Introduction
The margay (Leopardus wiedii) and the ocelot (tory no fulfilled the assumption of closure (not ad) are two medium-sized felids of the Neotropic. They are sympatric through their distribution ranges and occur in several habitats (Sunquist and Sunquist 2002). Leopardus pardalis is 2 to 3 times larger than L. wiedii (Sunquist and Sunquist 2002; Wilson and Mittermeier 2009), and shows plasticity in habitat use and feeding habits (Sunquist and Sunquist 2002; de Oliveira et al. 2010). L. pardalis is relatively common and its habitat use, diet, and activity pattern overlaps with those of other felids such as Puma concolor (Cougar) and Panthera onca (Jaguar; Moreno et al. 2006; Di Bitetti et al. 2010; Davis et al. 2011; but see Gomez-Ortiz et al. 2015). Some studies indicate that L. pardalis exerts a strong influence on other smaller felids in the Neotropic (de Oliveira et al. 2010). Particularly, L. pardalis abundance appears to negatively impact the abundance and activity patterns of other species by aggressive intraguild interactions or resource limitation (Cuellar et al. 2006; de Oliveira et al. 2010). Negative interactions exert a strong influence on population size, activity patterns and coexistence between species, but may decrease by spatial or temporal segregation (Donadio and Buskirk 2006; Ritchie and Johnson 2009).
Little research has been conducted on these felids in mountain tropical forests. Particularly the Sierra Norte, in southeastern Mexico, is a mosaic of natural vegetation including tropical, subtropical forest, temperate, and cloud forests. There are also areas of human activity such as agroecosystems and conservation areas (Arriaga et al. 2000). Several felids inhabit this region: L. pardalis, L. wiedii, Puma yagouaroundi (Yaguarundi) and Lynx rufus (Bobcat), as well as two larger felids: P. concolor and Panthera onca. The population size and activity pattern of these felids are still largely incomplete and their understanding is essential for the development and evaluation of conservation strategies in mountain tropical forests. Our goal was to estimate the density and activity pattern of L. wiedii and L. pardalis in Sierra Norte of Oaxaca. Specifically, we expected that L. pardalis had a high density relative to other felids due to its ecological plasticity (Sunquist and Sunquist 2002; de Oliveira et al. 2010). We also expected segregation in the activity patterns between both felids as a mechanism to reduce negative intraguild interactions (Ritchie and Johnson 2009; de Oliveira et al. 2010).
Materials and methods
Study area. The study area is located in the Sierra Norte region, Oaxaca, Mexico, in the community of San Isidro Yolox (17° 38' N, -96° 25' W; Figure 1). The region is part of the Sierras Norte-Mixe Oaxaca Priority Land Region (Arriaga et al. 2000). The climate is warm and humid. Mean annual temperature varies from 16 °C to 26 °C and precipitation ranges from 2500 to 4000 mm (Trejo 2004). The rainy season stretches from July to December, but there is rainfall throughout the year. The dominant vegetation types are semi-evergreen forest and cloud forest. Semi-evergreen forest areas alternate with agriculture land, pastures, open areas and human settlements. Cloud forests show little fragmentation and human intervention, being among the best preserved areas of natural vegetation in Mexico (Arriaga et al. 2000).

Figure 1 Location of Sierra Norte in southwestern Mexico (A), and study site at Sierra Norte, Oaxaca (B). Black dots indicate the position of camera traps in semi-evergreen and cloud forests (C).
From July 2014 to June 2015, we placed camera traps in 22 sites in the semi-evergreen forest and 22 sites in the cloud forest. The altitude of sites ranged from 480 to 1,050 and from 1,250 to 2000 m, respectively. We placed unbaited traps at 20 to 30 cm above ground and at an average distance of 0.5 ± 0.1 km between them. Camera trap models used were Bushnell Trophy Cam® and 990i Digital Game Camera Moultrie®.
Data analysis. In order to estimate population size, we identified each Leopardus individual according to patterns of rosettes, spots and strips on flanks. Males were identified by the presence of testes. We quantified the residence time of each individual as the time from the first to the last record, and considered as a transient individual any individual recorded only in a single month. Because photographs of both sides of the individuals were obtained in different numbers, only the most abundant side were used in the analysis to estimated population size by the Cormack-Jolly-Seber probabilistic model (Lebreton et al. 1992). Previously, we determined whether the populations were statistically open or closed with the program CloseTest (Stanley and Richards 2005). The Cormack-Jolly-Seber model estimates only two parameters: survival probability (φ) and capture probability (p). Both parameters can either vary or remain constant over time, so four candidate models emerge: 1) both parameters constant (φp); 2) constant φ and p varies through time (in this case expressed in years: 2014 and 2015; φpt); 3) φ varies through time and constant p (φtp); and 4) both φ and p vary through time (φtpt; Lebreton et al. 1992; Table 1). We used the program MARK version 8.1 (White and Burnham 1999) for the construction and analysis of the models and used the Akaike Information Criterion modified for small samples to select the best (final) model from the set of four candidate models. We estimated population size (N) as the number of identified organisms divided by the probability of capture of the final model. We extrapolated the population size to an area of 100 km2. We calculated the effective sampling area as the polygon defined by all trapping stations and a boundary strip. Strip width was defined as the mean maximum distance traveled by an individual that was recorded more than once.
Table 1 Selection of Cormack-Jolly-Seber models for L. wiedii and L. pardalis in Sierra Norte, southwestern Mexico. The models include time as a covariate to survival probability (φ) and capture probability (p).

aAkaike information criterion modified for small samples
bDifference between the respective model and the best model
cRelative contribution of each model regarding the sum of four models
*Best model selected for each species
In order to describe activity patterns, we divided the 24-h period into one-hour segments, and classified each independent record within those intervals. We defined an independent record as all photographs belonging to one species taken by each sampling station within a one-hour span. We assessed the level of activity overlapping between species by the coefficient of overlapping Δ1, a coefficient specially developed for circular data, such as those obtained with camera traps, which ranges from 0 (no overlap) to 1 (complete overlap) (Ridout and Linkie 2009). We also calculated the 95 % confidence intervals for the coefficient from 10, 000 replicates by the bootstraps method. The statistical analysis was performed with the Overlap package in R, version 3.3.1.
Results
With an effort of 12,800 trap-days, we obtained 141 independent records of L. wiedii and 68 records of L. pardalis, as well as records of another three felid species: P. concolor (n = 81), P. onca (n = 11), and P. yagouaroundi (n = 3). We obtained 86 % of L. wiedii records in the cloud forest and 97 % of L. pardalis records in the semi-evergreen forest. To estimate the density, we removed 50 % of the total records obtained for both species due to poor photographic quality, and we identified five individuals of L. pardalis and 16 individuals of L. wiedii. The capture history no fulfilled the assumption of closure (not additions or losses over the period of study) for L. wiedii (x2 = 5.53, P = 0.93) and L. pardalis (x2 = 0.93, P = 0.96). For both species, the best model was φp (i. e., survival and capture probabilities were constant throughout the survey; Table 1). According to this model, the estimated abundance was 51.5 individuals for L wiedii and 8.3 individuals for L. pardalis, and the density was 81 individuals/100 km2 and 7.8 individuals/100 km2, respectively (Table 2).
Table 2 Density and associated data according of the best model (i. e, (pp) for L. wiedii and L. pardalis in Sierra Norte, southwestern Mexico.

a Individuals/100 km2
b Mean maximum distance traveled by an individual captured in two or more occasions.
Of these 16 individuals of L. wiedii identified, four were females, four males and eight of indeterminate sex. We recorded two different cubs following the female closely. In the first case (27 January 2015; 05:29), the body size of the cub was about one-third of the body size of the female. In the second (2 February 2015; 05:57), the body size of the cub was similar to that of the female, and perhaps it was one year old. In the case of L. pardalis, two were females, two males and one of indeterminate sex. A female with its cub was recorded once (11 July 2014, 23:40 h). The cub followed the female closely, and its body size was about half the body size of the female, so it likely was less than one year old. Fifty six percent of individuals of L. wiedii and 40 % of individuals of L. pardalis were recorded for a month in the area. One female of L. wiedii had the longest residence time (12 months) and the mean residence time was 4.4 ± 1.4 months. One male of L. pardalis remained in the zone for 13 months and the mean residence time was 5.17 ± 2.3 months.
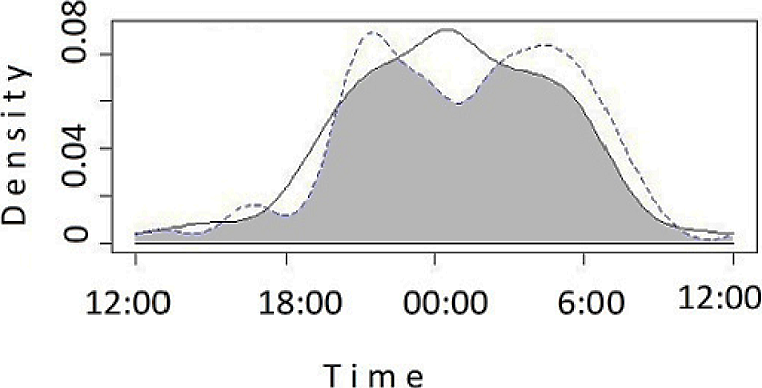
Both species were mostly nocturnal (ca 82 % of the records; n = 68 for L. pardalis, n = 141for L. wiedii). The period of peak activity of L. wiedii occurred around midnight (00:00 h), while L. pardalis was most active from 03:00 to 05:00 and from 19:00 to 21:00 (Figure 2). Since L. pardalis had fewer records in the cloud forest (n = 2), we assessed the overlap between species only in the semi-evergreen forest (n = 20 for L. wiedii; n = 66 for L. pardalis). The overlap coefficient was 0.75 (95 % Confidence interval CI = 0.63-0.90). Additionally, we assessed the activity overlapping of L. wiedii between cloud forest and semi-evergreen forest, obtaining a high overlap coefficient (Δ1 = 0.77, CI = 0.63-0.90).
Discussion
The density of L. pardalis was low (7.8 individuals/100 km2) at Sierra Norte relative to Neotropical regions of Central and South America (3 to 160 individuals/100 km2; Di Bitetti et al. 2008). Density studies for L. wiedii were scarce, but the estimated density and number of recorded individuals in our study area were higher (81 individuals/100 km2) compared to other regions where density has been estimated by capture-recapture with camera trap data, such as Sierra Nan-chititla (12 individuals/100 km2; López-Hernández 2010) or Los Chimalapas (68 individuals/100 km2; Pérez-Irineo and Santos-Moreno 2016) in the central and southeastern Mexico, respectively.
Several factors may affect the density and activity pattern in felids, such as predators, prey availability, interactions between species, human presence, or quality and type of habitat (Di Bitetti et al. 2010; de Oliveira et al. 2010). In our study area, the presence of large predators may not be a key factor affecting Leopardus density, since the record rate of larger predators (/. e., P. onca and P. concolor) was similar in both vegetation types. Some studies have indicated that large differences in body size minimize the risk of negative interaction between felids (Donadio and Buskirk 2006; Ritchie and Johnson 2009), and other studies did not record evidence of spatial avoidance between L. wiedii or L. pardal/s and larger predators (Hodge 2014). Further studies are required to determine whether the influence of larger felids is similar in the habitat of L. wiedii and L. pardalis.
Most L. pardalis records were obtained in the semi-evergreen forest, and L. wiedii was recorded more frequently in the cloud forests. There are few studies in cloud forests for felids; however, the record rate of L. wiedii was higher compared to L. pardalis in mountain tropical forests or cloud forests (Hodge and Arbogast 2016; Vanderhoff et al. 2011). Leopardus wiedii inhabits tropical forests, but is also reported in premontane moist forests and cloud forests, and is more strongly associated with dense forests than any other Neotropical felid (Wilson and Mittermeier 2009). In contrast, L. pardalis mainly inhabits lowland tropical forests under 2,000 m, including moist and dry forest, swampy savanna and dense thorny chaparral, but is rare in temperate forest (Sunquist and Sunquist 2002; Wilson and Mittermeier 2009). The records of both species may reflect the suitable vegetation types according to their habitat preferences. We also recorded that medium-sized prey of L. pardalis had a higher record rate in the semi-evergreen forest, such as Cuniculus paca (paca), Dasyprocta mexicana (agouti), and Dasypus novemcinctus (armadillo). Meanwhile, more small-sized prey (e. g, mice and small birds), common in the diet of L. wiedii, were most abundant in the cloud forest compared to the semi-evergreen forest in Sierra Norte.
Furthermore, the low density of L. pardalis may allow L. wiedii to attain a higher density in Sierra Norte. This is consistent with observations in other regions: the abundance of L. wiedii was higher in regions with absence or low density of ocelots, likely as a result of low interspecific competition (Carvajal-Villarreal et al. 2012; Kasper et al. 2016; Vanderhoff et al. 2011). Other medium-sized felids also showed a similar density pattern in sites with low density of ocelots, such as L. geoffroyi in central Argentina and Bolivia (Geoffroy's cat; Caruso et al. 2012; Cuellar et al. 2006), and L. tigrinus in the Brazilian Atlantic forest (Oncilla; Oliveira-Santos et al. 2012).
Leopardus pardalis and L. wiedii are primarily nocturnal, and we recorded that the activity of both species was consistent with previous observations in other regions (Perez-Irineo and Santos-Moreno 2016; Vanderhoff et al. 2011). Our results suggest that both species may coexist in the absence of pronounced temporal partitioning in semi-evergreen forests. The activity pattern of L. wiedii was similar in both semi-evergreen and cloud forests, seemingly unaffected by the presence of L. pardalis. In contrast, other studies indicate that small felids show a different activity pattern in sites where large felids are more abundant relative to sites with lower abundance of large felids (Oliveira-Santos et al. 2012).
Separately, the population of both species was breeding and a small part consisted of resident individuals. Transient individuals may use the site as a corridor, as suggested for other regions, with individuals moving from less favorable to more favorable patches (Pérez-Irineo and Santos-Moreno 2016; Vanderhoff et al. 2011).
Factors including differences in vegetation type and presence of a large number of transient individuals may influence the density of Leopardus in Sierra Norte. In addition, dense vegetation cover and low anthropogenic disturbance of the cloud forest, coupled with the lower abundance of L. pardalis, contributed to the higher density of L. wiedii. Both species have lost some of their original distribution range and are cataloged as endangered in Mexico (SEMARNAT 2010), but internationally L. wiedii is listed by the IUCN as near threatened (De Oliveira et al. 2015). We provided information about the density and activity of medium-sized felids, as well as on the factors that potentially affect these patterns in tropical mountain forest environments.











 text new page (beta)
text new page (beta)