Services on Demand
Journal
Article
Indicators
Related links
Share
Revista mexicana de ciencias agrícolas
Print version ISSN 2007-0934
Rev. Mex. Cienc. Agríc vol.13 n.3 Texcoco Apr./May. 2022 Epub Aug 08, 2022
https://doi.org/10.29312/remexca.v13i3.2845
Articles
Induced variability in morphological characteristics of Euphorbia fulgens Karw. ex Klotzsch
1Departamento de Fitotecnia-Universidad Autónoma Chapingo. Carretera México-Texcoco km 38.5, Chapingo, Estado de México. CP. 56230. Tel. 595 9521500. (mpereznicolas01@gmail.com; lozcol@gmail.com; carlos951215@hotmail.com; mgise310@gmail.com).
2Facultad de Ciencias Agropecuarias-Universidad Autónoma del Estado de Morelos. Av. Universidad 1001, Cuernavaca, Morelos. CP. 62210. Tel. 777 1345402. (ijac96@yahoo.com.mx).
3Instituto Nacional de Investigaciones Nucleares. Carretera México-Toluca s/n, La Marquesa, Ocoyoacac, México. CP. 52750. Tel. 55 53297200.
Euphorbia fulgens is an ornamental species that, in Mexico, has little variability in the color of flowers, so this work had as an objective to evaluate the effect of 60Co gamma radiation on seeds and morphological characteristics of adult plants, as well as to obtain mutants of different colors in the flowers. Seeds were collected in communities of Oaxaca (M0), irradiated at nine doses (50, 100, 150, 200, 250, 300, 450, 600, 750 Gy) and established under greenhouse until the adult stage (M1), giving follow-up until the development of their offspring (M2). Emergence, survival and 27 morphological characteristics corresponding to vegetative and reproductive structures in both generations were evaluated. The percentage of emergence and the number of plants that survived decreased as the radiation dose increased. Radiation doses of 250 Gy or higher caused seed germination percentages to be less than 50%. The treatments produced differences in the variables: number of branches with inflorescences and color of petaliferous appendages. The dose of 300 Gy generated branched individuals with different inflorescence color, although it affected survival. The phenotypic distribution in progenitors and descendants was similar, showing differences in number of cymes, number of cyathia and color of petaliferous appendages. Irradiation is a suitable technique to generate variation in the color of the inflorescences in this species.
Keywords: color; gamma rays; induced mutagenesis; ornamental plant
Euphorbia fulgens es una especie ornamental que en México presenta poca variabilidad en el color de las flores, por lo que este trabajo tuvo como objetivo evaluar el efecto de radiación gamma 60Co en semillas y en caracteres morfológicos de plantas adultas, así como obtener mutantes de diferentes colores en las flores. Se colectaron semillas en comunidades de Oaxaca (M0), fueron irradiadas a nueve dosis (50, 100, 150, 200, 250, 300, 450, 600, 750 Gy) y se establecieron bajo invernadero hasta la etapa adulta (M1) dando seguimiento hasta el desarrollo de su descendencia (M2). Se evaluó la emergencia, la sobrevivencia y 27 caracteres morfológicos que corresponden a estructuras vegetativas y reproductivas en ambas generaciones. El porcentaje de emergencia y el número de plantas que sobrevivieron disminuyó conforme aumentó la dosis de radiación. Dosis de radiación de 250 Gy o superiores provocaron que los porcentajes de germinación de las semillas fueran inferiores al 50%. Los tratamientos produjeron diferencias en las variables número de ramas con inflorescencias y color de apéndices petalíferos. La dosis de 300 Gy generó individuos ramificados y con diferente color de inflorescencias, aunque afectó la sobrevivencia. La distribución fenotípica en progenitores y descendientes fue similar presentando diferencias en número de cimas, número de ciatios y color de apéndices petalíferos. La irradiación es una técnica adecuada para generar variación en el color de las inflorescencias en esta especie.
Palabras clave: color; mutagénesis inducida; planta ornamental; rayos gamma
Introduction
Induced mutagenesis is a technique by which 671 mutant varieties of ornamentals have been generated, mostly corresponding to Chrysanthemum spp. (285 varieties), Rosa spp. (67 varieties), Dahlia spp. (35 varieties), Alstroemeria spp. (35 varieties) and Streptocarpus spp. (30 varieties) and were generated mainly in China, the Netherlands, Japan, India and Germany (FAO/IAEA, 2020). This technique has been widely used because most ornamental plants are vegetatively propagated, allowing easy reproduction of mutants (Rangaiah, 2006; Yamaguchi, 2018), and is justifiable when there is not enough natural variability, therefore, it is necessary to induce it to have a wide range of individuals with desirable characteristics.
The advantages of this process are: the possibility of generating variability; the speed with which an individual with desirable characters can be obtained, that is, it reduces the time compared to traditional improvement (De la Cruz, 2010) and as they are not considered genetically modified organisms (GMOs), this method is free of regulatory restrictions (Parry et al., 2009). The disadvantages are: mutations occur unpredictably throughout the genome, occur at a point and it is difficult to obtain a mutant with multiple desired characteristics, a large number of seeds or vegetative parts are required for the effect of a mutagenic agent to be identified; it is difficult to identify a small number of individuals with novel phenotypes within a large population and many mutations do not have a detectable effect on plants (De la Cruz, 2010).
Induced mutations are those caused by exogenous agents called mutagens, which can be chemical or physical; among the chemicals are ethyl methanesulfonate (EMS), dimethyl sulfate and diethyl sulfate, methylnitrosourea (MNU) and ethylnitrosourea (ENU) (Oladosu et al., 2016) and among the physical agents, we find x-rays, gamma rays (Jain, 2005; Yamaguchi et al., 2008), ultraviolet light (Ahloowalia and Maluszynski, 2001) and carbon ions (Wu et al., 2009).
In ornamental plants, the most commonly used mutagens in improvement are the physical ones (Jain, 2005; Wu et al., 2009; Yamaguchi, 2018; Yamaguchi et al., 2018; Hernández-Muñoz et al., 2019), since they can stimulate germination, accelerate the development of plants, favor resistance to different types of stress, mainly diseases, and cause changes in the color, shape and size of flowers (Maluszynski et al., 2001; Ahloowalia and Maluszynski, 2001; Chopra, 2005; Datta and Teixeira, 2006). In species where no prior information is available, the first thing to do is a radiosensitivity study, which consists of evaluating the sensitivity of cells, tissues, seeds and vegetative structures to the mutagenic agent to determine the mean lethal dose. Radiosensitivity varies according to species, ploidy level, degree of tissue differentiation and moisture content (De la Cruz, 2010).
In seed-propagated plants, mutagenized populations are generated by exposure of seeds (M0) to the mutagen, the obtaining of autogamous or allogamous plants (M1) that will give rise to seeds (M2). Since mutations are usually a recessive event, it is recommended to perform selection in the M2 generation, in which mutations in recessive homozygous condition become evident (Chikelu, 2013). M2 plants continue their development and M3 seeds are obtained, at this stage, the population is still segregating and not all M3 plants will carry the mutations identified in M2.
The mutagenized population can fix a mutation until the M6 generation, generating almost homozygous material, although in the process up to half of the mutations present in M1 disappear (Parry et al., 2009; Ukai and Nakagawa, 2011).
In Mexico, experiments where it has been determined that 60Co gamma radiation stimulates germination in seeds of Laelia autumnalis (Lex.) Lindl. have been carried out (Hernández-Muñoz et al., 2017). In tuberose (Polianthes tuberosa L.), its effect on tubers and plants has been evaluated in vitro (Estrada-Basaldua et al., 2011), in chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitam.), mutants with slow-developing, dwarfs and mottled-leaved genotypes were produced (Castillo-Martínez et al., 2015), while by irradiating bulbs of Tigridia pavonia (L. f.) DC., it was possible to modify the color of the flower (Díaz-López et al., 2003).
In sunflower (Helianthus annuus L.) and roselle (Hibiscus sabdariffa L.), gamma radiation influenced seed germination (Díaz-López et al., 2017), a similar behavior in cape gooseberry (Physalis peruviana L.) for seed germination and plant height, as well as yield components (Antúnez et al., 2020a, b) and finally, in poinsettia (Euphorbia pulcherrima Willd. ex Klotzsch), it influenced emergence, seed characteristics and morphological parameters in adult plants (Canul-Ku et al., 2012), with the poinsettia variety Juanita being registered in 2016 as a pot flower obtained by mutagenesis (Canul-Ku et al., 2019).
Euphorbia fulgens is a species that is marketed as an ornamental in Europe and other countries, mainly as a cut flower, although it is also offered as a potted plant. Its common name is scarlet plume, and it is considered a short-day plant. Various cultivars with different colors of inflorescences: orange, pink, red, white, salmon, peach and yellow, are marketed; while the color of the leaves can be green, dark red or purple.
In Mexico, where it originates, in wild populations only inflorescences with shades ranging from orange to red are found (Pérez-Nicolás et al., 2021). So, it is possible that such varieties originated through induced mutagenesis. Thus, the ‘Albora’ variety was developed in the Netherlands by X-ray (40Gy) irradiation of cuttings, was officially approved in 1976 and its main improved attribute is the color of the inflorescences (FAO/IAEA, 2020). Considering the above, the objective of this research was to evaluate the effect of 60Co gamma radiation on seeds and detect changes in morphological characteristics of adult plants of E. fulgens in the M1 and M2 generations to obtain mutants with different color of petaliferous appendages.
Materials and methods
The experiment was carried out in a glass greenhouse located at the Chapingo Autonomous University, which is located at 19° 20’ north latitude and 98° 53’ west longitude, at 2 240 m altitude. The temperature and relative humidity were recorded with a HOBO® U12-012 data logger (Onset Computer Corporation, Massachusetts, USA), the average temperature varied between 16 and 24 °C and the relative humidity between 38 and 68%. In April 2018, Euphorbia fulgens fruits were collected at two sites in the state of Oaxaca, site 1 is located in San Jerónimo Coatlán and site 2 in Santiago Jamiltepec.
The fruits were placed in brown paper bags until they dried and released their seeds. The seeds were deposited in glassine bags and kept at room temperature; 200 seeds were chosen from each site, which were distributed in 10 bags containing 20 seeds each (M0). On May 4 of the same year, the seeds were irradiated in a Transelektro irradiator (LGI-01, manufactured in Hungary) with a source of 60Co, located at the National Institute for Nuclear Research (ININ, for its acronym in Spanish). Nine doses of radiation with gammas of 60Co (50, 100, 150, 200, 250, 300, 450, 600, 750 Gy) plus the control without irradiation were established, having 40 seeds per treatment.
That same day, the irradiated seeds (M1) were sown under a completely random design in expanded polystyrene trays of 200 cavities, using peat/perlite (50:50 v/v) as a substrate. The M1 plants at the stage of first pair of opposite true leaves (late May, early June) were transplanted into pots of 11 x 11 x 15 cm, using peat/expanded perlite/vermiculite (40:30:30 v/v/v) as substrate. At this stage, starter fertilizer Ultrasol® N15-P30-K15+M. E. AQTEX, 0.5 g L-1, was added to the irrigation water every eight days. At the end of July, August and September, multipurpose Ultrasol® N18-P18-K18 + M. E. AQTEX, 1 g L-1, was applied every eight days.
In early October of the same year, the plants were switched to 25 x 25 cm black polyethylene bags that contained substrate of oak leaf soil/coconut fiber/vermicompost/black soil/pine bark (30:30:20:10:10). Preventive applications with fungicides were made to avoid the appearance of fungi with Benomyl, 1.5 g L-1; to avoid the appearance of black fly, Bifenthrin, 1.5 ml L-1, was used, alternating with Cypermethrin, additionally, blue and yellow chromatic traps were placed. However, the pest that appeared was the red spider mite (Tetranychus urticae), so it was controlled with Abamectin 1.5 ml L-1 and Bacillus thuringiensis 0.5 g L-1. Weed control was performed manually.
In the M1 generation, the percentage of emergence and the number of plants that survived to the adult stage with two to three fruits developed were recorded. In this phenological stage, there was an M1 population of 137 plants and 27 variables were recorded in all of them; a tape measure was used to measure plant height (cm) and by means of a ruler, the following variables were measured: petiole length (cm), blade length (cm) and blade width (cm).
These data were used to calculate the ratio between blade width and blade length (cm) and the ratio between petiole length and blade length (cm). The number of branches with cyathia, the number of cymes and the number of hermaphrodite cyathia were also counted, the following was measured: internode length (cm), inflorescence length (cm), length of the longest cyme (cm), length of internodes in the inflorescence (cm), peduncle length (cm), involucre length (cm), petaliferous appendage length (cm), petaliferous appendage width (cm), pedicel length (cm), ovary length (cm), style length (cm), stamen length (cm), fruit length (cm) and fruit diameter (cm), which was measured with a Pretul® vernier.
Finally, the following variables were recorded based on the guide for varieties of E. fulgens of the International Union for the Protection of New Varieties of Plants (UPOV, 1988): the color of the petaliferous appendages, the color of the blades of the upper third of the part with cymes (red or green), the intensity of the red color in the same blades, as well as the color of the blades in the lower third of the part with cymes (red or green) for which the color charts of The Royal Horticultural Society (2001) were used.
Subsequently, at the end of December 2018 and at the beginning of January 2019 and given that this species is allogamous (Pérez-Nicolás, 2020), random crosses were carried out to guarantee the production of seeds, between individuals obtained from the M1 Generation; then in March and April 2019, the fruits were harvested, the seeds were sown on May 12, 2019, and their development was followed until the adult stage (M2 Generation). It should be noted that both the sowing and the development of the plants was carried out under the same conditions as the previous cycle (M1 generation).
In the M2 generation, the percentage of emergence and the number of plants that survived to the adult stage with two to three fruits developed were recorded. In this phenological stage, the characterization of 48 adult plants was carried out during the last week of November, when most of the plants were flowering.
Based on the results of the M1 generation, it was determined to measure the 17 variables in all individuals of the M2 generation: plant height with a tape measure; stem diameter with a Pretul® vernier, and with a ruler, petiole length, blade length, blade width, internode length, inflorescence length, length of the longest cyme, length of internodes in the inflorescence, peduncle length (cm), involucre length (cm), petaliferous appendage length (cm) and petaliferous appendage width (cm). The leaves before the inflorescence, number of cymes and the number of cyathia were counted. Finally, the color of petaliferous appendages was taken with the color charts of The Royal Horticultural Society (2001).
In both generations, the vegetative variables were taken in the second third of the plant of three leaves and three internodes and the reproductive structures of the second third of the inflorescence of three cymes and of three cyathia.
To evaluate the M1 generation, an analysis of variance (Anova) was performed between the different treatments (0, 50, 100, 200 and 300 Gy) and when statistical differences between treatments were detected, Tukey’s mean comparison test (p≤ 0.05) was applied, using the Infostat statistical package version 2020 (Di Rienzo et al. 2008). To evaluate the phenotypic characteristics in the M1 and M2 generations, 13 variables were considered, and a descriptive statistical analysis was made through frequency tables and graphs in Excel.
Results and discussion
In the M1 generation, at both collection sites, the control treatment (without radiation) showed the highest percentage of emergence, which decreased as the radiation dose increased. A simple linear regression model per collection site was calculated between the radiation dose and the percentage of emergence, which explained 86% of the observed behavior. At site 1, for each Gray of 60Co radiation, the emergence decreased by 0.08%, while at site 2, it decreased by 0.03% (Figure 1).
The percentage of emergence for the control at site 1 was 80%, while at site 2, it was 55%. At both sites, from 250 Gy, between 55% and 56% of the plants died before reaching the adult stage. At the highest doses (450, 600 and 750 Gy), one to three seedlings emerged, most of them had hypocotyls and cotyledons that were deformed and without chlorophyll. At doses of 750 Gy, an individual from each site survived, so it was not possible to calculate the mean lethal dose; however, considering the regression curves for both sites, LD50 can be estimated at 275.41 Gy, for site 1, and at 401 Gy, for site 2.
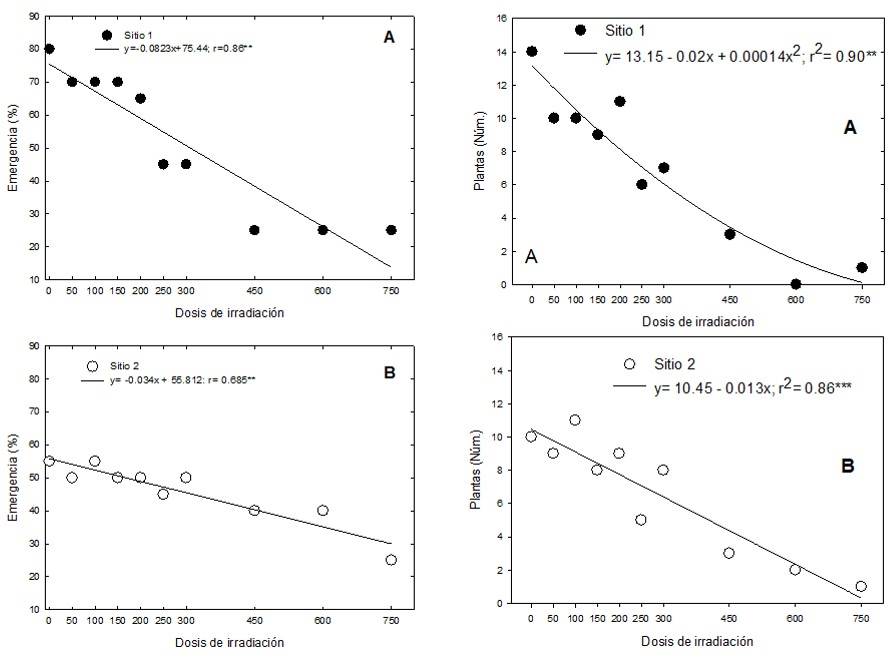
Figure 1 Percentage of emergence of seedlings and survival of plants of E. fulgens, from seeds irradiated with 60 Co gamma rays, from two collection sites in Mexico. A) site 1; and B) site 2.
Plants that survived the highest doses had malformations in leaves and inflorescences (Figure 1). The results obtained in the present study coincide with Díaz-López et al. (2017), who, in roselle and sunflower, when increasing the radiation dose, obtained lower germination percentages compared to the control, in the same way, Canul-Ku et al. (2012), in poinsettia, obtained lower percentages of emergence when increasing the radiation dose.
The survival of plants shown at high doses of radiation in this work can be explained by the fact that the seeds that underwent irradiation come from wild plants, which have greater plasticity to adapt to sudden changes in their habitat compared to cultivated species.
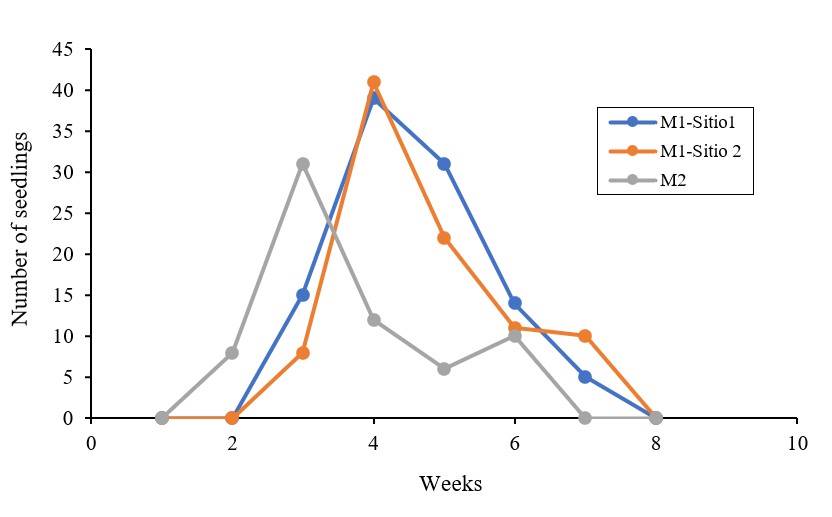
In the M1 generation, the emergence for both collection sites occurred 15 days after sowing, in a total of seven weeks, while in the M2 generation, the emergence occurred seven days after sowing, in a total of six weeks (Figure 2), so there was a decrease in the time of emergence under the same cultivation conditions.
The analysis of variance showed no significant differences between treatments for most of the variables considered for both vegetative and reproductive structures. In the number of branches with flowers, the 300 Gy treatment was significantly different from the 200 Gy treatment, while the rest of the treatments did not produce significant differences. For the variable color of the petaliferous appendages, the 300 Gy treatment was significantly different from the control and the 50 Gy and 100 Gy treatments (Table 1). However, in works with irradiated seeds of wild poinsettia, it has been possible to detect immediate effects, finding significant differences in seven characteristics, among them, plant height and cyathium diameter (Canul-Ku et al., 2012).
Table 1 Morphometric variables in plants of Euphorbia fulgens (M1) at different doses of 60 Co gamma radiation.
| Variable | 0 Gy | 50 Gy | 100 Gy | 150 Gy | 200 Gy | 300 Gy |
| Height | 101.88a | 102.97a | 92.05a | 98.34a | 100.31a | 98.54a |
| Petiole length | 3.1a | 2.73a | 2.87a | 3.1a | 3.21a | 3.05a |
| Blade length | 7.86a | 7.92a | 7.49a | 7.86a | 8.12a | 7.65a |
| Blade width | 2.36a | 2.3a | 2.29a | 2.35a | 2.41a | 2.27a |
| Blade width/blade length | 0.3a | 0.3a | 0.31a | 0.3a | 0.30a | 0.3a |
| Petiole length/blade length | 0.4a | 0.35a | 0.38a | 0.39a | 0.39a | 0.4a |
| Internode length | 1.57a | 1.56a | 1.76a | 1.63a | 1.57a | 1.54a |
| Number of branches with inflorescences | 1ab | 1ab | 1ab | 0.94ab | 0.89b | 1.21a |
| Inflorescence length | 27.5a | 20.99a | 20.43a | 20.06a | 21.08a | 27.27a |
| Number of cymes | 16.2a | 12.6a | 12.6a | 12.06a | 12.67a | 16.56a |
| Length of the longest cyme | 5.43a | 5.26a | 4.79a | 4.78a | 4.43a | 5.2a |
| Number of cyathia | 72.85a | 58.67a | 67.25a | 53.06a | 62.83a | 7.64a |
| Length of internodes in the inflorescence | 1.09a | 0.97a | 1.07a | 0.96a | 1.02a | 1.20a |
| Peduncle length | 1.12a | 1.22a | 1.13a | 1.14a | 0.96a | 1.19a |
| Involucre length | 0.31a | 0.31a | 0.31a | 0.28a | 0.27a | 0.3a |
| Petaliferous appendage length | 0.53a | 0.53a | 0.54a | 0.51a | 0.48a | 0.56a |
| Petaliferous appendage width | 0.49a | 0.49a | 0.52a | 0.46a | 0.44a | 0.51a |
| Pedicel length | 0.85a | 0.83a | 0.81a | 0.79a | 0.72a | 0.79a |
| Style length | 0.3a | 0.29a | 0.3a | 0.28a | 0.27a | 0.3a |
| Ovary length | 0.11a | 0.17a | 0.12a | 0.1a | 0.09a | 0.1a |
| Stamen length | 0.65a | 0.62a | 0.63a | 0.59a | 0.58a | 0.66a |
| Fruit length | 0.47a | 0.23a | 0.46a | 0.36a | 0.37a | 0.39a |
| Fruit diameter | 0.44a | 0.21a | 0.42a | 0.34a | 0.34a | 0.36a |
| Number of fruits | 2.6a | 2a | 2.9a | 1.56a | 2.28a | 3.43a |
| Number of seeds | 4.75a | 2.44a | 4.4a | 2.13a | 4.28a | 4.64a |
| Color of petaliferous appendages | 1.55b | 1.33b | 1.20b | 1.69ab | 1.78ab | 2.57a |
| Intensity of the color of the leaf blade in the upper third of the inflorescence | 1.3a | 1.11a | 1.5a | 1.25a | 1.23a | 1.21a |
abc= doses with different letters in the direction of the row are statistically different (p< 0.05).
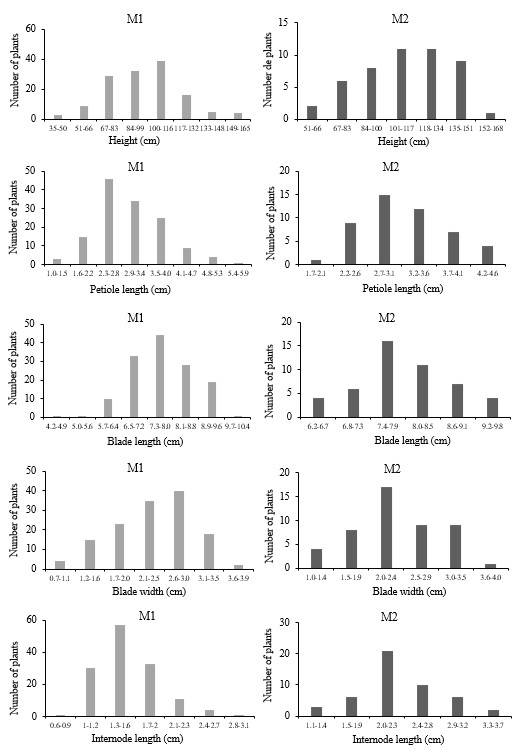
In the M1 generation, of 400 established seeds that correspond to site one and site two, 34.25% survived, of these plants 296 M1 seeds were obtained, of which 16.21% survived, which correspond to the M2 generation, since most were not viable. In both generations, similar characteristics were obtained in vegetative structures (Figure 3). The variable height in the progenitors (M1) ranged between 35 and 165 cm, the interval with the highest frequency was 100 to 116 cm, while in the offspring (M2), it ranged from 50 to 168 cm, and most were in the range of 101-134 cm. The petiole length in the M1 progenitors ranged from 1 to 6 cm, the interval with the highest number of plants was 2.2 to 2.7 cm, in the progeny, M2, from 1.7 to 4.6 cm, most of the plants were in the range of 2.5-2.9 cm (Figure 3).
The blade length in the M1 generation was between 4.2 and 10.4 cm, the interval with the highest frequency was 7.3 to 8 cm and in the M2 generation, from 6.1 to 9.7 cm, where the interval with the highest number of plants was 7.3 to 7.9 cm. The blade width from 0.7 to 3.9 cm, the interval with the highest frequency was 2.6 to 2.9 cm and in the offspring, it ranged from 1 to 4 cm, the interval with the highest number of plants was 2 to 2.4 cm. The internode length from 0.6 to 3.1 cm, the interval with the highest number of plants was 1.3 to 1.6 cm, and in the offspring from 1.1 to 3.7 cm and the interval with the highest number of plants was 1.9 to 2.2 cm (Figure 3).
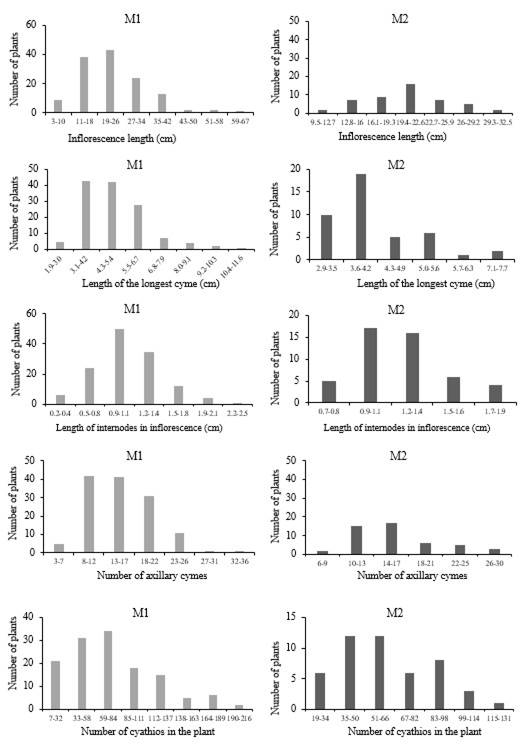
In the variables of reproductive structures, we found variation between progenitors and offspring, with fewer cymes in progenitors, but a greater number of cyathia with respect to the offspring (Figure 4). The inflorescence length in the M1 generation ranged from 3 to 67 cm, with most in the range of 19-26 cm and in the M2 generation from 9.5 to 32.5 cm, the interval with the highest number of plants is 19.4 to 22.6 cm. The length of the longest cyme in progenitors was from 1.9 to 11.6 cm and most were in the range of 3.1-4.2 cm and in their offspring, it was from 2.9 to 7.2 cm, and most were in the range of 3.6-4.2 cm. In the M1 generation, there were from 3 to 36 cymes, but most were in the range of 8-12 cymes, in the M2 generation, from 6 to 30 cymes and most were in the range of 14-17 cymes (Figure 4).
The length of internodes in the inflorescence in the progenitors was between 0.2 to 2.5 cm and most were in the range of 9.9-1.1 cm and in the offspring, it was from 0.8 to 1.9 cm, and most were from 9.9 to 1.3 cm. The number of cyathia counted in each plant of the M1 generation was from 7 to 216, most were from 59 to 84 cyathia. In the M2 generation, it was from 19 to 131 and most had from 35 to 66 cyathia. The length of the petaliferous appendage in the M1 generation was from 0.4 to 0.7 but most were 0.5 and, in their offspring, it was from 0.3 to 0.8 and most were from 0.4 to 0.5 (Figure 4). The width of the petaliferous appendage in progenitors was from 0.3 to 0.7 and most were 0.5, while in their offspring, it was from 0.3 to 0.7 and most in the range of 0.4-0.5 cm.
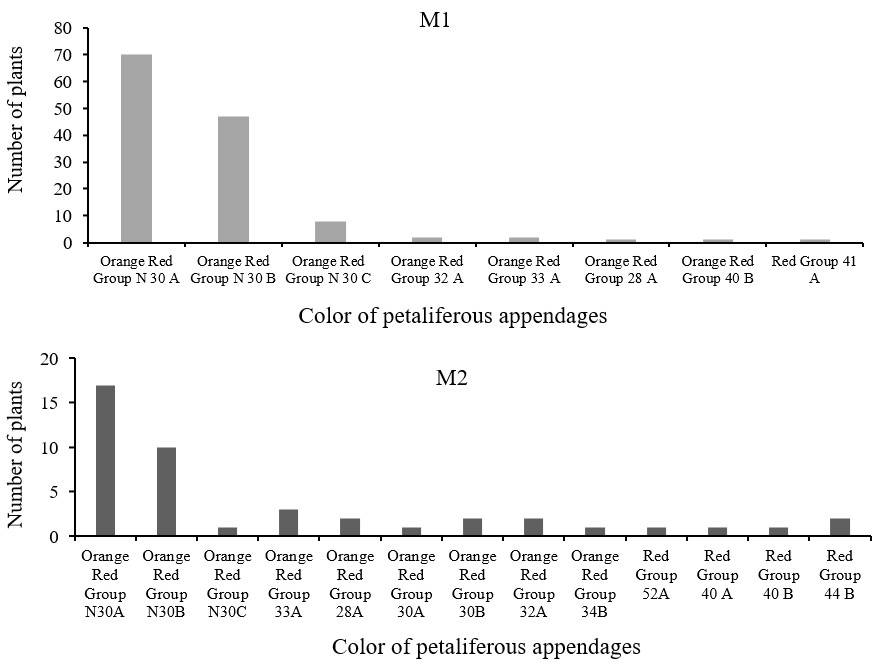
Regarding the variable color, in the progenitors, there were 8 different colors, with Orange Red Group N30 A being the most common, while in the offspring, there were 13 different colors, the most common was the same color as the progenitors (Figure 5 and 6).

Figure 6 Inflorescences of E. fulgens in greenhouse cultivation. A. M1 generation. B. M2 generation.
In a previous study where morphological characteristics of individuals from wild populations and from a first cycle of cultivation of plants obtained from unirradiated seeds were evaluated, the variable color did not show significant differences (Pérez-Nicolás et al., 2021). Although using a radiation dose of 300 Gy, it was possible to obtain mutants with different color of petaliferous appendages, which was one of the main objectives of the present study, the survival of the plants was low and there were malformations because the damage caused by radiation to the genetic material is greater, so it is convenient to conduct experiments with 100 Gy or less, since these doses did not affect the survival of the plants.
In addition, it is recommended to use more seeds in doses that led to coloration changes, considering that the induced mutation rate is around 1x10-4 (De la Cruz, 2010). However, as it is a microendemic species of restricted distribution, it would be best to irradiate cuttings at low doses, which could be obtained from the materials generated in this work and thus not have to extract material from wild populations and cause alteration.
Conclusions
At the radiation doses used in this work, 50-750Gy, total lethality was not reached. Based on the radiosensitivity curves obtained, LD50 is estimated at 275 Gy for site 1 and 401 Gy for site 2. The doses applied influenced the percentage of emergence and survival of the plants, but there was no effect on most of the morphometric variables evaluated, only on the number of branches with flowers and the color of the petaliferous appendages. Radiation influenced the variation of the color of inflorescences since the M1 generation, and this was greater in the M2 generation. In relation to the generations of mutagenesis, the phenotypic characteristics in the progenitors (M1) were similar to those shown by their offspring (M2) under the same cultivation conditions, highlighting that in the M2 generation there was greater color variation.
Literatura citada
Ahloowalia, B. S. and Maluszynski, M. 2001. Induced mutations. A new paradigm in plant breeding. Euphytica. 118:167-173. [ Links ]
Antúnez-Ocampo, O. M.; Cruz-Izquierdo, S.; Sandoval-Villa, M.; Santracruz-Valera, A.; Mendoza-Onofre, L. E.; Peña-Lomelí, A. y De la Cruz-Torres, E. 2020a. Peso y caracteres cuantitativos de la calidad en frutos de plantas M1 de Physalis peruviana L. provenientes de semillas irradiadas con 60Co. Agrociencia. 54(5):691-703. [ Links ]
Antúnez-Ocampo, O. M.; Cruz-Izquierdo, S.; Sandoval-Villa, M.; Santracruz-Valera, A.; Mendoza-Onofre, L. E.; Peña-Lomelí, A. and De la Cruz-Torres, E. 2020b. Growth dynamics of morphological and reproductive of Physalis peruviana L. M1 plants obtained from sedes irradiated with Gamma rays. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 48(1):200-209. Doi:10.15835/nbha48111745. [ Links ]
Canul-Ku, J.; García-Pérez, F.; Campos-Bravo, E.; Barrios-Gómez, E. J.; De la Cruz-Torres, E.; García-Andrade, J. M.; Osuna-González; F. de J. y Ramírez-Rojas, S. 2012. Efecto de la irradiación sobre nochebuena silvestres (Euphorbia pulcherrima Willd. ex Klotzsch) en Morelos. Rev. Mex. Cienc. Agríc. 3(8):1495-1507. [ Links ]
Canul-Ku, J.; García-Pérez, F.; Barrios-Gómez, E. J. y Rangel-Estrada, S. E. 2019. Juanita, nueva variedad de nochebuena para interior derivada por mutagénesis. Rev. Fitotec. Mex. 42(2):191-192. Doi: 10.35196/rfm.2019.2.191. [ Links ]
Castillo-Martínez, C. R.; De la Cruz, T. E.; Carrillo-Castañeda, G. y Avendaño-Arrazate, C. H. 2015. Inducción de mutaciones en crisantemo (Dendranthema grandiflora) usando radiación gamma y etil metano sulfonato. Agroproductividad. 8(2):60-64. [ Links ]
Chikelu, M. 2013. Induced mutations unleash the potential of plant genetic resources for food and agriculture. Agronomy. 3(1):200-231. Doi:10.3390/agronomy3010200. [ Links ]
Chopra, V. L. 2005. Mutagenesis: investigating the process and processing the outcome for crop improvement. Current Sci. 89(2):353-359. [ Links ]
Datta, S. K. and Teixeira, J. A. 2006. Role of induced mutagenesis for development of new flower colour and type in ornamentals. In: floriculture, ornamental and plant biotechnology: advances and topical issues. Teixeira da Silva, J. (Ed.). Global Science Books. London, United Kingdom. 640-645 pp. [ Links ]
De la Cruz, E. 2010. Aplicación de la energía nuclear en el mejoramiento de pseudocereales nativos de México. In: contribuciones del Instituto Nacional de Investigaciones Nucleares al avance de la ciencia y la tecnología en México. Mojica Duque G. (ed). Instituto Nacional de Investigaciones Nucleares. Estado de México, México. 407-420 pp. [ Links ]
Díaz-López, E.; Pichardo, R.; De la Cruz, T.; Norman, M. T.; Sandoval, R. F. y Vázquez-García, L. 2003. Variabilidad inducida en Tigridia pavonia (L.f.) D.C. var. Sandra por irradiación de bulbos con rayos gamma de 60Co. Rev. Chapingo Ser. Hortic. 9(2):235-241. [ Links ]
Díaz-López, E.; Morales-Ruiz, A.; Olivar-Hernández, A. and Loeza-Corte, J. M. 2017. Gamma irradiation effect of 60Co on the germination of two subtropical species in the Tehuacán-Cuicatlán Valley. Inter. J. Adv. Eng. Res. Sci. 4(8):56-61. Doi. 10.22161/ijaers.4.8.10. [ Links ]
Di Rienzo, J. A.; Casanoves, F.; Balzarini, M. G.; González, L.; Tablada, M. y Robledo, C. W. 2008. InfoStat, versión 2008, Grupo InfoStat, FCA. Universidad Nacional de Córdoba, Argentina. 336 p. [ Links ]
Estrada-Basaldua, J. A.; Pedraza-Santos, M. E.; Cruz-Torres, E.; Martínez-Palacios, C.; Sáenz-Romero, C. y Morales-García, J. L. 2011. Efecto de rayos gamma 60Co en nardo (Polianthes tuberosa L.). Rev. Mex. Cienc. Agríc. 2(3):445-458. [ Links ]
FAO/IAEA. 2020. Mutant variety database. Vienna, Austria: Food and Agriculture Organization of the United Nations and International Atomic Energy Agency. https://mvd.iaea.org/#!Search. [ Links ]
Hernández-Muñoz, S.; Pedraza-Santos, M. E.; López, P. A.; De la Cruz-Torres, E.; Martínez-Palacios, A.; Fernández-Pavia, S. P. y Chávez-Bárcenas, A. T. 2017. Estimulación de la germinación y desarrollo in vitro de Laelia autumnalis con rayos gamma. Rev. Fitotec. Mex. 40(3):271-283. [ Links ]
Hernández-Muñoz, S.; Pedraza-Santos, M. E.; López, P. A.; Gómez-Sanabria, J. M. y Morales-García, J. L. 2019. Mutagenesis in the improvement of ornamental plants. Rev. Chapingo Ser. Hortic. 25(3):151-167. Doi: 10.5154/r.rchsh.2018.12.022. [ Links ]
Jain, M. S. 2005. Major mutation-assisted plant breeding programs supported by FAO/IAEA. Plant Cell, Tissue and Organ Culture. 82:113-123. Doi: 10.1007/s11240-004-7095-6. [ Links ]
Maluszynski, M.; Nichterlein, K.; Van-Zanten, L. and Ahloowalia, B. S. 2000. Official released mutant varieties-the FAO/IAEA database. Mutation Breeding. 12:1-88. [ Links ]
Oladosu, Y.; Rafii, M. Y.; Absullah, N.; Hussin, G.; Ramli, A.; Rahim, H.; Miah, G. y Usman, M. 2016. Principles and application of plant mutagenesis in crop improvement: a review. Biotechnol. Biotechnol. Equip. 30(1):1-16. Doi: 10.1080/13102818.2015.1087333. [ Links ]
Parry, M. A.; Madgwick, P. J.; Bayon, C.; Tearall, K.; Hernández-López, A.; Baudo, M.; Rakszegi, M.; Hamada, W.; Al-Yassin, A.; Ouabbou, H.; Labhilili, M. and Phillips, A. L. 2009. Mutation discovery for crop improvement. J. Exp. Bot. 60(10):2817-2825. Doi: 10.1093/jxb/erp189. [ Links ]
Pérez-Nicolás, M. 2020. Análisis de la diversidad de Euforbias nativas de México para su uso sustentable como ornamentales. Colegio de Postgraduados. Tesis de Doctorado en Ciencias. Montecillo, Texcoco, México.72-91 pp. [ Links ]
Pérez-Nicolás, M.; Colinas-León, M. T.; Alia-Tejacal, I. y Peña-Ortega, G. 2021. Fenología y potencial ornamental de Euphorbia fulgens Karw. ex Klotzsch en México. Acta Agrícola y Pecuaria. E0071016. Doi: 10.30973/aap/2021.7.0071016. [ Links ]
Pérez-Nicolás, M.; Colinas-León, T.; Alia-Tejacal, I.; Peña-Ortega, G.; González-Andrés, F. and Beltrán-Rodríguez, L. 2021. Morphological variation in scarlet plume (Euphorbia fulgens Karw ex Klotzsch, Euphorbiaceae), and underutilized ornamental resource of Mexico with global importance. Plants. 10(10):2020. Doi:10.3390/plants10102020. [ Links ]
Rangaiah, S. 2006. Induced genetic variation for days to flowering and maturity following hybridization and mutagenesis in chilli (Capsicum annuum L.). Karnataka J. Agric. Sci. 19:382-384. [ Links ]
The Royal Horticultural Society. 2001. Color chart. The Royal Horticultural Society: London, UK. [ Links ]
Ukai, Y. and Nakagawa, H. 2011. Strategies and approaches in mutant populations development for mutant selection in seed propagated crops. In: plant mutation breeding and biotechnology. Shu, Q. Y., Forster, B. P. y Nakagawa, H. (Ed). Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture. Vienna, Austria. 209-221 pp. [ Links ]
UPOV. 1988. Guidelines for the conduct of tests for distinctness, homogeneity and stability. Euphorbia fulgens. Geneva, Switzerland: international union for the protection of new varieties of plants. https://www.upov.int/genie/es/details.xhtml?cropId=2262. [ Links ]
Yamaguchi, H. 2018. Mutation breeding of ornamental plants using ion beams. Breed. Sci. 68(1):71-78. Doi: 10.1270/jsbbs.17086. [ Links ]
Yamaguchi, H.; Shimizu, A.; Degi, K. and Morishita, T. 2008. Effects of dose rate of gamma ray irradiation on mutation induction and nuclear DNA content in Chrysanthemum. Breed. Sci. 58(3):331-335. Doi: 10.1270/jsbbs.58.331. [ Links ]
Wu, D.; Hou, S.; Qian, P.; Sun, L. D.; Zhang, Y. C. and Li, W. J. 2009. Flower color chimera and abnormal leaf mutants induced by 12C6+heavy ions in Salvia splendes Ker-Grawl. Sci. Hortic. 121(4):462-467. Doi: 10.1016/j.scienta.2009.02.022. [ Links ]
Received: November 01, 2021; Accepted: February 01, 2022











 text in
text in