences in soil nitrogen between bare areas and under nurse canopies have been documented for other nurse species in the Sonoran Desert (Franco & Nobel 1989, Nobel 1989). In our study, the leguminous O. tesota may have a direct influence on N concentration, as it has a symbiosis with bacteria that convert atmospheric N to ammonia, which in turn may have a positive influence for the growth of AMF (Bukovská et al. 2016). In turn, AMF may also have an important contribution absorbing and transporting P and N for its own requirements and the facilitated plants (Lambers & Oliveira 2019). Regarding the enrichment of assimilable phosphorous in the soil under the nurse plant, it is well known the significant role of AMF making the phosphorous available for plants (Lambers & Oliveira 2019). In the soil, most phosphate is in an organic and immobile form, which is not absorbed by plants. Once the plants deplete the phosphorous around their roots, extraradical hyphae can reach the organic P and transform it, in coordination with some soil bacteria, in an inorganic form available for plants (Fall et al. 2022). Thus, the nurse plant and the associated AMF may be responsible for the greater concentration of N and P under O. tesota.
In this study we provide evidence of greater mycorrhization in four species of columnar cacti from the Sonoran Desert growing under the canopy of O. tesota. Likewise, the documented enrichment of soil quality under O. tesota supports the importance of nurse plants in structuring desert plant communities (Valiente-Banuet & Verdu 2007). It is likely that AMF play a fundamental part in the facilitation process in desert ecosystems. The influence of microbes on soil enrichment and water absorption has been recognized, but it is still understudied (Auge 2001). Further studies on the role of AMF in desert environments, particularly the role of mycorrhizal networks in nutrient and water exchange between desert plants, require special attention.











 text new page (beta)
text new page (beta)



