Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.15 n.2 Texcoco Feb./Mar. 2024 Epub June 21, 2024
https://doi.org/10.29312/remexca.v15i2.3221
Articles
Low-temperature-resistant sugars in avocado rootstocks
1Colegio de Postgraduados-Campus Monteillo. Carretera México-Texcoco km 36.5, Montecillo, Texcoco, Estado de México, México. CP. 56230.
In Mexico, due to the demand for fruit, orchards of avocado (Persea americana Mill.) variety ‘Hass’ of subtropical climate are erroneously established in cold areas, affecting their production; an alternative is to use cold-tolerant rootstocks of the Mexican race, tolerance related to the increase of sugars in areas of demand, which it shares through grafting with the ‘Hass’ variety; therefore, in 2021, the segregants of the ‘rootstocks’ duke 7, tepetl, aceitoso, and colecta 1 were evaluated at the College of Postgraduates; analyzing glucose, fructose, sucrose and starch contents in vegetative shoots at 1, 7, and 14 days of treatment; in chamber 1 (treatment) with luminosity of 380 μmol m-2 s-1 and temperatures with light of 15.61 °C, and darkness of 4.40 °C; and chamber 2 (control) with luminosity of 367 μmol m-2 s-1 and temperatures with light of 23.2 °C, and darkness of 19.29 °C, considering the factor of cold and low luminosity, it was observed that, chlorophyll in the leaves shows growth without photosynthetic deficiency in plants in both chambers; the glucose content of the ‘Hass’ variety varies according to the glucose content in the rootstock; the fructose content increases in grafted and non-grafted materials, acting as an osmoprotectant, the sucrose content increases in the grafted aceitoso material and the starch content is not affected; as a result, duke 7 and tepetl were the materials with the highest concentration of glucose and fructose under cold conditions.
Keywords: cold tolerant; fructose; glucose; ‘Hass’; segregating
Debido a la demanda de fruta, en México, las huertas de aguacate (Persea americana Mill.) variedad ‘Hass’ de clima subtropical, se establecen erróneamente en zonas frías, afectando su producción; una alternativa es usar portainjertos de la raza mexicana tolerantes al frio, tolerancia relacionada con el incremento de azúcares, en áreas de demanda, que comparte a través del injerto con la variedad ‘Hass’, por lo que, en 2021 se evaluaron en el Colegio de Posgraduados los segregantes de los ‘portainjertos’ duke 7, tepetl, aceitoso y colecta 1, analizando contenido de glucosa, fructosa, sacarosa y almidón en brotes vegetativos, a los 1, 7 y 14 días de tratamiento, en la cámara 1 (tratamiento) con luminosidad de 380 µmol m-2 s-1 y temperaturas con luz de 15.61 °C y oscuridad de 4.4 °C y la cámara 2 (testigo) con luminosidad de 367 µmol m-2 s-1 y temperaturas con luz de 23.2 °C y oscuridad de 19.29 °C, considerando el factor frío y la baja luminosidad, se observó que la clorofila en las hojas, presenta un crecimiento sin deficiencia fotosintética en las plantas de ambas cámaras, el contenido de glucosa de la variedad ‘Hass’ varía de acuerdo al contenido de glucosa en el portainjerto, el contenido de fructosa, aumenta en los materiales injertados y no injertados, actuando como un osmoprotector, el contenido de sacarosa aumenta en el material aceitoso injertado y el contenido de almidón, no es afectado, resultando, duke 7 y tepetl los materiales con mayor concentración de glucosa y fructosa, en condiciones de frío.
Palabras clave: fructosa; glucosa; ‘Hass’; segregante; tolerantes al frío
Introduction
The avocado varieties grown are the result of hybridization between races of the species (Knight, 2002). The ‘Hass’ variety is a hybrid of the Guatemalan race but with genes of the Mexican race and fruits with a large storage and transport capacity that favor its postharvest management characteristics (Crane et al., 2013). In 2020, avocado production in Mexico was 2.39 million tons (PROFECO, 2021). This production of avocado fruit involves a process that begins with the selection of the rootstock, which is responsible for providing the commercial variety with a root support and maintaining its genetic characteristics, without the variability obtained from the variety propagated by seed, since the success or failure of a plantation depends on it (Barrientos-Priego et al., 2000).
Avocado materials from subtropical areas, such as the ‘Hass’ variety, are more susceptible to low temperatures (Crane et al., 2013), so an alternative in cold conditions is the use of rootstocks of the Mexican race as a possible solution to the effects of cold (Lockard and Schneider, 1981). Lacono et al. (1998) mention that, together, the graft and the rootstock form characteristics in the plant, which are the result of the characteristics of each.
Affirming the influence of rootstock on the variety, Mickelbart et al. (2007) found significant differences in nutrient uptake because of different clonal rootstocks. Bergh (1992) mentions that the Mexican race has contributed genes that favor cold tolerance, because there is a concentration of soluble sugars that reduce the freezing point of the intracellular solution (Poirier et al., 2010).
Therefore, the increase or decrease in the concentration of sugars in the plant is an acclimatization response, such as the glucose content in the leaves of vegetative shoots considered tissues of demand, which can change its concentration due to environmental, biochemical, and physiological factors (Rolland et al., 2002), or the increase in fructose content that acts as an osmoprotectant against adverse environmental conditions due to heat, cold, or water stress (Marschall et al., 2019) and thus continue to use sucrose as the main source of energy to continue their biochemical processes (Hopkins and Huner, 2004).
Therefore, in this research, avocado segregants were selected from four materials of the Mexican race (duke-7, tepetl, aceitoso, and colecta 1), grafted and non-grafted with ‘Hass’, to evaluate, under a 14-day cold treatment, the concentration of glucose, fructose, sucrose, and starch as possible sugars involved in tolerance to low temperatures.
Materials and methods
We began with the sowing of seeds in September 2020 and later in February 2021, we performed the side-veneer grafting with a ‘Hass’ scion in the greenhouse area of the ‘La Cruz’ experimental center of the Salvador Sánchez Colin-CICTAMEX, SC Foundation, located in Coatepec Harinas, State of Mexico, at 18° 55’ 10.4” north latitude, 99° 45’ 39.7” west longitude, with an altitude of 2 100 m and we concluded with the transfer of the plant in May 2021 to the cold chambers located at the College of Postgraduates, Montecillo, Texcoco, State of Mexico, at 19° 27’ 36.1” north longitude, 98° 54’ 22.5” west latitude, with an altitude of 2 250 m.
Experimental design
A randomized complete block design was used with an arrangement of divided plots, where large plots represent the factor of temperature (factor A). Chamber 1 was the cold treatment, with an average temperature of 15.61 °C during daylight hours, with a light intensity of 380 μmol m-2 s-1 and an average temperature in darkness of 4.4 °C (data recorded with a datalogger); on the other hand, chamber 2, considered the control, kept an average temperature of 23.2 °C in light, with a light intensity of 367 μmol m-2 s-1 and an average temperature in darkness of 19.29 °C (data recorded with a datalogger). In the small plots (factor B), 20 avocado materials grafted with the ‘Hass’ variety and 20 non-grafted materials and factor C, which includes 10 plants of each material used (aceitoso, colecta 1, tepetl, and duke 7), using a total of 40 rootstocks in each chamber. The results between chambers and the results by material were compared using the LSD test in the SAS (Statistical Analysis System) version 9.4 program.
Plant growth measurement
At the beginning of the experiment, the height of each plant was measured in centimeters with the help of a ruler, marking the base of the stem at the level of the container substrate to ensure a correct second measurement at the end of the experiment.
Sample preparation
The leaves of the vegetative shoots of the plants were cut at 1, 7 and 14 days, considering that the perception of cold begins on day 1, and a possible start of acclimatization at 7 and 14 days; they were stored in aluminum sachets at -20 °C, the fresh weight (between 0.1 g and 0.13 g) was recorded with a Scientech, SA 120 electronic analytical balance. Samples were ground directly in 1.5 ml Eppendorf tubes with 500 μl of 80% ethanol, then centrifuged at 10 000 rpm for 10 min in a Dlab D3024 24-tube Eppendorf centrifuge, and the supernatant was extracted for placement in water vapor immersion for 1 h at 80 °C.
Glucose, fructose, and sucrose reading
A reaction mixture (Table 1) was prepared, and 200 μl was placed in each well of a reading plate, 2.5 μl of sample was added, and then 10 μl of each enzyme previously dissolved independently in 1.5 ml of reaction mixture (Table 2) was applied; finally, the samples were read with the help of a Thermo Scientific multiskan FC.
Table 1 Reaction mixture for 10 ml.
| Hepes 0.5 M pH 8.0 | KCl | MgCl | ATP | NAD+ | Hexokinase | H2O |
|---|---|---|---|---|---|---|
| 2 ml | 2.5 ml | 150 μl | 8.5 mg | 2.83 mg | 10 μ | 5.4 ml |
Information from the College of Postgraduates (2021).
Starch determination
One milliliter of dimethyl sulfoxide (DMSO) was added to the samples and they were homogenized with a vortex (Analog vortex Mixer) for 30 s, placed in a bain-marie for 30 min at boiling point; they were again homogenized, and the supernatant was extracted to be placed in two 2 ml Eppendorf tubes, placing 100 μl in each, and 450 μl with and without enzyme was added to each tube.
The 100 μl samples with and without enzyme were prepared at 5:00 pm and left overnight at 55 °C, left to cool at room temperature and centrifuged at 7 000 revolutions per minute for 5 min; from the fully identified 2 ml Eppendorf tubes, 10 μl of sample was extracted and placed in the holes (wells) of the plates, to add 200 μl of reaction mixture and proceed to the next process (Table 3).
Table 3 Process for reading starch.
| Enzyme | Amount (µl) | Incubation time (min) | Temperature (°C) | |
|---|---|---|---|---|
| Reading 1 | Hexokinase | 10 | Direct | Room |
| Reading 2 | Dehydrogenase | 10 | 35 | 35 |
| In starch, the standard curve was generated, calibrated to 20µl only for glucose |
Source: information from the College of Postgraduates (2021).
Results and discussion
Plant growth
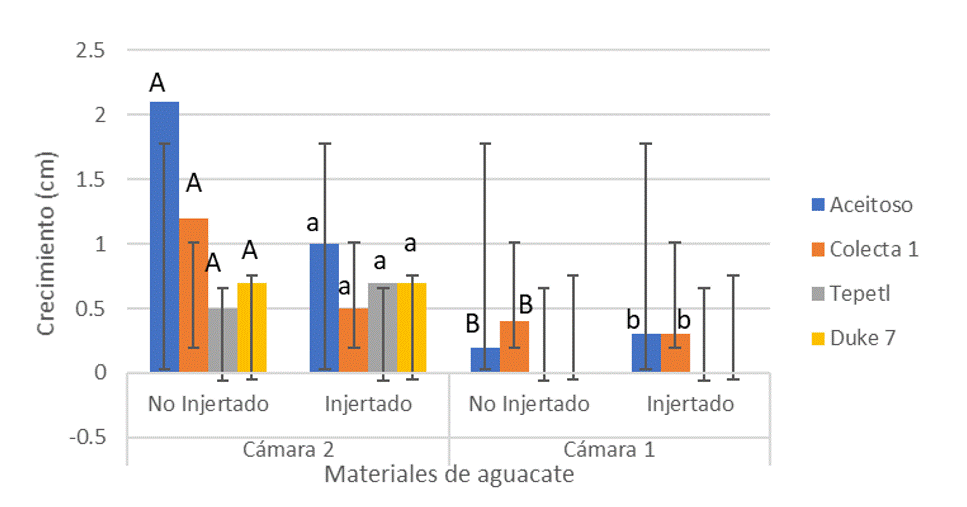
The growth results of the plants in chamber 2 (control) with temperatures with light of 23.20 °C, and darkness of 19.29 °C showed an increase in size at 14 days in the non-grafted and grafted materials; as mentioned by Lahav and Trochoulias (1982), the vegetative development of avocado plants takes place in the optimal temperature range of 20-30 °C, where there is a maximum net assimilation of CO2 favoring the growth of the entire plant.
On the contrary, under the conditions of chamber 1 with temperatures with light of 15.61 °C, and darkness of 4.4 °C, little or no growth was recorded in the non-grafted and grafted materials; therefore, the effect of avocado tree growth and development is significantly reduced at temperatures below 10 °C (Whiley et al., 1990).
According to the comparison of means with a significance of α= 0.05, the growth of the non-grafted and grafted materials of chamber 2 (control), with values of 1.12 cm with a σ= 0.713 cm and 0.72 cm with a σ= 0.206 cm, respectively, were significantly higher than those obtained in the non-grafted and grafted materials of chamber 1 (cold treatment), with values of 0.3 cm in the non-grafted materials with a σ= 0.191 cm, and grafted materials with a σ= 0.173 cm (Figure 1).
Chlorophyll content
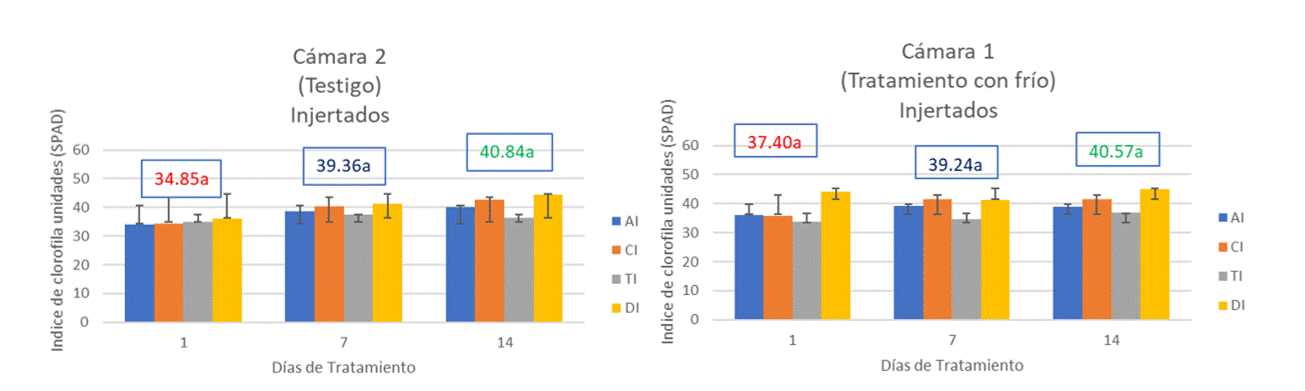
Concerning the chlorophyll indices, (SPAD) units, of the non-grafted materials of chamber 2 (control), they show a general average reading of 40.46 units with a σ= 3.29 units, compared to 39.48 units with a σ= 0.65 units of the non-grafted materials of chamber 1 (cold treatment); according to Coelho et al. (2010), the range of 35.2 and 42.2 SPAD units is suitable for diagnosing a good level of N in potato (Solanum tuberosum).
Likewise, Taiz and Zeiger (2004) mention that the intensity of the green of the leaves is correlated with the chlorophyll content and the concentration of nitrogen; therefore, it can be mentioned that the chlorophyll content in the materials of both chambers is not affected by light intensity or cold treatment. According to the comparison of means with a significance α= 0.05, between the non-grafted materials of both chambers, there is no significant difference (Figure 2).

Figure 2 Reading of SPAD units of non-grafted materials of aceitoso (AN); colecta 1 (CN); tepetl (TN); and duke 7 (DN), comparing the same treatment day number between the two chambers.
The materials established in chamber 1 (cold treatment) did not present alterations in the maturation of the evaluated leaves, coinciding with the results of chamber 2 and with Gianquinto et al. (2003), who mention the positive correlation of the SPAD indices with the availability of nitrogen in the leaves; it can be said that the process of leaf maturation goes through different morphological stages, from their emergence to their senescence, from demanding to being sources of nutrients, as well as their response to environmental variables (Dickson et al., 2000), indicating that the low-temperature factor does not affect nitrogen content at the demand stage.
For the grafted materials of chamber 2 (control), with an average of 38.35 units and a σ= 3.11 units, compared to the grafted materials of chamber 1 (cold treatment), with an average of 39.07 units and a σ= 1.59, it can be mentioned that, according to the comparison of means with a significance of α= 0.05, there are no significant differences in the chlorophyll content between the grafted materials of both chambers (Figure 3).
Sugar content
Glucose
Analyses carried out on leaves of young shoots, which, according to Liu et al. (2002), should be leaves less than 20 days after shooting, show that the concentration of glucose in the leaves of vegetative shoots increases under the conditions of chamber 1 (cold treatment), because glucose is used in the synthesis of compounds such as organic acids, amino acids, and lipids (Geigenberger et al., 2005), building blocks for biomass accumulation and plant development; therefore, the glucose content in the leaves of vegetative shoots as demand tissues is affected by environmental, biochemical, and physiological factors that together determine the amount of photosynthates that can be unloaded into the demand tissues (Rolland et al., 2002).
In accordance with the above, the glucose concentration in conditions of chamber 1 (cold treatment) presents average values of 9.74 μmoles g-1 fresh weight for day 1, 14.87 μmoles g-1 fresh weight on day 7, and 12.64 at 14 days, compared to the control, with average values of 5.02 μmoles g-1 fresh weight for day 1, 8.06 μmoles g-1 fresh weight on day 7, and 7.17 μmoles g-1 fresh weight at 14 days; it can be mentioned that, according to the comparison of means with a significance of α = 0.05, the 3 average values of the cold treatment are significantly higher than those of the control (Figure 4).

Figure 4 Effect of cold treatment on the glucose content in young leaves of materials of aceitoso (AN and AI); colecta 1 (CN and CI); tepetl (TN and TI); and duke 7 (DN and DI), comparing the same treatment day number between both chambers. The number above each set of bars of the rootstocks is the average value across all rootstocks. The comparison of means (LSD) is between chambers on the same day of treatment.
Fructose
The fructose content in the leaves of vegetative shoots is related to the carbon reserve of higher plants (Marschall et al., 2019); these reserves are the fructose content of the materials established in chamber 2 (control); however, under stressful conditions due to cold, these carbohydrates are associated with tolerance of different types of stress, acting as osmoprotectants against adverse environmental conditions due to heat, cold or water stress (Marschall et al., 2019).
With average values of the materials established in chamber 1 (cold treatment) of 7.65 μmoles g-1 fresh weight for day 1, 9.91 μmoles g-1 fresh weight for day 7, and with 12.52 μmoles g-1 fresh weight for the 14 days, compared with chamber 2 (control) values of 3.28 μmoles g-1 fresh weight for day 1, 4.6 μmoles g-1 fresh weight for day 7, and 5.79 μmoles g-1 fresh weight at 14 days; it can be mentioned that, when comparing the means, with a significance of α= 0.05, the plants in chamber 1 (cold treatment) increased their fructose content in the leaves of the vegetative shoots from day 1 of treatment compared to the average values of the chamber 2 (control) (Figure 5).

Figure 5 Effect of cold treatment on the fructose content of young leaves of materials of aceitoso (AN and AI); colecta 1 (CN and CI); tepetl (TN and TI); and duke 7 (DN and DI), comparing the same treatment day number between both chambers. The number above each set of bars of the rootstocks is the average value across all rootstocks.
Sucrose
The transport of photoassimilates to sinks is carried out in the form of sucrose, which is one of the most mobile biochemical compounds, depending on the species of plant and the type of loading and unloading in the phloem (via symplast or apoplast) (Minchin and Lacointe, 2005); therefore, the effect of low temperatures may or may not affect its mobility in the young leaves of the vegetative shoots; sucrose is used by the plant as the main source of biochemical energy, when it is synthesized in the cytosol from phosphorylated glucose and fructose (Hopkins and Huner, 2004), by the symplast route, sucrose travels through ducts (plasmodesmata) and via the apoplast, sucrose can be incorporated by a specific transporter or can be hydrolyzed by a cell wall invertase (Padilla and Martínez, 2007).
According to the characteristics of sucrose and the average values of the materials in chamber 1 (cold treatment) of 0.32 μmoles g-1 fresh weight at day 1, 0.43 μmoles g-1 fresh weight at 7 days, and 0.58 μmoles g-1 fresh weight at 14 days, and when compared with the average values of chamber 2 (control) of 0.15 for day 1, 0.21 μmoles g-1 fresh weight at 7 days and 0.41 μmoles g-1 fresh weight at 14 days; it can be mentioned that, according to the comparison of means with a significance of α= 0.05, the results of days 1 and 7 of the cold treatment were significantly higher than those of the control and for the results of day 14, there is no significant difference (Figure 6).

Figure 6 Effect of cold treatment on the sucrose content in young leaves of materials of aceitoso (AN and AI); colecta 1 (CN and CI); tepetl (TN and TI); and duke 7 (DN and DI), comparing (LSD) the same treatment day number between both chambers. The number above each set of bars of the rootstocks is the average value across all rootstocks.
Starch
Starch is composed of amylopectin (85-70%) and amylose (15-30%), respectively (Mclauchlan et al., 2001); its metabolism in sinks (seeds, roots, tubers, developing leaves) is associated with the ratio of starch/sucrose synthesis at the source, export efficiency, sucrose transport, and sink priority and potency (Minchin and Lacointe, 2005). Young leaves from avocado vegetative shoots (sinks) showed a low concentration in starch content in both chambers.
Baguma et al. (2003) mention that the leaves are tissues that accumulate transient starch; therefore, the samples of the avocado materials under the conditions of chamber 1 (cold treatment) recorded average values of 1.71 μmoles g-1 fresh weight for day 1, 1.33 μmoles g-1 fresh weight at 7 days, and 1.07 μmoles g-1 fresh weight at 14 days; on the other hand, in chamber 2 (control), the mean values were 1.31 μmoles g-1 fresh weight for day 1, 1.59 μmoles g-1 fresh weight at 7 days, and 1.05 μmoles g-1 fresh weight at 14 days; therefore, according to the comparison of means with a significance of α= 0.05, it can be mentioned that, there are no significant differences between the materials in chamber 1 (cold treatment) and the control (Figure 7).

Figure 7 Effect of cold treatment on the starch content in young leaves of materials of aceitoso (AN and AI); colecta 1 (CN and CI); tepetl (TN and TI); and duke 7 (DN and DI), comparing the same treatment day number between both chambers. The number above each set of bars of the rootstocks is the average value across all rootstocks.
Aceitoso
The sugar content in the aceitoso material under temperature and light conditions of chamber 1 (Table 4), recorded an average glucose content (AcG) significantly higher in the non-grafted plants on days 1 and 7 and for the grafted plants on days 1, 7 and 14, compared to chamber 2 (control). Nonetheless, for fructose (AcF), the non-grafted and grafted plants in the three samples taken at 1, 7, and 14 days were significantly higher than those of the control. For sucrose (AcS), the averages in non-grafted plants were significantly higher on day 1 and for grafted plants on days 1, 7, and 14; for starch (AcSt), in non-grafted plants, the sample on day 1 was significantly higher and in grafted plants, it was significantly higher on days 1 and 7.
Table 4 Average values of glucose (AcG), fructose (AcF), sucrose (AcS), and starch (AcSt) contents of the non-grafted and grafted aceitoso material.
| Material | Variable | Chamber 2 (control) | Chamber 1 (treatment) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 7 days | 14 days | 1 day | 7 days | 14 days | |||
| Aceitoso non-grafted (AN) | AcG (µmoles g-1) | 2.22b | 7.3b | 10.31a | 8.82a | 10.65a | 12.29a | |
| AcF (µmoles g-1) | 1.95b | 5.14b | 5.69b | 4.46a | 7.7a | 9.83a | ||
| AcS (µmoles g-1) | 0.05b | 0.17b | 0.36a | 0.12a | 0.22b | 0.46a | ||
| AcSt (µmoles g-1) | 0.8b | 1.42a | 1.17a | 1.77a | 0.78b | 0.85b | ||
| Aceitoso grafted (AI) | AcG (µmoles g-1) | 3.86b | 6.92b | 5.2b | 9.28a | 13.71a | 8.2a | |
| AcF (µmoles g-1) | 4.02b | 3.59b | 5.13b | 8.28a | 9.9a | 10.79a | ||
| AcS (µmoles g-1) | 0.12b | 0.29b | 0.31b | 0.85a | 1.08a | 1.12a | ||
| AcSt (µmoles g-1) | 0.96b | 0.71b | 1.05a | 1.68a | 1.69a | 0.86b | ||
Means with the same letter in rows and day of sampling are not significantly different (LSD test).
Colecta 1
The sugar content in the colecta 1 material (Table 5), under the temperature and low luminosity conditions of chamber 1, had an average glucose content (AcG) significantly higher in the result of day 7 in the non-grafted plants and for the grafted plants, a significantly higher difference only in the sample taken at 14 days; fructose (AcF) is significantly higher in both non-grafted plants and grafted plants; sucrose (AcS) for non-grafted plants is significantly higher only in the sample on day 7, and for grafted plants, it was significantly higher in the sample on day 1; finally, starch (AcSt) showed a significant difference in the sample on day 1 in the non-grafted plants and for the grafted materials, it is significantly higher in the sample on day 7.
Table 5 Average values of glucose (AcG), fructose (AcF), sucrose (AcS), and starch (AcSt) contents of the non-grafted and grafted Colecta 1 material.
| Material | Variable | Chamber 2 (control) | Chamber 1 (treatment) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 7 days | 14 days | 1 day | 7 days | 14 days | |||
| Colecta 1 non-grafted (CN) | AcG (µmoles g-1) | 3.46a | 9.96b | 10.65a | 3.87a | 14.34a | 8.44a | |
| AcF (µmoles g-1) | 1.49b | 3.72b | 5.65b | 5.56a | 8.25a | 10.39a | ||
| AcS (µmoles g-1) | 0.31a | 0.23b | 0.51a | 0.27a | 0.53a | 0.34b | ||
| AcSt (µmoles g-1) | 0.85b | 1.32a | 0.84a | 1.88a | 1.95a | 1a | ||
| Colecta 1 grafted (CI) | AcG (µmoles g-1) | 6.88a | 10.92a | 5.25b | 5.79a | 13.11a | 8.3a | |
| AcF (µmoles g-1) | 5.64b | 4.61b | 5.65b | 7.79a | 11.09a | 11.14a | ||
| AcS (µmoles g-1) | 0.1b | 0.21a | 0.4a | 0.24a | 0.25a | 0.33a | ||
| AcSt (µmoles g-1) | 1.51a | 2.19a | 1.06a | 1.27a | 1b | 0.94a | ||
Means with the same letter in rows and day of sampling are not significantly different (LSD test).
Tepetl
The sugar content in the tepetl material, under the temperature and low luminosity conditions of chamber 1, registered an average glucose content (AcG) significantly higher in the results of day 7 and 14 in the non-grafted plants, compared to the control; for plants grafted with ‘Hass’, they only showed a significant difference in the samples taken on days 7 and 14; fructose (AcF) was significantly higher in the three samples carried out in both non-grafted and grafted plants; sucrose (AcS) was significantly higher for non-grafted plants in the sample on day 7 and for grafted plants, it is significantly higher in the sample on day 14; finally, the starch content (AcSt) did not show a significant difference compared to the control in both non-grafted and grafted plants (Table 6).
Table 6 Average values of glucose (AcG), fructose (AcF), sucrose (AcS), and starch (AcSt) contents of the non-grafted and grafted tepetl material.
| Material | Variable | Chamber 2 (control) | Chamber 1 (treatment) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 7 days | 14 days | 1 day | 7 days | 14 days | |||
| Tepetl non-grafted (TN) | AcG (µmoles g-1) | 4.32a | 8.67b | 7.82b | 5.49a | 17.48a | 25.47a | |
| AcF (µmoles g-1) | 1.38b | 4.54b | 5.57b | 5.9a | 9.62a | 13.04a | ||
| AcS (µmoles g-1) | 0.19a | 0.15b | 0.53a | 0.29a | 0.63a | 0.54a | ||
| AcSt (µmoles g-1) | 1.54a | 1.98a | 1.12a | 2.1a | 1.62a | 1.77a | ||
| Tepetl grafted (TI) | AcG (µmoles g-1) | 5.74a | 4.51b | 5.23b | 5.58a | 14.71a | 13.53a | |
| AcF (µmoles g-1) | 4.8b | 4.4b | 5.15b | 8.08a | 11.2a | 15.48a | ||
| AcS (µmoles g-1) | 0.17a | 0.33a | 0.42b | 0.05b | 0.1b | 1.46a | ||
| AcSt (µmoles g-1) | 1.72a | 2.36a | 1.33a | 1.78a | 0.88b | 1.24a | ||
Means with the same letter in rows and day of sampling are not significantly different (LSD test).
Duke 7
The sugar content in this material, under the conditions of temperature and low luminosity of chamber 1, showed an average glucose content (AcG) significantly higher in the samples of days 1, 7, and 14 in the non-grafted and grafted plants, compared to the control; in the case of the fructose content (AcF), it is significantly higher in the samplings carried out on day 1, 7, and 14 in the non-grafted plants and in the grafted ones. In relation to the sucrose content (AcS), for the non-grafted plants, it is significantly higher only in the sample of day 1, and for the grafted plants, it is significantly higher only in the samples of day 1 and 7; finally, the average content of starch (AcSt) in the non-grafted plants only showed significant difference in the sample of day 7, and in the grafted materials, no significant differences were recorded in the three samples (Table 7).
Table 7 Average values of glucose (AcG), fructose (AcF), sucrose (AcS), and starch (AcSt) contents of the non-grafted and grafted duke 7 material.
| Material | Variable | Chamber 2 (control) | Chamber 1 (treatment) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 day | 7 days | 14 days | 1 day | 7 days | 14 days | |||
| Duke 7 non-grafted (DN) | AcG (µmoles g-1) | 6.02b | 5.93b | 6.7b | 17.03a | 13.93a | 12.48a | |
| AcF (µmoles g-1) | 1.33b | 4.36b | 5.66b | 7.52a | 10.15a | 13.63a | ||
| AcS (µmoles g-1) | 0.14b | 0.22a | 0.29a | 0.23a | 0.27a | 0.25a | ||
| AcSt (µmoles g-1) | 1.18a | 0.79b | 0.97a | 1.55a | 1.37a | 0.88a | ||
| Duke 7 grafted (DI) | AcG (µmoles g-1) | 7.67b | 10.3b | 6.24b | 22.02a | 21.03a | 12.41a | |
| AcF (µmoles g-1) | 5.59b | 6.46b | 7.81b | 13.59a | 11.36a | 15.88a | ||
| AcS (µmoles g-1) | 0.14b | 0.1b | 0.43a | 0.46a | 0.32a | 0.13b | ||
| AcSt (µmoles g-1) | 1.91a | 1.94a | 0.9a | 1.67a | 1.33a | 1.05a | ||
Means with the same letter in rows and day of sampling are not significantly different (LSD test).
Conclusions
Low temperature stress affects the sugar content, significantly increasing the concentration of glucose and fructose in the vegetative shoots of avocado materials of the Mexican race (aceitoso, colecta 1, tepetl and duke 7) grafted and not grafted with the ‘Hass’ variety, being associated with cold tolerance as a natural osmoprotectant.
Considering that the materials with the highest concentration of glucose and fructose under cold conditions were duke 7 and tepetl, they are recommended for establishment as rootstocks of the ‘Hass’ variety in areas with temperatures ranging between 15.61 °C average during the day and 4.4 °C average at night.
The materials that showed growth under cold conditions were aceitoso and colecta 1, grafted and non-grafted; nevertheless, the growth of plants without an increase in the concentration of sugars (glucose and fructose) in vegetative shoots makes them more susceptible to cold damage.
Bibliografía
Baguma, Y. K.; Sun, Ch.; Ahlandsberg, S.; Mutisya, J.; Palmqvist, S.; Rubaihayo, P. R.; Magambo, M. J.; Egwang, T. G.; Larsson, H. and Jansson, C. 2003. Expression patterns of the gene encoding starch branching enzyme II in the storage roots of cassava (Manihot esculenta crantz). Plant Sci. 164(5):833-839. [ Links ]
Barrientos-Priego, A. F.; Muñoz-Pérez, R. B.; Borys, M. W. y Martínez-Damián, M. T. 2000. Cultivares y portainjertos del aguacate. In: el aguacate y su manejo integrado. 35-54 pp. [ Links ]
Bergh, B. O. 1992. The origin nature and genetic, improvement of avocado. California Avocado Society Yearbook. 76(1):61-75. [ Links ]
Crane, J. H.; Douhan, G. W.; Faber, B. A.; Arpia, M. L.; Bender, G. S.; Balerdi, C. F. and Barrientos, A. F. 2013. El aguacate. Botánica. Cultivares y portainjertos. Producción y usos. Ed. Universitarias de Valparaiso. Chile. 243-271 pp. [ Links ]
Coelho, F. S.; Fontes, P. C. R.; Puiatti, M.; Neves, J. C. L. y Silva, M. C. C. 2010. Dose de nitrogenioassociada a produtividade de batata e índices do estado de nitrogenionafolha. Revista Brasileira de Ciencia do Solo. 34(4):1175-1183. [ Links ]
Dickson, R. E.; Tomlinson, P. T. and Isebrands, J. G. 2000. Allocation of current photosynthate and changes in tissue dry weight within northern red oak seedlings: individual leaf and flush carbon contribution during episodic growth. Canadian Journal of Forest Research. 30(8):1296-1307. [ Links ]
Geigenberger, P.; Kolbe, A. and Tiessen, A. 2005. Redox regulation of carbon storage and partitioning in response to light and sugars. Journal of Experimental Botany. 56(416):1469-1479. [ Links ]
Gianquinto, G.; Sambo, P. and Bona, S. 2003. The use of SPAD-502 chlorophyll meter for dynamically optimizing the nitrogen supply in potato crop. A methodological Approach. Acta Horticulturae. 32(607):197-204. [ Links ]
Hopkins, W. G. and Huner, N. 2004. Introduction to plant physiology. New York: John Wiley. 173-194 pp. [ Links ]
Knight, R. J. 2002. History, distribution and uses. In: Whiley, A. W.; Schaffer, B. and Wolstenholme, B. N. Ed. The avocado: botany, production and uses, 1st end. CAB International, Wallingford, UK. 1-14 pp. [ Links ]
Lahav, E. and Trochoulias, T. 1982. The effect of temperature on growth and dry matter production of avocado plants. Australian Journal of Agricultural Research. 33(3):549-558. [ Links ]
Lacono, F.; Buccella, A. and Peterlunger, E. 1998. Water stress and rootstock influence on leaf gas exchange of grafted and ungrafted grapevines. Scientia Horticulturae. 75(1):27-39. [ Links ]
Liu, X.; Mickelbart, M. V.; Robinson, P. W.; Hofshi, R. and Arpaia, M. L. 2002. Photosynthetic characteristics of avocado leaves. Acta Horticulturae . 575:865-874. [ Links ]
Lockard, R. G. and Schneider, G. W. 1981. Stock and scion relationships and the dwarfing mechanism in apple. Horticultural reviews. 3(7):315-375. [ Links ]
Marschall, M.; Sütő, S. and Szőke, S. 2019 Comparative ecophysiological study of the seasonally dependent non-structural carbohydrate pool of the fractal accumulating Helianthus tuberosus, Cichorium intybus and Dactylis glomerata. Acta biol. Plant. Agriensis. 7(1):81-115. [ Links ]
Mclauchlan, A.; Ogbonnaya, F. Ch.; Hollingsworth, B.; Mcneil M. D; Gale, K.; Henry, R. J.; Holton, T.; Morell, M.; Rampling, L.; Sharp, P.; Shariflou, M. R.; Jones, M. E. and Appels, R. 2001. Development of robust PCR-based DNA markers for each homoeo-allele of granule-bound starch synthase and their application in wheat breeding programs. Aust. J. Agric. Res. 52(11-12):1409-1416. [ Links ]
Mickelbart, M. V.; Mesleer, S. and Arpaia, M. L. 2007. Slinityinduced changes in ion concentration of Hass avocado trees on three rootstocks. Journal of Plant Nutrition. 30(1):105-122. [ Links ]
Minchin, P. E. and Lacointe, A. 2005. New understanding on phloem physiology and possible consequences for modelling long distance carbon transport. New Phytol 166(3):771-779. [ Links ]
Padilla, Ch. D. y Martínez, B. E. 2007. Factores involucrados en la distribución de azúcares en las plantas vasculares: comunicación entre los tejidos fuente y demanda. Departamento de bioquímica. Conjunto E. Facultad de Química. Universidad Nacional Autónoma de México (UNAM). 26(3):99-105. [ Links ]
Poirier, M.; Lacointe, A. and Améglio, T. 2010. A semi physiological model of cold hardening and dehardening in walnut stem. Tree Physiol. 30(12):1555-1569. [ Links ]
PROFECO. 2021. Producción de Aguacate. https://www.gob.mx/profeco/documentos/ para-aguacates-los-demexico?state=published#:~:text=En%202020%2C%20la%20p. [ Links ]
Rolland, F.; Moore, B. and Sheen, J. 2002. Sugar sensing and signaling in plants. Plant Cell. S185-S205. [ Links ]
Taiz, L. and Zeiger, E. 2004. Fisiología vegetal. 3ra . Ed. Porto Alegre: Artmed. 709-719 pp. [ Links ]
Whiley, A. W. 1990. CO2 assimilation of developing shoots of cv ‘Hass’ avocado (Persea americana Mill.) a preliminary report. South African avocado growers’ association yearbook 13(1):28-30. [ Links ]
Received: November 01, 2023; Accepted: February 01, 2024











 text in
text in