Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.15 n.3 Texcoco Apr./May. 2024 Epub Aug 25, 2024
https://doi.org/10.29312/remexca.v15i3.3676
Essays
Methods of rearing and reproduction predatory mites of the order Mesostigmata
1Laboratorio de Entomología ‘Biol. Sócrates Cisneros Paz’-Facultad de Biología-Universidad Michoacana de San Nicolás de Hidalgo. Edificio B4, Ciudad Universitaria, Francisco J. Múgica S/N, Col. Felicitas del Río, Morelia, Michoacán. CP. 58030. (0802133k@umich.mx; 1719791h@umich.mx).
2Unidad Académica Multidisciplinaria Mante-Universidad Autónoma de Tamaulipas. E. Cárdenas González No. 1201 Pte, Jardín, Ciudad Mante, Tamaulipas. CP. 89840. (mapatcg@gmail.com).
3Laboratorio de Fitopatología-Facultad de Agrobiología-Universidad Michoacana de San Nicolás de Hidalgo. Paseo Lázaro Cárdenas 2290, Emiliano Zapata, Melchor Ocampo, Uruapan, Michoacán. CP. 60170. (blanca.lara@umich.mx).
Due to the irrational use of chemicals for pest control in intensive crops, the diversity of communities of beneficial organisms, such as the predatory mites of the order Mesostigmata, has been alarmingly diminished,. These arachnids have taken on agricultural relevance because most of their members have zoophagic feeding habits and naturally regulate harmful populations of insects, nematodes and other mites that inhabit plants. For more than 40 years, predatory mites have been introduced regularly for biocontrol work and it is estimated that more than 60% of programs use these organisms; however, the commercial products available are limited to a few species, which vary in effectiveness according to the conditions of the crop, availability of prey and competition for niches with other predatory organisms; in addition, their production is protected under trade secrecy in most cases. With this, the opportunity opens up to explore native or even already commercialized species in order to evaluate their predatory capacity at the research level or as usual tools for pest regulation; a first challenge for this purpose is propagation and culture methods. The methods for mite culture can be divided into two: maintenance on plants and in confinement; although each technique has been developed for a particular species or a certain purpose, knowing their basic design promises to bring us closer to a new line of study for their introduction and reintroduction into agricultural systems.
Keywords: agricultural pests; biological control; Blattisociidae; Laelapidae; Phytoseiidae; natural enemies
Debido al uso irracional de productos químicos para el control de plagas en los cultivos intensivos, se han disminuido de manera alarmante la diversidad de comunidades de organismos benéficos, tal es el caso de los ácaros depredadores del orden Mesostigmata. Estos arácnidos han tomado relevancia agrícola debido a que, la mayoría de sus miembros presentan hábitos alimentarios zoofágicos y regulan de manera natural poblaciones perjudiciales de insectos, nematodos y otros ácaros que habitan las plantas. De manera regular, desde hace más de 40 años se introducen los ácaros depredadores para los trabajos de biocontrol y se estima que más de 60% de los programas utilizan estos organismos; sin embargo, los productos comerciales disponibles están limitados a unas pocas especies, las cuales varían en efectividad según las condiciones del cultivo, disponibilidad de presas y competencia de nichos con otros organismos también depredadores, además, su producción está resguardada bajo secreto empresarial en la mayoría de los casos. Con esto, se abre la oportunidad para explorar especies nativas o incluso ya comercializadas, con el fin de evaluar su capacidad depredadora a nivel de investigación o como herramientas habituales para la regulación de plagas, un primer reto para este fin es la propagación y los métodos de cultivo. Los métodos para cultivar ácaros se pueden dividir en dos: mantenimiento sobre plantas y en confinamiento, aunque cada técnica se ha desarrollado para una especie en particular o un fin determinado, el conocer su diseño básico, promete acercarnos a una nueva línea de estudio para su introducción y reintroducción en los sistemas agrícolas.
Palabras clave: Blattisociidae; control biológico; enemigos naturales; Laelapidae; Phytoseiidae; plagas agrícolas
Since their appearance in biological control more than 40 years ago, predatory mites have been considered highly prized natural enemies in agriculture (Knapp et al., 2018). In the commercialization of biological control agents, predatory mites are the second most important, with 13.1% of the species on the market; nevertheless, their releases into agricultural systems exceed 60% (Knapp et al., 2013; 2018). Nonetheless, given the economic value of these organisms, the protocols for establishing massive rearing are limited by companies and protected under patent (Moreira and de Moraes, 2015; Kumar et al., 2015).
This manuscript concentrates general research data on the establishment of rearing of predatory mites of the order Mesostigmata, which have been studied with the intention of being introduced into agricultural systems to regulate populations of harmful invertebrates and arthropods.
The order Mesostigmata in agriculture
Most of the species that make up this order are zoophagous and some have already been extensively studied for the control of harmful organisms with both phytophagous and edaphic habits (Chaires-Grijalva, 2012; Moreira and de Moraes, 2015) (Figure 1).

Figure 1 Microhabitats of Mesostigmata mites and their potential in pest regulation. A) thrips; B) whitefly; C) phytophagous mites; D) nematodes; E) immature thrips; F) oribatid mites.
They are generally small to medium in size, ranging from 0.2 to 5 mm in length. Their life cycle includes five stages: egg, larva, protonymph, deutonymph, and adult; it has been discovered that, in certain species, not all developmental instars are obligate predators and may require another food source such as pollen or mycelium (Chaires-Grijalva, 2012; Walter and Proctor, 2013; Chaires-Grijalva et al., 2016).
About 12 000 species have been described in this taxon (Lindquist et al., 2009) and about a quarter are made up of the family Phytoseiidae; important biological control agents that inhabit vegetation (Moreno and Mancebón, 2011; Lam et al., 2019) (Figure 1). On the other hand, most of the members of this order are edaphic and are reported as predators of various organisms that make up the mesofauna, such as other mites, nematodes (Figure 1D), springtails, and small soil insects including thrips prepupae and pupae (Figure 1E) (Castro-López, 2018).
The families Laelapidae, Blattisociidae, Ascidae and Parasitidae stand out (Messelink and van Holstein-Saj, 2008; Navarro-Campos et al., 2012; Castilho et al., 2015; Moreira and de Moraes, 2015), which are currently being studied for their predatory potential in different soil pest organisms.
Family Phytoseiidae
Phytoseiid predators are considered one of the most important groups of natural enemies used in biological control worldwide (Schoeller et al., 2020) as they can regulate the populations of different species of pest arthropods such as: thrips (Thysanoptera: Thripidae) (Figure 1A), whiteflies (Homoptera: Aleyrodidae) (Figure 1B), and other phytophagous mites of the family Tetranychidae (Figure 1C) (Knapp et al., 2013; 2018; Schoeller et al., 2020).
Due to their agricultural relevance, harvests and determinations are intensified worldwide; up to 2021, 2 550 species were identified and the number is expected to increase (Demite et al., 2023). They can feed on pollen, which allows them to establish themselves in crops when the prey is at low densities; they can occasionally be found on the soil (epigeal habit), feeding on arthropods such as the immature stages of thrips (Lindquist, 2009; Walter and Proctor, 2013). They have been documented to tolerate a wide range of climatic conditions and their establishment is successful in most crops (Knapp et al., 2018).
Predatory mites with edaphic habits
Despite the high rates of predation and the interesting prospect of being introduced into biological control programs, edaphic Mesostigmata have been scarcely studied (Walter and Proctor, 2013). They usually consume mites that feed on decaying organic matter, bacteria, and mycelium, such as oribatids (Oribatida) and phytophages, which can damage the parenchyma of plant tissues (Prostigmata and Astigmata) (Figure 1F) (Walter and Proctor, 2013).
In contrast to its lack of exploration, there is the family Laelapidae, where some members of the genus Stratiolaelaps (Berlese, 1916) are marketed by at least eleven companies in the Americas and Europe (Moreira and de Moraes, 2015) for the control of fungus gnats species (Bradysia spp.; Diptera: Sciaridae), prepupae and pupae of thrips, such as Frankliniella occidentalis (Pergande) (Figure 1E), and shore fly [Scatella stagnalis (Fallen); Diptera: Ephydridae], among other pests that inhabit the soil or its surface (Lam et al., 2019).
Studies in Europe and Brazil have shown that this family has a predation capacity between 50 and 70% of the edaphic phases of thrips under laboratory conditions (Castro-López, 2018). Although the behavior and potential of predatory families such as Blattisociidae and Rhodacaridae for pest nematode control in Mexico has already been explored, information is still limited (García-Ortíz et al., 2015).
They can exhibit epigeal behavior when they are prepared for the phoresis mechanism; that is, they have the ability to attach themselves to the body of other animals, usually insects, birds, or mammals, without being direct parasites of them, they use hosts as a means of transport to move from one place to another and look for new food resources or habitats (Lindquist, 2009; Chaires-Grijalva et al., 2016).
Predatory mite rearing
The reproduction of predatory mites has been complicated due to the requirements of these organisms (Rodríguez et al., 2013). It is estimated that the average duration of a generation of Mesostigmata is usually 8 to 10 days at 22-24 °C (McMurtry and Croft, 1997). However, successful establishment and development time depend on temperature, relative humidity, prey species, and support substrate (Rodríguez-Aragón et al., 2018).
There are two basic techniques for rearing predatory mites. The first refers to the rearing on host plants, where they are offered as a primary source of food the prey with which they generally share their habitat (Bustos-Rodríguez, 2013; Rodríguez et al., 2013; Rodríguez-Aragón et al., 2018; Bulnes and Orozco, 2020) (Figure 2).

Figure 2 Process for rearing phytoseiids on plants. Diagram based on the rearing methodology for P. persimilis (Rodríguez-Aragón et al., 2018).
The second most referenced technique for rearing is carried out in a confined manner, where mites are restricted to small areas within containers with natural or artificial substrates and varied prey (Rodríguez et al., 2013; Ballal et al., 2021).
Methods of reproduction on plants
When phytoseiids are chosen for reproduction, they are generally kept on vegetation, such are the cases of the species Phytoseiulus persimilis (Athias-Henriot) and Amblyseius swirskii Athias-Henriot (Rodríguez-Aragón et al., 2018; Bulnes and Orozco, 2020). Rearing predators with this technique includes three basic stages: 1) obtaining plant material; 2) reproduction of the prey mite; and 3) obtaining the mite Phytoseiidae (Rondon et al., 2005), the phytoseiid can have a commercial origin or captured from the field (naturalized predator).
Once the predatory mite has become established, it is captured for storage and then released again into another crop; the conventional way of storing is in transparent bottles with vermiculite and food for later release (in approximately 1 to 3 days), so as not to diminish the effectiveness of predation (Rodríguez-Aragón et al., 2018) (Figure 2).
The most suitable host plants for the sustenance of the rearing of any species of predatory mites are those whose leaves remain turgid for a relatively long time, the most recommended are the following: beans [Phaseolus vulgaris L. (Fabaceae)], castor oil plant [Ricinus communis L. (Euphorbiaceae)], pepper [Capsicum annuum L. var. Annum (Solanaceae)] and citrus [Citrus spp. (Rutaceae)] (Bustos-Rodríguez, 2013; Rodríguez et al., 2013; Kumar et al., 2015; Rodríguez-Aragón et al., 2018; Bulnes and Orozco, 2020; Ballal et al., 2021).
This method is low-cost, and once optimized, provides large populations of the predator; nevertheless, it requires a lot of work during the rearing process (Rodríguez-Aragón et al, 2018; Bulnes and Orozco, 2020) (Figure 2). Although this technique is ideal for mass or commercial rearing, it is not exempt from contamination; therefore, monitoring is added as a fundamental labor force (Bustos-Rodríguez, 2013; Rodríguez-Aragón et al., 2018; Bulnes and Orozco, 2020).
Recurring prey includes: phytophagous mites, thrips, and lepidopteran larvae. The red spider mite or two-spotted spider (Acari:Tetranychidae) is the most commonly referenced for phytoseiid rearing, but it is not the only food source, this family includes abundant species of generalist predators and some omnivores (McMurtry and Croft, 1997; Moreno and Mancebón, 2011).
Methods of rearing in confinement
To define which predatory mite is appropriate to be introduced into a crop or to start a rearing, knowing general aspects about its biology and that of its prey is essential, it is in this context that in vitro culture has been key to discovering the predatory activity of edaphic species and their potential for the biological control of soil pests. Some studies that have been carried out on these mites have arisen from fortuitous invasions in experimental laboratory units.
For example, the mite Blattisocius tarsalis (Berlese) of the family Blattisociidae was found while feeding on eggs of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) (Gavara et al., 2022) and was subsequently evaluated for the control of the potato moth Tecia solanivora Povolný (Lepidoptera: Gelechiidae), the results were favorable with a decrease of up to 98% of the eggs of this insect.
Another case is that of Lasioseius penicilliger (Berlese), this mite, which is also a member of the family Blattisociidae, was isolated from a soil sample along with root-knot nematodes of the genus Meloidogyne (Tylenchida: Meloidogynidae) (Ortíz et al., 2015). This predator demonstrated strong results in decreasing nematode colonies by 80% and 95%.
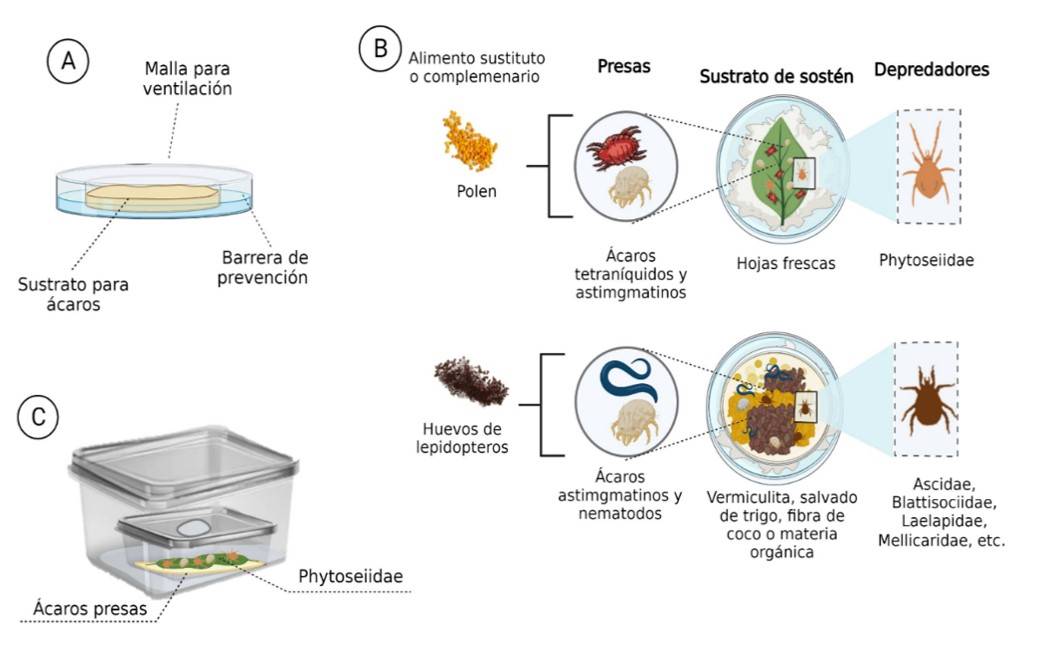
Confinement systems provide a great advantage for the studying of the predatory capacity of mesostigmatid mites. Colonies are kept in Petri dishes with three essential conditions; 1) space with substrate for the maintenance and reproduction of the mite and its prey or food; 2) isolation barrier, it can be water, glycerol, or damp cotton to prevent leakage or contamination; and 3) ventilation to promote aeration and reduce fungal contamination due to excess humidity (Figure 3A).
Predatory mite rearing can be kept on artificial or natural substrates (Ballal et al., 2021). Some methodologies incorporate combinations of different materials, such as vermiculite, wheat bran, coconut fiber, and perlite, all media must be inert; that is, disinfected prior to the release of predators or their prey (Bustos-Rodríguez, 2013; Rodríguez et al., 2013; Ballal et al., 2021) (Figure 3B).
These small units are also suitable for the maintenance of species of the family Phytoseiidae and have been used to reduce costs. Young and fresh leaves of one of the aforementioned host plants can be incorporated into the substrate.
The massive rearing of phytoseiids in confinement have controlled conditions in bioclimatic chambers, the small areas, generally inside containers, with dimensions close to 40 cm long by 10 cm high and the incorporation of water barriers serve to maintain high relative humidity, an ideal condition to increase the population of Phytoseiidae (Rodríguez, 2013; Ballal et al., 2021), as in the case of the methodology for the reproduction of the native predator Neoseiulus indicus (Narayanan and Kaur) from containers, inside containers, designed by Ballal et al. (2021) (Figure 3C).
Although these techniques are practical, they do not exempt the risk of disease or external contamination as the leaves are difficult to change once decomposed. In phytoseiids, in addition to T. urticae, soil mites such as Carpoglyphus lactis (Acari: Astigmatina) (Ballal et al., 2021), used for the rearing of the phytoseiid Neoseiulus californicus (Mcgregor), are used.
To sustain soil predators, edaphic organisms are mainly used, such as: Tyrophagus putrescentiae (Schrank) (Acaridae), used as a frozen and fresh food source for the predator Blattisocius mali (Oudemans) (Blattisociidae) (Pirayeshfar et al., 2022; Asgari et al., 2022), and predators of the families Laelapidae and Rhodacaridae (Barbosa and de Moraes, 2016). Soil nematodes are a recurrent food in the rearing of soil predators (García-Ortíz et al., 2015). Other less conventional prey, but with a positive impact on phytoseiid and edaphic mesostigmatid populations, are the eggs of E. kuehniella (Bulnes and Orozco, 2020; Gavara et al., 2022).
This food can be considered as a substitute or complementary food resource since in order to give better results, they must be in combination with prey, and pollen should also be used (Bulnes and Orozco, 2020; Gavara et al., 2022). Pollen-enriched diets have yielded good results in commercial and research populations (Bustos-Rodríguez, 2013; Rodríguez-Aragón et al., 2018; Bulnes and Orozco, 2020; Pirayeshfar et al., 2022), as in the predators A. swirskii, Neoseiulus cucumeris Oudeman, and B. mali (Kumar et al., 2015; Bulnes and Orozco, 2020; Gavara et al., 2022).
The most recommended are pollen from corn and Typha spp. (Typhaceae) (Bustos-Rodríguez, 2013; Bulnes and Orozco, 2020) in the case of phytoseiids; however, for Blattisociidae, olive pollen has also been used (Pirayeshfar et al., 2022) (Figure 3B). It is recommended to experiment with the effect of supplements (Rodríguez-Aragón et al., 2018; Bulnes and Orozco, 2020) in order to optimize rearing in captivity. For example, using commercial wildflower pollen or the inclusion of Artemia spp. cysts promise improvements in cost, rates of oviposition and fecundity, as has been observed in the rearing of A. swirskii (Vangansbeke et al., 2014; Riahi et al., 2017).
On the other hand, mites require shelters and places to oviposit; for this, coverslips can be used with cotton strands arranged like Tetranychidae’s web and that resemble their areas of natural oviposition, small roof-shaped pieces built with sheets of transparent acetates or square pieces of black felt can be arranged (Bustos-Rodríguez, 2013; Rodríguez et al., 2013). Other important recommendations are: 1) avoid constant handling of containers with colonies; and 2) maintain dark conditions (García-Ortiz et al., 2015; Ballal et al., 2021); nevertheless, this last requirement will vary depending on the predator’s habits.
Conclusions
Although most protocols for reproducing predatory mites are kept under trade secrets, the techniques documented can be grouped into two main strategies, on the plant and in confinement, the selection of an ideal methodology must consider costs, available infrastructure, prospects for the cultivation of predators, and research objectives. Due to the diversity of crops and the increase in their pests, the production of any natural enemy is a challenge at all scales.
With general knowledge about rearing methods for mesostigmatid predatory mites, research into the biology and ecology of species with agricultural potential is favored and it can provide timely information for the containment of harmful organisms. The mites of the order Mesostigmata that have been mainly used are those of the family Phytoseiidae; however, species with edaphic behavior demonstrate an enormous capacity for the regulation of populations of soil and foliage pest organisms, where they could fit successfully into biological control programs.
Bibliografía
Asgari, F.; Safavi, S. A. and Moayeri, H. R. S. 2022. Life table parameters of the predatory mite, Blattisocius mali Oudemans (Mesostigmata: blattisociidae), fed on eggs and larvae of the stored product mite, Tyrophagus putrescentiae (Schrank). Egyptian Journal of Biological Pest Control. 32(1):118-127. Doi.org/10.1186/s41938-022-00616-5. [ Links ]
Ballal, C. R.; Gupta, S. K.; Gupta, T. and Varshney, R. 2021. A simple protocol for rearing a native predatory mite Neoseiulus indicus. Current Science. 120(12):1923-1926. 10.18520/cs/v120/i12/1923-1926. [ Links ]
Barbosa, M. F. and Moraes, G. J. 2016. Potential of astigmatid mites (Acari: astigmatina) as prey for rearing edaphic predatory mites of the families Laelapidae and Rhodacaridae (Acari: mesostigmata). Experimental and Applied Acarology. 69(3):289-296. Doi: 10.1007/s10493-016-0043-4. [ Links ]
Bulnes, D. R. y Orozco, J. 2020. Producción masiva del ácaro depredador Amblyseius swirskii (Athias-Henriot) (Acari, Phytoseiidae) y su aplicación en campo: revisión de literatura. Escuela Agrícola Panamericana, Zamorano Honduras. Tesis de Licenciatura. https://bdigital.zamorano.edu/items/ee85783f-94d3-4ff7-b0b0-66de84671989. [ Links ]
Bustos-Rodríguez, H. A. 2013. Desarrollo de un modelo de simulación de una cría masiva del ácaro depredador Phytoseiulus persimilis (Parasitiformes: phytoseiidae). Universidad Militar Nueva Granada. 9-20.http://hdl.handle.net/10654/12444 [ Links ]
Castilho, R. C.; Venancio, R. and Narita J. P. Z. 2015. Mesostigmata as biological control agents, with emphasis on Rhodacaroidea and Parasitoidea. Ed. Prospects for biological control of plant feeding mites and other harmful organisms. 19(1):1-31. 10.1007/978-3-319-15042-0-1. [ Links ]
Castro-López, M. A. 2018. Ácaros Mesostigmata como potenciales controladores de Thrips tabaci Lindeman en el cultivo de cebolla Allium cepa L. Universidad Nacional de Colombia, Facultad de Agronomía. Tesis de maestría. 35-67 pp. https://repositorio.unal.edu.co/handle/unal/63820. [ Links ]
Chaires-Grijalva, M. P. 2012. Gamásidos (Acari: Mesostigmata). Ed. Ácaros de importancia en el suelo. Colegio de Postgraduados. Montecillo, Estado de México. 110-126 pp. ISBN: 978-607-715-059-6. [ Links ]
Chaires-Grijalva, M. P.; Estrada-Venegas, E. G.; Equihua-Martínez, A.; Moser, J. C. and Blomquist, S. R. 2016. Trophic habits of Mesostigmatid mites associated with bark beetles in Mexico. Journal of the Acarological Society of Japan. 25(1):161-S167. 10.2300/acari.25.Suppl-161. [ Links ]
Demite, P. R.; Moraes, G. J.; McMurtry, J. A.; Denmark, H. A. and Castilho, R. C. 2023. Phytoseiidae Database. http://www.lea.esalq.usp.br/phytoseiidae/. [ Links ]
García-Ortiz, N.; Marcelino, L. A.; Gives, P. M.; Arellano, M. E. L.; Garfias, C. R. B. y Garduño, R. G. 2015. Actividad depredadora in vitro de Lasioseius penicilliger (Arachnida: Mesostigmata) contra tres especies de nemátodos: Teladorsagia circumcincta, Meloidogyne sp. y Caenorhabditis elegans. Veterinaria México. 2(1):1-9. https://www.scielo.org.mx/scielo.php?pid=S244867602015000100003&script=sci-abstract . [ Links ]
Gavara, J.; Cabello, T.; Gallego, J. R.; Hernández-Suarez, E. and Piedra-Buena, D. A. 2022. Evaluation of the egg predator Blattisocius tarsalis (Mesostigmata: Blattisociidae) for the biological control of the potato tuber moth Tecia solanivora under storage conditions. Agriculture. 12(7):1-14. https://doi.org/10.3390/ agriculture12070920. [ Links ]
Knapp, M.; Van-Houten, Y.; Hoogerbrugge, H. and Bolckmans, K. 2013. Amblydromalus limonicus (Acari: Phytoseiidae) as a biocontrol agent: literature review and new findings. Acarologia. 53(2):191-202. https://doi.org/10.1051/acarologia/20132088. [ Links ]
Knapp, M.; Van-Houten, Y.; Van-Baal, E. and Groot, T. 2018. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarology. 58(1):72-82. https://doi.org/10.24349/acarologia/20184275. [ Links ]
Kumar, V.; Xiao, Y.; McKenzie, C. L. and Osborne L. S. 2015. Early establishment of the Phytoseiid mite Amblyseius swirskii (Acari: Phytoseiidae) on pepper seedlings in a predator-in-first approach. Experimental and Applied Acarology . 65(4):465-481 https://doi.org/10.1007/s10493-015-9895-2. [ Links ]
Lam, W.; Paynter, Q. and Zhang, Z. Q. 2019. Predation, prey preference and reproduction of predatory mites Amblydromalus limonicus (Garman), Amblyseius herbicolus (Chant) and Neoseiulus cucumeris (Oudemans) (Mesostigmata: phytoseiidae) on immature Scricothrips staphylinus Haliday (Thysanoptera: thripidae), a biocontrol agent of gorse. Experimental and Applied Acarology. 24(3):508-519. https://doi.org/10.11158/saa.24.3.14. [ Links ]
Lindquist, E. E.; Krantz, G. W. and Walter, D. E. 2009. Order Mesostigmata. Ed. A manual of acarology, third edition. Texas Tech University Press, Lubbock. 124-232 pp. [ Links ]
McMurtry, J. A. and Croft, B. A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Annual Review of Entomology. 42(1):291-321. [ Links ]
Messelink, G. and Holstein-saj, R. 2008. Improving thrips control by the soil dwelling predatory mite Macrocheles robustulus (Berlese). IOBC/WPRS Bull. 32(1):135-138. [ Links ]
Moreira, G. F. and de Moraes, G. J. 2015. The potential of free living laelapid mites (Mesostigmata: Laelapidae) as biological control agents. Ed. Prospects for biological control of plant feeding mites and other harmful organisms . 19(1):77-102. https://doi.org/10.1007/978-3-319-15042-0-3. [ Links ]
Moreno, I. P. y Mancebón, V. S. M. 2011. Importancia y uso de los ácaros fitoseidos (Acari, Phytoseiidae) en el manejo agroecológico de plagas. Manejo agroecológico de sistemas. Benemérita Universidad Autónoma de Puebla (BUAP). México. 69-92 pp. https://www.researchgate.net/publication/299657957-Importancia-y-uso-de-los-acaros-fitoseidos-Acari-Phytoseiidae-en-el-Manejo-Agroecologico-de-Plagas. [ Links ]
Navarro-Campos, C.; Pekas, A.; Moraza, M. L.; Aguilar, A. and García-Marí, F. 2012. Soil-dwelling predatory mites in citrus: Their potential as natural enemies of thrips with special reference to Pezothrips kellyanus (Thysanoptera: thripidae). Biological Control. 63(2):201-209. https://doi.org/10.1016/j.biocontrol.2012.07.007. [ Links ]
Ortiz, N. G.; Marcelino, L. A.; Gives, P. M.; Arellano, M. E. L.; Garfias, C. R. B. y Garduño R. G. 2015. Actividad depredadora in vitro de Lasioseius penicilliger (Arachnida: Mesostigmata) contra tres especies de nemátodos: Teladorsagia circumcincta, Meloidogyne sp. y Caenorhabditis elegans. Veterinaria México . 2(1):1-9. http://dx.doi.org/10.21753/vmoa.2.1.340. [ Links ]
Pirayeshfar, F.; Moayeri, H. R. S.; Silva, G. L. and Ueckermann, E. A. 2022. Comparison of biological characteristics of the predatory mite Blattisocius mali (Acari: Blattisocidae) reared on frozen eggs of Tyrophagus putrescentiae (Acari: Acaridae) alone and in combination with cattail and olive pollens. Systematic and applied acarology. 27(3):399-409. 10.1186/s41938-022-00616-5. [ Links ]
Riahi, E.; Fathipour, Y.; Talebi, A. A. and Mehrabadi M. 2017. Natural diets versus factitious prey: comparative effects on development, fecundity and life table of Amblyseius swirskii (Acari: phytoseiidae). Systematic and Applied Acarology . 22(5):711-723. https://doi.org/10.11158/saa.22.5.10. [ Links ]
Rodríguez-Aragón, S. M.; Vargas, Y. A. M.; Sabogal, A. E. y Rodríguez, M. L. 2018. Investigación, desarrollo y registro de enemigos naturales para control biológico. Caso: Phytoseiulus persimilis. 2(1):716-741. http://hdl.handle.net/ 20.500.12324/34079. [ Links ]
Rodríguez, H.; Montoya, A. Y.; Pérez, M. y Ramos, M. 2013. Reproducción masiva de ácaros depredadores Phytoseiidae: retos y perspectivas para Cuba. Revista de Protección Vegetal. 28(1):12-22. http://scielo.sld.cu/scielo.php?script=sci-arttext&pid=S1010-27522013000100002. [ Links ]
Schoeller, E. N.; McKenzie, C. L. and Osborne, L.S. 2020. Comparison of the Phytoseiid mites Amblyseius swirskii and Amblydromalus limonicus for biological control chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae). Exp. Appl. Acarol. 82(3):309-318. [ Links ]
Vangansbeke, D.; Nguyen, D. T.; Audenaert, J.; Verhoeven, R.; Deforce, K.; Gobin, B.; Tirry, L. and Clercq, P. 2014. Diet-dependent cannibalism in the omnivorous Phytoseiid mite Amblydromalus limonicus. Biological control. 74(1):30-35. https://doi.org/10.1016/j.biocontrol.2014.03.015. [ Links ]
Walter, D. E. and Proctor, H. C. 2013b. Mites in soil and litter systems. In: mites: ecology, evolution and Behaviour. Springer, Dordrecht. 161-212 pp. https://doi.org/10.1007/978-94-007-7164-2-8 . [ Links ]
Received: March 01, 2024; Accepted: May 01, 2024











 text in
text in