Introduction
Freshwater molluscs belong to the most threatened species worldwide and their habitats are highly impacted by several human activities (Modesto et al., 2017). In North America, freshwater molluscs, especially gastropods and mussels, are the most imperiled of all aquatic biota, even more than fishes and crayfishes (Johnson et al., 2013; Lysne et al., 2008). But, despite their importance in the aquatic food web (Covich, 2010; Covich et al., 1999), our knowledge of extant Mexican freshwater gastropods and their geographical distributions is still incomplete.
Thompson (2011) estimated that 85% of Mexico had not been explored for land and freshwater snails and the estimated number of undescribed species remains very high. The first extensive checklist of Mexican freshwater molluscs, which included 121 species of gastropods, was published by Contreras-Arquieta (2000). Unfortunately, this compilation was published in a journal difficult to access, and was discontinued after a few volumes. As a consequence, several species mentioned by Contreras-Arquieta were not included by Thompson (2011). Thompson (2011) listed 1,502 native species of non-marine (continental) gastropods, which included 133 freshwater species and 15 subspecies. The checklists of Contreras-Arquieta and Thompson, together, included 157 freshwater gastropods but neither noted their conservation status. Several new species have been described from Mexico since 2011.
Here, we present a checklist of the freshwater gastropods of Mexico based on all available published sources. It includes 193 native freshwater species, an increase of 36 species (19%) over the mentioned checklists. There are 219 new state records, including information from Aguascalientes and Tlaxcala that previously lacked any records, an increase of 130%.
The checklist utilizes updated taxonomy in addition to information on distribution, threats, centers of endemicity, and conservation status. The checklist makes possible a direct comparison between the faunas of Mexico, and those of the USA and Canada, and will serve as a part of a future synopsis of all freshwater gastropods of North America and their conservation status.
Materials and methods
The general layout of the checklist (Table 1) follows Johnson et al. (2013) that presented a revised list of the distribution and conservation status of freshwater gastropods of Canada and the USA. The literature survey included all available publications on Mexican freshwater gastropods published from 1849 to 2019. In only a few cases, our own unpublished distribution records were used. We followed the family classification of Bouchet et al. (2017), with some modifications concerning hydrobiid sensu lato families (e.g., Wilke et al., 2013). Subspecies are not recognized. The revision was based mainly on biodiversity websites including WoRMS (World Register of Marine Species), the International Union for Conservation of Nature Red List (IUCN, 2018), MolluscaBase (MolluscaBase, 2018) and the most recent revisions of particular families, e.g., Gómez-Berning et al. (2012) and MolluscaBase for the family Pachychilidae and Taylor (2003) for the Physidae. Species occurrences in states were determined using primary literature, including published local checklists and available local Conabio (National Commission for the Knowledge and Use of Biodiversity) reports and our own records (Avendaño-Gil et al., 2010; Campos et al., 2013; Conabio, 2016; Contreras-Arquieta, 2000 [Littoridinops monroensis (Frauenfeld, 1863), L. tenuipes (Couper, 1844), Ferrissia californica (Rowell, 1863), Gyraulus circumstriatus (Tryon, 1866), Micromenetus brogniartianus (Lea, 1842)]; Cózatl-Manzano & Naranjo-García, 2007; Estrada-Loreto, 2011; Hershler et al., 1999 [Tryonia imitator (Pilsbry, 1899)]; Lam-Gordillo et al., 2012; López-López et al., 2009; Baqueiro et al., 2006; Naranjo-García, 2003; Ortega-Chávez et al., 2007 [Lymnaea stagnalis (Linnaeus, 1758)]; Naranjo-García & Meza Meneses, 2000 [Ecrobia truncata (Vanatta, 1924), Gundlachia radiata (Guilding, 1828)]; Pérez-Rodríguez & Vicente-Velázquez, 2003; Ramos-Solís, 2018; Rangel-Ruiz, 2000 [Drepanotrema depressissimum (Moricand, 1839), D. surinamense (Clessin, 1884)]; Rangel-Ruiz & Gamboa-Aguilar, 2005; Reguero & García-Cubas, 1989; Rosenberg et al., 2009; Serrano-Pinto & Caraveo-Patiño, 2002).
Table 1 Checklist of Mexican freshwater gastropods (see abbreviations and definitions in Materials and methods).
| Taxa | Endemic | N-Rank | IUCN | Hotspot | Distribution |
|---|---|---|---|---|---|
| Family Neritidae | |||||
| Clypeolum latissimum (Broderip, 1833) | - | N4 | ─ | ─ | COL, GRO |
| Neritina virginea (Linnaeus, 1758) | - | N5 | LC | ─ | CAMP, QR, TAB, TAMPS, VER, YUC |
| Vitta clenchi (Russell, 1940) | - | NUQ | ─ | ─ | VER |
| Vitta usnea (Röding, 1798) | - | N4 | ─ | ─ | CAMP, VER |
| Family Ampullariidae | |||||
| Pomacea cerasum (Hanley, 1854) | E | NU | ─ | ─ | TAB |
| Pomacea flagellata (Say, 1829) | - | N4N5 | ─ | ─ | CAMP, CHIS, TAB, VER |
| Pomacea cumingii (King & Broderip, 1831) | E | NUQ | ─ | ─ | CHIS, VER |
| Pomacea catemacensis (H. B. Baker 1922) | LE | N2Q | ─ | PP, S | VER |
| Pomacea picta (Reeve, 1856) | E | NUQ | ─ | ─ | GRO |
| Family Viviparidae | |||||
| Viviparus inornatus (Binney, 1865) | LE | N1 | ─ | S | NL |
| Family Pachychilidae | |||||
| Amnipila pila (Pilsbry & Hinkley, 1910) | LE | N1 | ─ | C | SLP |
| Pachychilus apheles F. G. Thompson, 1967 | LE | N1 | ─ | C, S | SLP |
| Pachychilus apis (I. Lea & H.C. Lea, 1851) | E | NU | ─ | ─ | VER |
| Pachychilus atratus Pilsbry & Hinkley, 1910 | E | N2N3 | ─ | C | SLP |
| Pachychilus chrysalis (Brot, 1872) | - | N4 | ─ | ─ | CHIS, TAB |
| Pachychilus corpulentus F. G. Thompson, 1967 | E | N2 | ─ | C | TAMPS |
| Pachychilus corvinus (Morelet, 1849) | - | N4 | ─ | PG | TAB |
| Pachychilus dalli Pilsbry, 1896 | E | NU | ─ | ─ | OAX |
| Pachychilus glaphyrus (Morelet, 1849) | - | NU | ─ | PG | TAB |
| Pachychilus graphium (Morelet, 1849) | - | NU | ─ | ─ | YUC |
| Pachychilus hellerii (Brot, 1862) | - | NU | ─ | ─ | OAX, TAB |
| Pachychilus humerosus Pilsbry & Hinkley, 1910 | LE | N1 | ─ | C | SLP |
| Pachychilus indiorum (Morelet, 1849) | - | N4 | ─ | ─ | CHIS, OAX, VER |
| Pachychilus largillierti (Philippi, 1843) | - | N4 | ─ | PG | CHIS |
| Pachychilus larvatus (Brot, 1877) | - | NU | ─ | ─ | OAX |
| Pachychilus liebmanni (Philippi, 1848) | - | N4 | ─ | ─ | OAX, VER |
| Pachychilus moctezumensis (Pilsbry & Hinkley, 1910) | LE | N1 | ─ | C | SLP |
| Pachychilus pilsbryi von Martens, 1899 | E | N3 | ─ | PG | TAB |
| Pachychilus pleurotoma Pilsbry & Hinkley, 1910 | E | N3 | ─ | C | SLP |
| Pachychilus pluristriatus (Say, 1831) | E | N1 | ─ | C | SLP |
| Pachychilus potomarchus Pilsbry, 1892 | E | NU | ─ | PG | TAB |
| Pachychilus radix (Brot, 1872) | - | NU | ─ | ─ | CHIS |
| Pachychilus rasconensis Thiele, 1928 | E | NUQ | ─ | ─ | SLP |
| Pachychilus rubidus (Lea, 1856) | E | NUQ | ─ | ─ | SLP |
| Pachychilus saussurei (Brot, 1874) | E | NX | ─ | ─ | HGO, TAMPS |
| Pachychilus schiedeanus (Philippi, 1843) | E | N3 | ─ | PP | VER |
| Pachychilus schumoi Pilsbry, 1931 | - | N4 | ─ | ─ | CHIS, OAX, TAB |
| Pachychilus suturalis Pilsbry & Hinkley, 1910 | LE | N3 | ─ | C | SLP |
| Pachychilus tristis Pilsbry & Hinkley, 1910 | LE | N1 | ─ | C | SLP |
| Pachychilus turati (A. Villa & G. B. Villa, 1854) | LE | N2 | ─ | PP | VER |
| Pachychilus vallesensis Hinkley, 1907 | E | N4 | ─ | ─ | SLP, TAMPS |
| Family Pleuroceridae | |||||
| Lithasiopsis crassus Thompson, 1959 | LE | N3 | ─ | C | TAMPS |
| Lithasiopsis darnelli Thompson, 1959 | LE | N3 | ─ | C | TAMPS |
| Lithasiopsis hinkleyi Pilsbry, 1910 | LE | N2N3 | ─ | C | SLP |
| Lithasiopsis mexicanus Pilsbry, 1910 | LE | N2N3 | DD | C | SLP |
| Family Assimineidae | |||||
| Angustassiminea californica (Tryon, 1865) | - | N1 | ─ | ─ | COL |
| Assiminea cienegensis Hershler, Liu & Lang, 2007 | LE | N2N3 | ─ | B | COAH |
| Family Cochliopidae | |||||
| Aroapyrgus alleei Morrison, 1946 | - | N4 | ─ | ─ | COL, TAB |
| Aroapyrgus clenchi (Goodrich & Van der Schalie, 1937) | - | N3 | ─ | ─ | TAB |
| Aroapyrgus guatemalensis (Fischer & Crosse, 1891) | - | NU | ─ | ─ | VER |
| Aroapyrgus mexicanus (Pilsbry, 1910) | LE | N2 | ─ | C | SLP |
| Aroapyrgus orizabensis (Crosse & Fischer, 1891) | LE | NU | ─ | PP | VER |
| Aroapyrgus pasionensis (Goodrich & Van der Schalie, 1937) | - | N4 | ─ | ─ | TAB |
| Balconorbis sabinasense Czaja, Cardoza-Martínez& Estrada-Rodríguez, 2019 | LE | N2 | B | COAH | |
| Chorrobius crassilabrum Hershler, Liu & Landye, 2011 | LE | N1 | ─ | B | COAH |
| Coahuilix hubbsi Taylor, 1966 | LE | N2N3 | CR | B | COAH |
| Coahuilix landyei Hershler, 1985 | LE | N2N3 | ─ | B | COAH |
| Cochliopina compacta (Pilsbry, 1910) | LE | N1 | DD | C | SLP |
| Cochliopina francesae (Goodrich & Van der Schalie, 1937) | - | N3 | ─ | ─ | TAB |
| Cochliopina infundibulum (Martens, 1899) | - | N4 | ─ | ─ | CHIS, TAB |
| Cochliopina milleri Taylor, 1966 | LE | N2N3 | VU | B | COAH |
| Cochliopina picta (Pilsbry, 1910) | LE | N2 | ─ | C | SLP |
| Cochliopina riograndensis (Pilsbry & Ferriss, 1906) | - | N4 | ─ | ─ | COAH, SLP, TAMPS |
| Emmericiella longa (Pilsbry, 1909) | LE | N1 | ─ | C | SLP |
| Emmericiella novimundi (Pilsbry, 1909) | LE | N1 | ─ | C | SLP |
| Eremopyrgus elegans Hershler, Liu & Landye, 2002 | LE | N1 | ─ | A | CHIH |
| Juturnia coahuilae (Taylor, 1966) | LE | N2N3 | ─ | B | COAH |
| Littoridina crosseana (Pilsbry, 1910) | - | NU | ─ | ─ | TAB, TAMPS |
| Littoridina orcutti (Pilsbry, 1928) | E | NU | ─ | ─ | GRO, NAY |
| Littoridinops monroensis (Frauenfeld, 1863) | - | N5 | ─ | ─ | CAMP |
| Littoridinops tampicoensis (Pilsbry & Hinkley, 1907) | LE | NU | ─ | ─ | TAMPS |
| Littoridinops tenuipes (Couper, 1844) | - | N4 | ─ | ─ | TAB |
| Mexicenotica xochii Grego, Angyal & Liévano- Beltrán, 2019 | LE | N1 | S | YUC | |
| Mexipyrgus carranzae Taylor, 1966 | LE | N2N3 | DD | B | COAH |
| Mexithauma quadripaludium Taylor, 1966 | LE | N2N3 | ─ | B | COAH |
| Minckleyella balnearis Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A | CHIH |
| Paludiscala caramba Taylor, 1966 | LE | N2N3 | VU | B | COAH |
| Phreatoceras taylori (Hershler & Longley, 1986) | - | N2N3 | DD | ─ | COAH |
| Pseudotryonia mica Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Pseudotryonia pasajae Hershler, Liu & Landye, 2011 | LE | N1 | ─ | S | DGO |
| Pyrgophorus coronatus (Pfeiffer, 1840) | - | N4 | ─ | ─ | OAX, QR, TAB, TAMPS |
| Pyrgophorus cisterninus (Küster, 1852) | E | NU | ─ | ─ | CAMP, YUC |
| Pyrgophorus spinosus (Call & Pilsbry, 1886) | - | NU | ─ | ─ | CHIH, COAH |
| Pyrgophorus cenoticus Grego, Angyal & Beltrán, 2019 | LE | N1 | ─ | S | YUC |
| Tepalcatia bakeri (Pilsbry, 1891) | LE | N3 | ─ | ─ | MOR |
| Tepalcatia polia (Thompson & Hershler, 1991) | LE | N2 | ─ | S | OAX |
| Tepalcatia tela Thompson & Hershler, 2002 | LE | N3 | ─ | ─ | MICH |
| Texadina sphinctostoma (Abbott & Ladd, 1951) | - | NU | ─ | ─ | CAMP, VER |
| Tryonia allendae Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia angosturae Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia chuviscarae Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia contrerasi Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia dugesiana (Morrison, 1945) | LE | NU | ─ | ─ | MICH |
| Tryonia hertleini (Drake, 1956) | LE | NH | ─ | A, S | CHIH |
| Tryonia imitator (Pilsbry, 1899) | - | NH | DD | ─ | BC |
| Tryonia julimesensis Hershler, Liu & Landye, 2011 | LE | NH | ─ | A, S | CHIH |
| Tryonia mariae (Morrison, 1945) | LE | N1 | ─ | A, S | CDMX |
| Tryonia minckleyi Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia molinae Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia ovata Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia peregrina Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia pilsbryi (Morrison, 1945) | LE | NU | ─ | ─ | COL |
| Tryonia porrecta (Mighels, 1845) | - | NU | LC | ─ | SON (BCS?) |
| Tryonia santarosae Hershler, Landye, Liu, De la Maza-Benignos, Ornelas & Carson, 2014 | LE | NH | ─ | A, S | CHIH |
| Tryonia seemani (Frauenfeld, 1863) | LE | N1 | ─ | S | DGO |
| Tryonia shikueii Hershler, Landye, Liu, De la Maza-Benignos, Ornelas & Carson, 2014 | LE | NH | ─ | A, S | CHIH |
| Tryonia taylori Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Tryonia zaragozae Hershler, Liu & Landye, 2011 | LE | N1 | ─ | A, S | CHIH |
| Family Hydrobiidae | |||||
| Cincinnatia integra (Say, 1829) | - | N2N3 | LC | ─ | COAH, SLP |
| Ecrobia truncata (Vanatta, 1924) | - | N4 | ─ | ─ | TAB, TAMPS, VER |
| Pyrgulopsis acarinatus (Hershler, 1985) | LE | N2N3 | ─ | B | COAH |
| Pyrgulopsis bernardina (Taylor, 1987) | - | N1 | EN | ─ | SON |
| Pyrgulopsis brandi (Drake, 1953) | LE | NX | ─ | A, S | CHIH |
| Pyrgulopsis californiensis (Gregg & Taylor, 1965) | - | NU | ─ | ─ | BC |
| Pyrgulopsis cedrosensis (Pilsbry, 1927) | LE | NH | ─ | S | BC |
| Pyrgulopsis chihuahua (Pilsbry, 1928) | E | N1 | ─ | A | CHIH |
| Pyrgulopsis manantiali (Hershler, 1985) | LE | N2N3 | ─ | B | COAH |
| Pyrgulopsis minckleyi (Taylor, 1966) | LE | N2N3 | ─ | B | COAH |
| Pyrgulopsis palomasensis (Pilsbry, 1895) | LE | N1 | ─ | A, S | CHIH |
| Pyrgulopsis patzcuarensis Pilsbry, 1891 | LE | NH | ─ | S | MICH |
| Pyrgulopsis thompsoni Hershler, 1988 | - | N2 | NT | ─ | SON |
| Family Lithoglyphidae | |||||
| Phreatomascogos gregoi Czaja & Estrada- Rodríguez, 2019 | LE | N2 | B | COAH | |
| Pterides bisinulabris Pilsbry, 1909 | LE | N1 | ─ | C | SLP |
| Pterides pterostoma Pilsbry, 1909 | E | NU | ─ | ─ | SLP |
| Pterides rhabdus Pilsbry, 1909 | LE | N1 | ─ | C | SLP |
| Family Valvatidae | |||||
| Valvata beltrami Contreras-Arquieta, 1993 | LE | N1 | ─ | S | NL |
| Valvata humeralis Say, 1829 | - | N4 | LC | ─ | CDMX, MICH, SLP |
| Family Lymnaeidae | |||||
| Galba bulimoides (Lea, 1841) | - | N4 | LC | ─ | BC, SON, TAMPS |
| Galba cubensis (Pfeiffer, 1839) | - | N4 | LC | ─ | BC, PUE, SLP, VER |
| Galba humilis (Say, 1822) | - | N4Q | LC | ─ | COAH, DGO, NL, SON, |
| Galba modicella (Say, 1825) | - | N4 | LC | ─ | BC, CHIH, SON |
| Galba obrussa (Say, 1825) | - | N5 | ─ | ─ | AGS, BCS, CHIH, COAH, SON, ZAC |
| Galba viator (Orbigny, 1835) | - | N4 | ─ | ─ | CAMP, JAL, TAB, VER |
| Ladislavella elodes (Say, 1821) | - | N5 | LC | ─ | BC, BCS, CHIH, CDMX, COAH, DGO, HGO, MOR, NAY, NL, SIN, SLP, SON, YUC, ZAC |
| Lymnaea stagnalis (Linnaeus, 1758) | - | N4 | ─ | ─ | EDOMÉX, PUE |
| Pseudosuccinea columella (Say, 1817) | - | N5 | LC | ─ | EDOMÉX, MICH, NAY, PUE, TLAX |
| Family Physidae | |||||
| Amecanauta jaliscoensis Taylor, 2003 | LE | N1 | ─ | S | JAL, (NAY) |
| Austrinauta elatus (Gould, 1853) | LE | N1 | ─ | S | NAY |
| Chiapaphysa grijalvae Taylor, 2003 | E | N4 | ─ | ─ | CHIS |
| Mayabina bullula (Crosse & Fischer, 1882) | LE | N1 | ─ | PP, S | VER |
| Mayabina polita Taylor, 2003 | E | N4 | ─ | ─ | CAMP, CHIS, TAB, YUC |
| Mayabina spiculata (Morelet, 1849) | E | N4 | ─ | ─ | CAMP, QR, YUC |
| Mayabina tapanensis (Crosse & Fischer, 1882) | - | N4 | ─ | ─ | CHIS, OAX |
| Mexinauta aurantia (Carpenter, 1857) | - | N4 | ─ | ─ | JAL, GRO, SIN |
| Mexinauta impluviatus (Morelet, 1849) | - | N4 | DD | ─ | CHIS, QR, TAB |
| Mexinauta nitens (Philippi, 1841) | - | N4 | ─ | ─ | CAMP, TAB, TAMPS, VER |
| Mexinauta princeps (Philippi, 1846) | - | N4 | ─ | ─ | QR, YUC |
| Physella acuta (Draparnaud, 1805) | - | N5 | LC | ─ | AGS, COAH, DGO, PUE |
| Physella boucardi (Crosse and Fischer, 1881) | - | NUQ | ─ | ─ | EDOMÉX |
| Physella gyrina (Say, 1821) | - | N4 | LC | ─ | COAH, DGO |
| Physella mexicana (Philippi, 1841) | - | N5 | ─ | ─ | CAMP, CDMX, COL, GRO, MICH, OAX, TAB, VER |
| Physella patzcuarensis (Pilsbry, 1891) | LE | N2 | ─ | S | MICH |
| Physella solidissima (Pilsbry, 1920) | LE | N3 | ─ | S | JAL |
| Physella squalida (Morelet, 1851) | - | N4Q | ─ | ─ | TAB |
| Physella virgata (Gould, 1855) | - | N4N5Q | ─ | ─ | COAH, QRO, ZAC |
| Ultraphysella sinaloae Taylor, 2003 | E | N4 | DD | ─ | NAY, SIN |
| Family Planorbidae | |||||
| Antillorbis aeruginosus (Morelet, 1851) | - | N4 | ─ | ─ | QR, SON |
| Biomphalaria belizensis (Crosse & Fischer, 1878) | - | NU | ─ | ─ | TAB |
| Biomphalaria boucardianus (Preston, 1907) | E | NU | ─ | ─ | NAY |
| Biomphalaria gracilenta (Gould, 1855) | - | NU | ─ | ─ | BCS |
| Biomphalaria havanensis (Pfeiffer, 1839) | - | N5 | LC | ─ | COAH, CAMP, CHIS, DGO, NL, OAX, QR, PUE, SLP, TAMPS, TAB, VER, YUC |
| Biomphalaria helophila (d'Orbigny, 1835) | - | N4 | LC | ─ | TAB, VER |
| Biomphalaria orbicula (Morelet, 1849) | - | N5 | ─ | ─ | CAMP, CDMX, OAX, QR, SLP, TAB, TAMPS, VER, YUC |
| Biomphalaria petenensis (Morelet, 1851) | - | NU | ─ | ─ | OAX |
| Biomphalaria retusus (Morelet, 1849) | E | N2 | ─ | ─ | CAMP, YUC |
| Biomphalaria subprona (Von Martens, 1899) | - | N2 | ─ | ─ | CHIS |
| Biomphalaria tepicensis (Von Martens, 1899) | E | NU | ─ | ─ | NAY, OAX |
| Drepanotrema anatinum (d'Orbigny, 1835) | - | N4 | ─ | ─ | TAB |
| Drepanotrema cimex (Moricand, 1839) | - | N5 | LC | ─ | TAB, QR, BCS, TAMPS, SLP |
| Drepanotrema cultratum (d'Orbigny, 1841) | - | N5Q | ─ | ─ | BCS, QR, SLP, TAMPS, YUC |
| Drepanotrema depressissimum (Moricand, 1839) | - | N4 | ─ | ─ | TAB |
| Drepanotrema kermatoides (d'Orbigny, 1835) | - | N2 | ─ | ─ | QR |
| Drepanotrema lucidum (Pfeiffer, 1839) | - | N2 | ─ | ─ | YUC |
| Drepanotrema sumichrasti (Crosse & Fischer, 1879) | - | NU | ─ | ─ | OAX |
| Drepanotrema surinamense (Clessin, 1884) | - | NU | ─ | ─ | TAB |
| Ferrissia californica (Rowell, 1863) | - | N4 | LC | ─ | BCS, COAH, DGO |
| Ferrissia rivularis (Say, 1817) | N3 | LC | ─ | BCS, NL | |
| Gundlachia radiata (Guilding, 1828) | - | N4 | ─ | ─ | COAH, NL, TAMPS |
| Gyraulus circumstriatus (Tryon, 1866) | - | N4 | LC | ─ | COAH, NL |
| Gyraulus deflectus (Say, 1824) | - | N4 | LC | ─ | COAH, QR |
| Gyraulus parvus (Say, 1817) | - | N4N5 | LC | ─ | DGO, MOR, PUE, SON |
| Hebetancylus excentricus (Morelet, 1851) | - | N4N5 | ─ | ─ | COAH, DGO, MICH, TAB, TLAX, VER, YUC |
| Helisoma anceps (Menke, 1830) | - | N4N5 | LC | ─ | COAH, DGO, NL, SON |
| Laevapex papillaris (Von Martens, 1899) | E | N4 | ─ | ─ | EDOMÉX, JAL, MICH, NL |
| Laevapex sallei (Bourguignat, 1857) | LE | N1 | ─ | S | VER |
| Menetus dilatatus (Gould, 1841) | - | N3N4 | LC | ─ | DGO, VER, ZAC |
| Micromenetus brogniartianus (Lea, 1842) | - | N3Q | ─ | ─ | COAH |
| Planorbella contrerasi (Pilsbry, 1920) | LE | N3 | ─ | S | JAL |
| Planorbella duryi (Wetherby, 1879) | - | N4 | ─ | ─ | DGO, PUE, TAB |
| Planorbella foveale (Menke, 1830) | - | N4N5 | ─ | ─ | CAMP, CHIS, SON, VER, YUC |
| Planorbella tenue (Dunker, 1850) | - | N5 | ─ | ─ | BC, BCS, CDMX, CHIH, DGO, EDOMÉX, GTO, JAL, MICH, SIN, SLP, VER |
| Planorbella trivolvis (Say, 1817) | - | N4N5 | LC | ─ | PUE, QR, TLAX |
| Planorbula armigera (Say, 1821) | - | N3 | LC | ─ | QR |
To avoid repetitions, we have refrained from detailed data (coordinates) of each type locality. Most of these data are already available from the checklist of Thompson (2011), and new records are cited in the text.
To facilitate direct comparisons with the conservation status of freshwater gastropods of Canada and USA, we used the NatureServe Conservation Rank System of Master et al. (2012). This methodology, rather than the IUCN ranking criteria, is the most frequently used standard in North America. Each species was assigned a conservation status on a scale from critically imperiled (N1) to secure (N5) using the NatureServe Rank Calculator and methodology (Master et al., 2012). The calculator facilitates the process of assigning status ranks through automation (Faber-Langendoen et al., 2009). However, as with other ranking approaches (e.g., IUCN’s Red List), the NatureServe Ranking was designed primarily for vertebrates, and the coverage is less complete for invertebrates like mollusks. For example, there are often difficulties with assigning ranks in categories such as “population size” or “area of occupancy” for many invertebrates. Nevertheless, in our opinion, despite the mentioned disadvantages, the NatureServe Conservation Rank System is (at least for invertebrates) more appropriate than the IUCN system. However, our checklist also provides data of all Mexican species that are included in the IUCN system.
For most species we used 2 of the 3 categories (rarity and threats) to assess extinction risk because data on the category “trends” were rarely available. Before each assessment, we evaluated extensive secondary literature, especially current Conabio and Semarnat reports, from which information on specific habitat characteristics and current threats were obtained. Because direct visits to all the type and occurrence localities were not possible, we also analyzed Google Earth and World Imagery (Agis) images of each known type locality or occurrence site to evaluate changes in land use. These updates were necessary, especially in case of small bodies of water like “pozas” (springs) or “cenotes” (sinkholes), because many type and other localities have recently experienced major disturbances such as increased water diversions for irrigating crops. Some of the species were described from only a single location along small rivers or their tributaries, although a broader distribution is likely. In these cases, we presumed a wider distribution. Regarding the endemic species of the Cuatro Ciénegas valley, we adjusted status after the automatic rank assignation. These changes conform to the methodology that expressly recommends reviews and adjustments after the automatic calculator assignation (Faber-Langendoen et al., 2009). Further detailed data on distributions are required to determine the true conservation status of these species.
However, despite these efforts, we were unable to assign a conservation assessment to 35 species (18.1%) because of a lack of necessary data or taxonomic uncertainty. These species have not been recorded since the 19th or early 20th centuries and could therefore be assessed only as NU (unable to assign rank). Because some of these species occur in heavily modified regions, we expect that they will be at least vulnerable or even possibly extirpated. We emphasize that our conservation ranks are a preliminary approach that serve as a first phase in the development of conservation strategies for Mexican freshwater gastropods. More data are required, especially regarding areas of occupancy, short-term trends, population sizes, and intrinsic vulnerability.
Definition of endemism. Species restricted to Mexico were defined as endemic and taxa with an especially narrow distribution in small lakes, single springs or spring complexes, caves or parts of small streams were defined as local endemics. According to the definition of Bezaury-Creel et al. (2000), we defined hotspots as an “exceptional concentration of endemic species experiencing exceptional loss of habitat”. In the last 2 decades, the hotspot approach has been successfully applied especially in freshwater fish ecosystems and has enabled definition of various areas of conservation (Contreras-MacBeath et al., 2014; Mittermeier et al., 2011).
To demonstrate hotspots of endemicity, instead of maps with delimitation of state boundaries, we used a global map of biogeographic regionalization of the Earth’s freshwater systems (Abell et al., 2008). The use of ecoregions has an advantage because those areas mainly represent natural drainage basins that allow a direct comparison with hotspots of other aquatic organism such as bivalves and fishes. As important as these clusters of endemics are for conservation, we also call attention to sites with only 1 or 2 endemic species.
For the local endemics, data on their distributions were obtained from the above-mentioned available literature sources and spatial records, converted to a dot format (*.shp) using ArcGis 9.3.1. These distributions were subsequently superimposed on the 1:8,000,000 ecoregions map of México. The resulting areas with concentrated spatial records within the respective basins were marked as hotspots using Arcmap 9.3.1.
Abbreviations and symbols. The checklist (Table 1) includes the family, the taxonomic name of each species, information on endemicity or local endemicity, NatureServe-Rank-Conservations status, IUCN categories (assessed by IUCN) and the distribution (by state) in Mexico. NatureServe-Rank-Conservation status. N1 = Critically imperiled, N2 = imperiled, N3 = vulnerable, N4 = apparently secure, N5 = secure, NH = possibly extirpated, NX = presumed extirpated, NU = unable to assign rank, Q = questionable taxonomy. IUCN categories and assigned conservation status. DD = Data deficiency, EX = extinct, EW = extinct in the wild, LC = least concern, NT = near threatened, VU = vulnerable. State abbreviations: AGS = Aguascalientes, BC = Baja California, BCS = Baja California Sur, CAMP = Campeche, CDMX = Ciudad de México, CHIH = Chihuahua, CHIS = Chiapas, COAH = Coahuila, COL = Colima, DGO = Durango, EDOMEX = Estado de México, GTO = Guanajuato, GRO = Guerrero, HGO = Hidalgo, JAL = Jalisco, MICH = Michoacán, MOR = Morelos, NAY = Nayarit, NL = Nuevo León, OAX = Oaxaca, PUE = Puebla, QR = Querétaro, QRO = Quintana Roo, SLP = San Luis Potosí, SIN = Sinaloa, SON = Sonora, TAB = Tabasco, TAMPS = Tamaulipas, TLAX = Tlaxcala, VER = Veracruz, YUC = Yucatán, ZAC = Zacatecas. E = Endemic (Mexico), LE = local endemic, A, B, C = hotspots, PG = potential hotspot Grijalva, PP = potential hotspot Papaloapan, S = single site endemic.
Results
To increase our knowledge of freshwater species diversity, Lysne et al. (2008) proposed the establishment of local working groups. Given the extensive geography of Mexico, we agree with this proposal. The first sampling results of our working group with records from the states of Coahuila, Durango, Aguascalientes and Quintana Roo have been incorporated into the current analysis. The list of freshwater gastropod species of Mexico presented here comprises 193 native species distributed among 61 genera and 13 families (Table 1). We could assess the conservation status of 158 species but for the remaining 35 an assessment was not possible because of the lack of necessary data or taxonomic uncertainty.
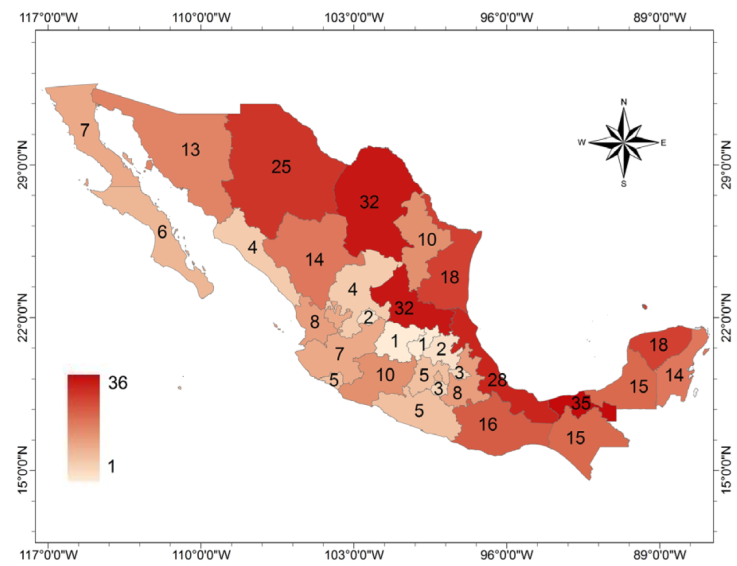
The number of records of native freshwater gastropods by state are shown in Figure 1. Since Mexico is still under-sampled, the totals at least partially reflect the current state of sampling rather than the real species diversity. States such as Tabasco (35 species), San Luis Potosí (32), Coahuila (32) and Veracruz (28) show a high diversity of known species probably because they have been more intensively sampled than states such as Guanajuato (1), Querétaro (1), Aguascalientes (2), or Hidalgo (2) where only a few records exist. However, this map suggests where future collections and studies are needed.
The Cochliopidae are the most diverse family with 61 species, followed by the Planorbidae (37) and Pachychilidae (31) (Table 2). Pachychilus is the most species-rich genus (31), followed by Tryonia (20) and Pyrgulopsis (11). The fauna contains 103 species endemic to Mexico. Of these endemics, 75 species have extremely limited distributions. Thirteen genera are endemic to Mexico, of which Amecanauta Taylor, 2003, Amnipila Pilsbry, 1956, Austrinauta Taylor, 2003, Chorrobius Hershler, Liu & Landye, 2011, Chiapaphysa Taylor, 2003, Mexicenotica Grego, Angyal and Liévano-Beltrán 2019, Mexipyrgus Taylor, 1966, Mexithauma Taylor, 1966, Minckleyella Hershler, Liu and Landye 2011, Paludiscala Taylor, 1966, Phreatomascogos Czaja and Estrada-Rodríguez 2019 and Ultraphysella Taylor, 2003, are monotypic; Coahuilix Taylor, 1966, has 2 species.
Table 2 Families, genera and the number of critically imperiled (N1), imperiled (N2), vulnerable (N3), possibly extirpated (NH), presumed extirpated (NX) and endemic Mexican freshwater gastropods.
| Family | Genera | Species | N1, N2, N3, NH, NX Species |
Endemic |
|---|---|---|---|---|
| Ampullariidae | 1 | 5 | 1 | 4 |
| Assimineidae | 2 | 2 | 2 | 1 |
| Cochliopidae | 21 | 61 | 42 | 44 |
| Hydrobiidae | 3 | 13 | 11 | 8 |
| Lithoglyphidae | 2 | 3 | 2 | 3 |
| Lymnaeidae | 4 | 9 | 0 | 0 |
| Neritidae | 3 | 4 | 0 | 0 |
| Pachychilidae | 2 | 31 | 14 | 20 |
| Physidae | 8 | 21 | 5 | 9 |
| Planorbidae | 12 | 37 | 11 | 8 |
| Pleuroceridae | 1 | 4 | 4 | 4 |
| Valvatidae | 1 | 2 | 1 | 1 |
| Viviparidae | 1 | 1 | 1 | 1 |
| Total | 61 | 193 | 94 | 103 |
Ladislavella elodes (Say, 1821) (Lymnaeidae) is the most widely distributed native species, occurring in 14 states, from Baja California to the Yucatán Peninsula. Other widely represented species are Planorbella tenue (Dunker, 1850), Biomphalaria havanensis (Pfeiffer, 1839), Biomphalaria orbicula (Morelet, 1849) (all Planorbidae) and Physella mexicana (Philippi, 1841) (Physidae). The most extensively distributed Mexican freshwater species is the non-native snail Melanoides tuberculata (Müller, 1774) (Thiaridae), which, as a non-native species, is not included in the checklist. This species lives in almost every freshwater site in the country (Naranjo-García & Olivera-Carrasco, 2014; Naranjo-García & Castillo-Rodríguez, 2017).
Of 193 native freshwater gastropods considered to be present in Mexico, 9 (4.7%) species are possibly or presumably extirpated, 40 (20.7%) are critically imperiled, 30 (15.5%) are imperiled, 15 (7.8%) are vulnerable and 64 (33.2%) are currently stable (Fig. 2).
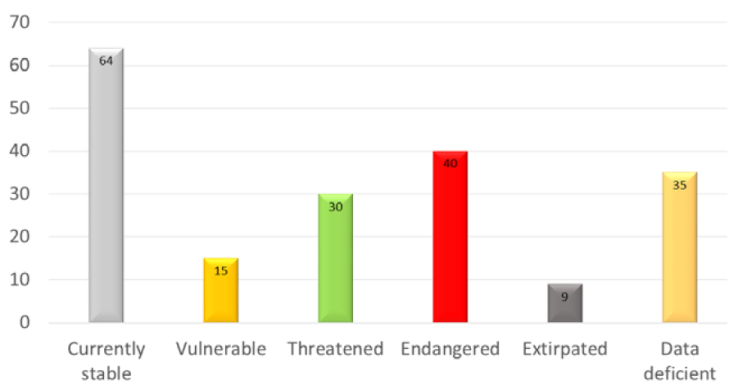
Figure 2 Conservation statuses for Mexican freshwater gastropods based on the NatureServe conservation rank system by Master et al. (2012).
Of the entire Mexican freshwater snail fauna, almost half (48.7%, 94 species) of the species are critically imperiled, imperiled, vulnerable or possibly/presumably extirpated. This general relationship of imperilment for Mexican snails is similar to that in the USA and Canada. Nevertheless, the percentage of Mexican endangered snails is lower than that of the USA and Canada, where almost 3 quarters are imperiled, critically imperiled or vulnerable (Johnson et al., 2013). This difference may be due to the inaccuracy of some of the sources, or because a large number (20%) of Mexican species are currently without a conservation rank. The most important difference is probably in terms of national conservation status designations among these 3 countries. While in the USA and Canada 74% of all species are imperiled, critically imperiled or vulnerable, in Mexico, so far, only 7 species (3.6%), all from the Cuatro Ciénegas basin, have been officially assigned as vulnerable (Semarnat, 2010).
Figure 3 shows the number of imperiled, critically imperiled or vulnerable species compared to the total number of species for all 13 freshwater gastropod families in Mexico. The Cochliopidae contains by far the highest number of endangered species of all families. This result is consistent with the data from the USA and Canada and the worldwide trend of hydrobioid families being the most imperiled of all gastropod families (Johnson et al., 2013; Miller et al., 2018). In Mexico, of all the 94 imperiled freshwater gastropods, 51 (54.3%) are hydrobioid species. This concentration indicates that the Cochliopidae and Hydrobiidae are a clear focus for needed conservation. The members of these families frequently have extremely restricted ranges in small habitats and specialized ecological niches.
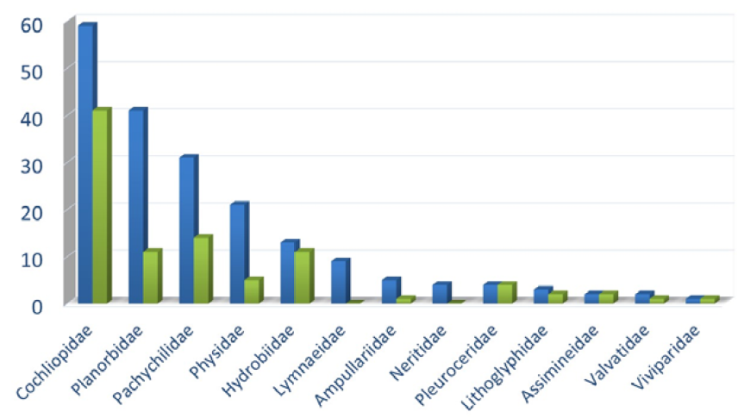
Figure 3 The number of critically imperiled, imperiled and vulnerable species (green) relative to the total number of species (blue) of freshwater gastropod families in Mexico.
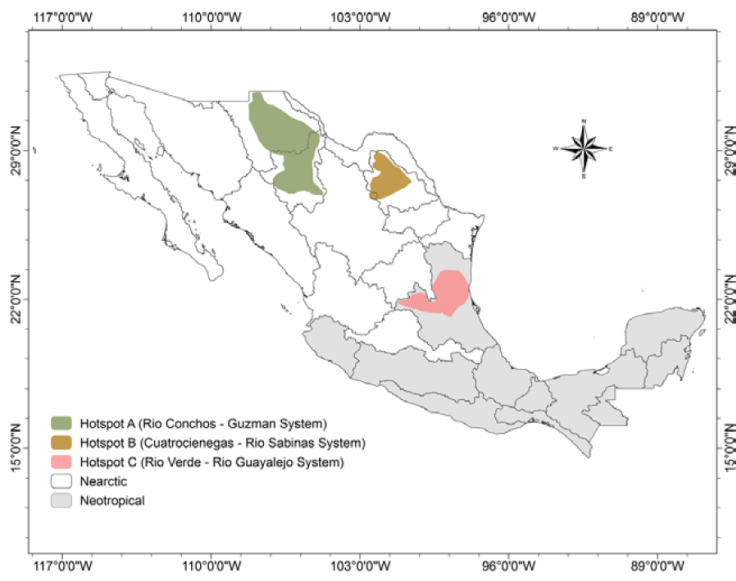
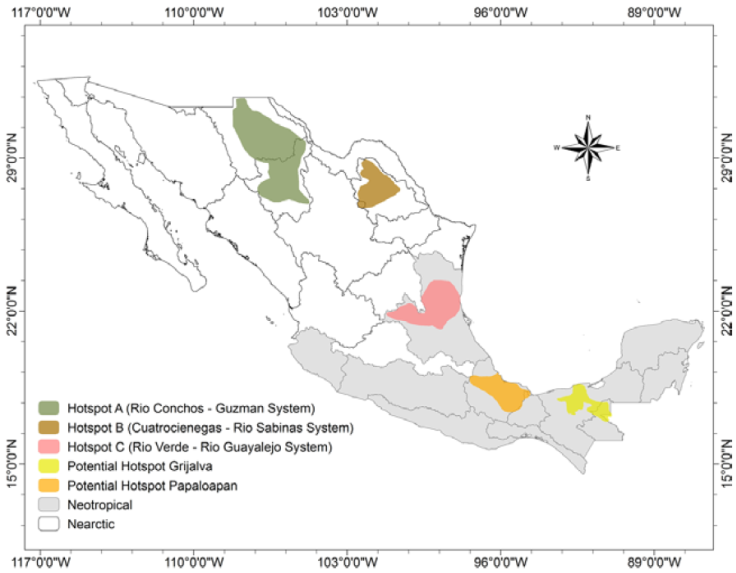
The geographical analysis of conservation ranks and distributions of all species in the checklist allowed us to define 3 hotspots of freshwater gastropod endemicity in Mexico (Fig. 4): hotspot A, Río Conchos - Guzmán Basin System (Chihuahua); hotspot B, Cuatro Ciénegas-Río Sabinas System (Coahuila); hotspot C, Río Verde - Río Guayalejo System (San Luis Potosí, Tamaulipas, Veracruz). Hotspots A and B are located in the Chihuahuan Desert. Hotspot C is in the less arid, central-eastern part of the country within the Pánuco ecoregion. Of the 75 local endemic species, 59 (78.7%) live within these hotspots.
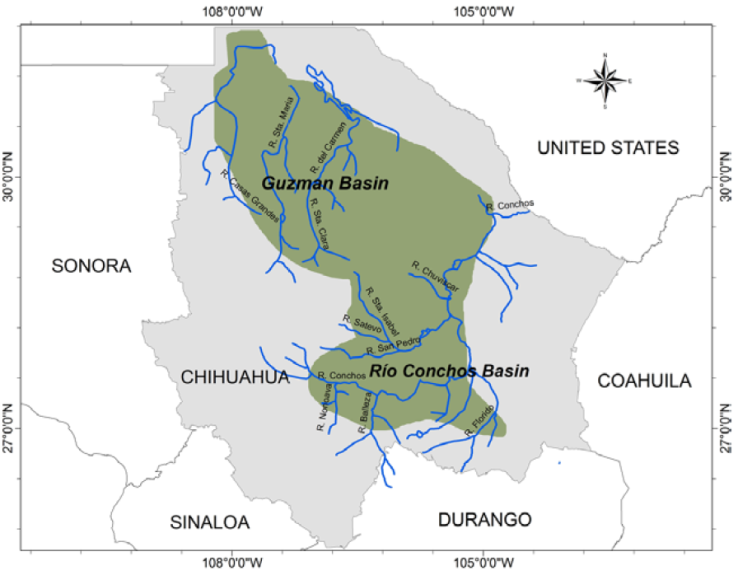
Hotspot A - Río Conchos-Guzman Basin System. Hershler et al. (2011) used the designation “insular habitat patch” in the Chihuahuan Desert for hotspot A (Fig. 5). The main streams are Río Conchos, Río Florido, Río Chuviscar, Río El Álamo, Río Parral, Río Balleza, and Río San Pedro in the Río Conchos basin, and Río Casas Grandes, Río Santa María, Río Santa Clara, Río Carmen, and several small springs, in the Guzmán basin. Of the freshwater biota, only the ichthyofauna -which also shows a high level of endemism (Contreras-MacBeath et al., 2014) - and the hydrobioid snails have been studied intensively in both basins. Of the springsnail genus Tryonia, 11 local endemic species were described from this region by Hershler et al. (2011). Hotspot A contains the highest number of Mexican freshwater gastropods with the highest conservation rank (15 species critically imperiled, N1). Some species such as Tryonia julimesensis or T. santarosae were already at the time of their description thought possibly to be extinct. Hershler et al. (2011) even considered the whole endemic springsnail fauna of the region to be at risk of extirpation.
Many type localities are aquifer-fed springs located in areas that are highly imperiled through human activities such as intensive agriculture, overexploitation of groundwater resources and increased mining (Arriaga-Cabrera et al., 2000; our own observations). These threats, associated with the snails’ limited vagility and narrow environmental tolerances, make springsnails extremely vulnerable (Burroughs et al., 2017; Hershler et al., 2014). Restricting groundwater extraction in hotspot A is therefore urgently needed. We propose that the region of hotspot A should have the highest priority for conservation in Mexico. This recommendation is supported by the assessment of the whole freshwater ecoregion of Río Conchos as of “critical” (the highest category) conservation status by Abell et al. (2000) and as “endangered” by Olson et al. (1998) based on fish faunas. Bezaury-Creel et al. (2000) considered the region as one with the highest conservation priority of all freshwater habitats in Mexico. In 2008, the lower and upper drainage basins of the Río Conchos and the upper basins of Río Carmen and Río María were designated as priority hydrological regions with biodiversity at high risk (Bezaury-Creel et al., 2017; Conabio, 2008). Twenty-one endemic species, mainly Cochliopidae, occur in hotspot A.
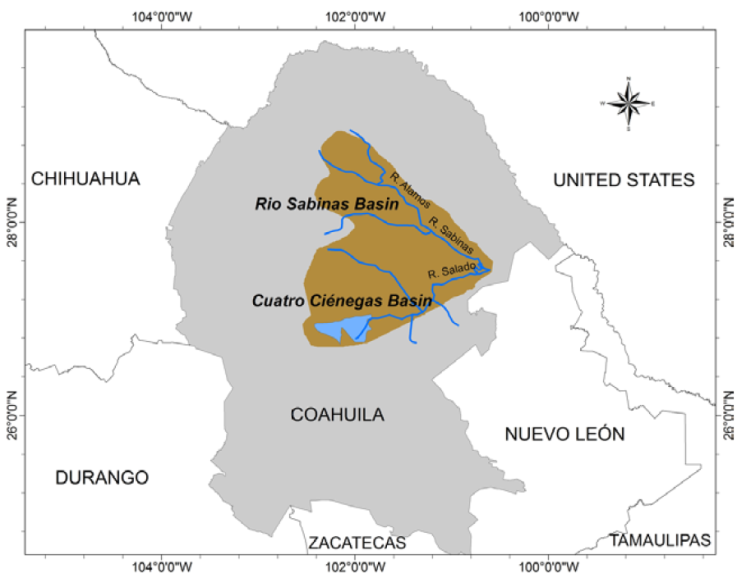
Hotspot B - Cuatro Ciénegas-Río Sabinas System. The small Cuatro Ciénegas valley was identified as one of 25 worldwide hotspots of gastropod diversity by Strong et al. (2008). All 7 Mexican freshwater species listed as endangered by the Mexican Secretary of Environment and Natural Resources (Semarnat, 2010) occur in this small basin (Fig. 6).

Photograph: J. L. Estrada-Rodríguez, May, 2017
Figure 6 Poza Becerra in Cuatro Ciénegas, Coahuila, Mexico. An endangered oasis with freshwater stromatolites and endemic gastropods in the Chihuahuan Desert.
We increased the geographical area of this hotspot (Fig. 7) because various endemic species have been described from adjacent locations near Cuatro Ciénegas. Other species occur in the neighboring Río Sabinas basin (Phreatomascogos gregoi and Balconorbis sabinasensis; Czaja, Cardoza-Martínez et al., 2019). Moreover, both basins form a common system that formerly had drained directly toward the Río Grande. Also, the ichthyofaunas of both basins are very similar (M.C. Fernando Alonso Rojo, pers. comm.). The wetlands of the Cuatro Ciénegas and Río Sabinas drainage basins are RAMSAR sites of international importance (Bezaury-Creel et al., 2017). Typical habitats of hotspot B are small springs (pozas), streams such as Río Sabinas, Río Salado de los Nadadores, Río Álamos, Río Monclova and underground aquifers containing stygobiont snails (species of Coahuilix and Paludiscala). The small core of hotspot B, the Cuatro Ciénegas valley (approx. 1,000 km2), is a complex of more than 700 springs, lakes, marshes and streams where 12 species (9 endemics) occur. This site is malacologically one of the extensively studied locations in North America because of the evolution of its snails and their interactions with freshwater stromatolites (Breitbart et al., 2009; Chaves-Campos et al., 2011; Elser et al., 2005; Hershler, 1985; Hershler & Minckley, 1986; Johnson et al., 2007; Taylor, 1966). Cuatro Ciénegas has also the highest richness of endemic fish species in Mexico among which some co-evolved with hydrobioid snails such as Mexipyrgus carranzae, Mexithauma quadripaludium and Pyrgulopsis minckleyi (Contreras-MacBeath et al., 2014; Covich, 2010). If the snails went extinct, this could be detrimental for the fishes because of their specialized relationships. There are many cases of co-extinctions or secondary extinctions in which the loss of one species leads to the loss of another (Brodie et al., 2014; Modesto et al., 2017). Nevertheless, although an extensive decline of one taxon can cause negative impacts in other groups, the complexities of possible co-extinction patterns among organisms are still poorly understood (Modesto et al., 2017).
Unfortunately, human activities, especially groundwater pumping, agriculture and non-native species continue to endanger the ecosystems of Cuatro Ciénegas and endemic species such as Juturnia coahuilae, Paludiscala caramba and Phreatoceras taylori have become rare in these springs (our own observations since 2009). Despite its small size, the valley was designated as a separate freshwater ecoregion with the highest level of global and regional conservation priority and a globally outstanding biological distinctiveness (Bezaury-Creel et al., 2000). Nevertheless, only Paludiscala caramba, Cochliopina milleri and Coahuilix hubbsi from Cuatro Ciénegas have been assessed in the IUCN Red List as vulnerable or critically endangered. Also, the NatureServe rank calculator assigned a similar rank to that assigned by IUCN, but we assigned to all endemic species of Cuatro Ciénegas a higher conservation rank (N2,N3) because although most populations seem stable, parts of the entire ecosystem are at risk (De la Maza-Benignos, 2017). This is in accordance with the methodology that expressly recommends reviews and adjustments after the automatic calculator assignation (Faber-Langendoen et al., 2009). At least 12 endemic species, mostly cochliopids, occur in hotspot B.
Hotspot C - Río Verde - Río Guayalejo System. Hotspot C, which contains the Río Verde and Río Guayalejo basins, is located in eastern Mexico in San Luis Potosí and Tamaulipas states, and includes the first and second-order tributaries of these rivers (Río Moctezuma, Río Tampaón, Río Tamasopo, Río Choy, Río del Cojo) (Fig. 8). The hotspot hosts a high number of endemic snail species (21), more than in hotspot A or B. Eleven of 30 Mexican Pachychilus species are restricted to this system and the family Pachychilidae is also the dominant family, followed by Cochliopidae and Hydrobiidae.
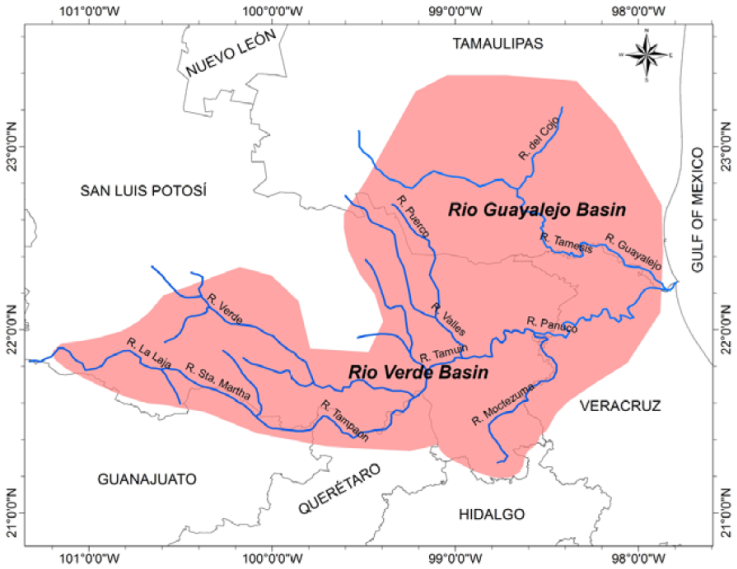
Figure 8 Endemicity hotspot C: Río Verde - Río Guayalejo system, San Luis Potosí, Tamaulipas and Veracruz.
Typical habitats are small streams but a few springs and caves are the type localities of endemic stygobiont cave snails (species of Emmericiella Pilsbry, 1909, and Pterides Pilsbry, 1909). Although the ranges of the species of this hotspot are generally wider than these both Chihuahuan Desert hotspots, the Río Verde-Río-Guayalejo basin is far more populated and therefore more imperiled by human activities, especially agriculture and industry. Only the headwaters of the Río Verde appear relatively undisturbed by human activities and their conservation statuses were assessed as “stable” by Abell et al. (2000). However, the remaining rivers of the Río Verde-Río Guayalejo basin are some of the most contaminated rivers in México (Arriaga-Cabrera et al., 2000). Aguilar et al. (2010) assigned one of the highest (extreme) levels of conservation priority of all freshwater habitats in Mexico to the whole Pánuco ecoregion.
Single site endemics. Thirty-nine local endemics are restricted to single sites within and outside these hotspots. Such endemic species with an extremely limited distribution occur throughout Mexico (Fig. 9). Here too, the Cochliopidae dominate together with planorbids such as Planorbella contrerasi (Pilsbry, 1920) and Physella solidissima (Pilsbry, 1920) (Physidae), which occur only in the Lago de Chapala, Jalisco. While some springsnails of the genera Pyrgulopsis and Tryonia occur in springs that are only a few meters in diameter, others, such as Pomacea catemacensis, Physella solidissima or Planorbella contreras live in large lakes (Lago de Catemaco, Veracruz and Lago de Chapala, Jalisco).
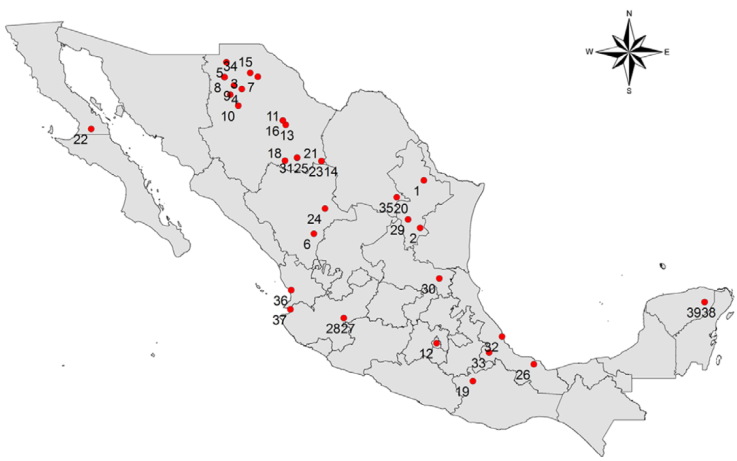
Figure 9 Mexican freshwater gastropods single site endemics.
1. Viviparus inornatus (Viviparidae), 2. Valvata beltrami (Valvatidae), 3. Tryonia zaragozae (Cochliopidae), 4. Tryonia taylori (Cochliopidae), 5. Tryonia shikueii (Cochliopidae), 6. Tryonia seemani (Cochliopidae), 7. Tryonia santarosae (Cochliopidae), 8. Tryonia peregrina (Cochliopidae), 9. Tryonia ovata (Cochliopidae), 10. Tryonia molinae (Cochliopidae), 11. Tryonia minckleyi (Cochliopidae), 12. Tryonia mariae (Cochliopidae), 13. Tryonia julimesensis (Cochliopidae), 14. Tryonia hertleini (Cochliopidae), 15. Tryonia contrerasi (Cochliopidae), 16. Tryonia chuviscarae (Cochliopidae), 17. Tryonia angosturae (Cochliopidae), 18. Tryonia allendae (Cochliopidae), 19. Tepalcatia polia (Cochliopidae), 20. Pyrgulopsis patzcuarensis (Hydrobiidae), 21. Pyrgulopsis palomasensis (Hydrobiidae), 22. Pyrgulopsis cedrosensis (Hydrobiidae), 23. Pyrgulopsis brandi (Hydrobiidae), 24. Pseudotryonia pasajae (Cochliopidae), 25. Pseudotryonia mica (Cochliopidae), 26. Pomacea catemacensis (Ampullariidae), 27. Planorbella contrerasi (Planorbidae), 28. Physella solidissima (Physidae), 29. Physella patzcuarensis (Physidae), 30. Pachychilus apheles (Pachychilidae), 31. Minckleyella balnearis (Cochliopidae), 32. Mayabina bullula (Physidae), 33. Laevapex sallei (Planorbidae), 34. Eremopyrgus elegans (Cochliopidae), 35. Chorrobius crassilabrum (Cochliopidae), 36. Austrinauta elatus (Physidae), 37. Amecanauta jaliscoensis (Physidae), 38. Mexicenotica xochii (Cochliopidae), 39. Pyrgophorus cenoticus (Cochliopidae).
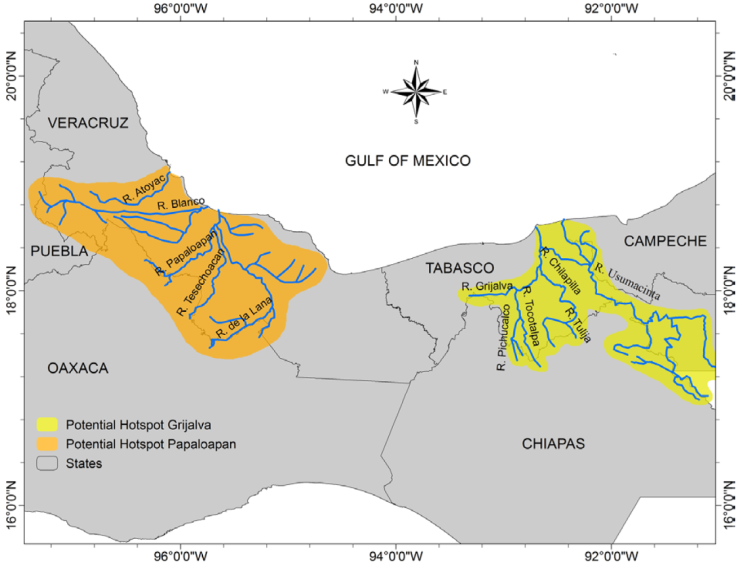
We are convinced that in the near future additional centers of freshwater snail diversity and endemism will be identified in the southern regions of Mexico. Thompson (2011) already pointed out the existence of such potential centers of land and freshwater snails in southern and western Mexico. The cores of such hotspots are already recognizable in the states of Tabasco and Veracruz, where the diversity is relatively high and various sites support local endemics (Fig. 10). For example, many Pachychilus species such as P. corvinus, P. glaphyrus, P. pilsbryi, and P. potomarchus, occur in the Río Grijalva-Río Usumacinta region, Tabasco, but almost all were described in the 19th century based only on morphological criteria of the shell and we have poor information on their distribution. Therefore, they have not received a conservation rank in the checklist but are surely local endemics. A molecular genetic examination of these taxa and more information on their distributions are urgently needed (M. Glaubrecht, personal communication). Every new study of the malacofauna shows that the aquatic ecosystems of this potential hotspot contain species-rich snail assemblages (e.g., Albarrán-Mélzer et al., 2017).
Also, the basin of Río Papaloapan with its tributaries in Veracruz (Fig. 11) could be a further hotspot if more detailed data on species distributions become available. Rare endemic species such as Pachychilus turati, P. schiedeanus, Mayabina bullula, Aroapyrgus orizabensis were described from streams and lakes of this under sampled region. Both potential hotspots with their rivers, marshes, estuaries and lagoons are generally less contaminated and have a lower human population than hotspots A, B and C (Contreras-MacBeath et al., 2014). However, besides the localized endemicity hotspots and potential centers of species diversity, many freshwater locations in Mexico are malacologically still unexplored. There are almost no records of mollusks from the important wetlands of Guerrero (rivers: Atoyac, Papagayo, and Ometepec), Oaxaca (Río Verde), Nayarit (San Blas-La Tovara wetlands) and Sinaloa (Río Baluarte), although these sites are known as ecosystems with high diversity of aquatic species (Bezaury-Creel et al., 2017; Conabio, 2008). Almost completely unexplored are the caves and subterranean habitats in the northern and southern (Yucatán Peninsula) part of the country (Grego et al., 2019). Only a few cave-dwelling and subterranean species of Paludiscala, Balconorbis, Coahuilix, Phreatoceras, Emmericiella, Phreatomascogos and Pterides have been reported from the extensive karst regions of Coahuila and San Luis Potosí states. In contrast, similar karst sites in Texas, USA, contain very rich phreatic snail assemblages (Hershler & Longley, 1986). Also, hundreds of “cenotes” (sinkholes) and caves in the Chiapas highlands and Yucatán Peninsula are probably good habitats for subterranean aquatic fauna, although only 1 gastropod, Pyrgophorus coronatus, a brackish water species, has been reported from these regions (Palacios-Vargas et al., 2015).
Biogeographically, Mexico is complex because 2 ecoregions overlap: the Nearctic in the north and Neotropical in the central and southern part. Hotspots A and B are in the Nearctic ecoregion, hotspot C is within the transitional zone to the Neotropical ecoregion (Figs. 4, 10). These 2 biogeographic ecoregions are reflected by the dominant taxa in the hotspots. In the Nearctic regions the dominant hydrobioid families of hotspots A and B contain species that mainly have present-day Nearctic distributions. In hotspot C (and in both additional potential centers of endemism) more families with a predominantly Neotropical distribution are found, such as the Pachychilidae and Ampullariidae. However, the transitional character of this hotspot is also reflected in the still-prevailing presence of Nearctic genera such as Cochliopina Morrison, 1946 (Cochliopidae), Lithasiopsis Pilsbry, 1910 (Pleuroceridae) and Cincinnatia Pilsbry, 1891 (Hydrobiidae). In hotspot C, Cincinnatia is at its most southern limit in North America (Czaja, Estrada-Rodríguez, Romero-Méndez, Estrada-Arellano et. al., 2017; Hershler & Thompson, 1996). Similar transitional patterns in the fish faunas have been reported in this region (Miller, 2005).
The degrees of endemicity of all hotspots are relatively low. This may be a result of the relatively short time in which these systems have been fragmented, and this seems to be confirmed by paleomalacological studies in the northern part of Mexico, where several locally restricted endemics had much wider distributions, and the now separate hotspots A and B in the middle Holocene were once connected (Czaja, Estrada-Rodríguez et al., 2014; Czaja, Estrada-Rodríguez, Romero-Méndez & Orona-Espino, 2017; Czaja, Estrada-Rodríguez, Romero-Méndez, Estrada-Arellano et al., 2017; Czaja, Estrada-Rodríguez, Romero-Méndez, Ávila-Rodríguez et al., 2017; Czaja, Palacios-Fest et al., 2014). The final division of the originally united region into the 2 present-day hotspots probably occurred in the latest Holocene, or even in historical times (Czaja, Covich et al., 2019). These transitional regions between the current hotspots support a uniform gastropod fauna of a small set of ubiquitous species, mainly planorbids and physids, known from many other similarly affected regions of the world (Neubauer et al., 2015). The key to understanding the present-day freshwater gastropod biogeography of Mexico lies probably also in the dramatic climatic changes during the middle and late Holocene.
Discussion
The principal threats faced by Mexican mollusks are, not surprisingly, the same as those in many other parts of the world and caused mainly by the same anthropogenic activities (Dudgeon et al., 2006: summarized by Köhler et al., 2012): 1) dam construction and other flow modifications (Figs. 12, 13), 2) water pollution mainly from inappropriate agricultural and industrial activities, 3) overexploitation of aquifers, 4) habitat degradation, and 5) the introduction of non-native species. All 5 interacting threats affect the freshwater ecosystems of the 3 hotspots. An special case in the transition area between hotspots A and B in northern Mexico with dramatic declines in diversity of freshwater gastropods was recently reported by Czaja, Covich et al. (2019). The impacts occurred mainly during the 20th Century and were so catastrophic that we can now call the region a "ghost" center of endemism (in the sense of Contreras-MacBaeth et al. [2014]), where 84% of the gastropod species of the springs and marshes in the area of Viesca was extirpated within a short time. Dam construction and overexploitation of surface and ground waters were the main causes for these local extinctions (Wolfe, 2013).

Photograph: J. L. Estrada-Rodríguez, May, 2017
Figure 12 River regulation (canalization) of the Río Nazas with urban pollution, near the city of Torreón, Coahuila, Mexico.

Photograph: J. L. Estrada-Rodríguez, May, 2017
Figure 13 Habitat loss as a consequence of dam construction on the Río Nazas, Torreón, Coahuila, Mexico.
A particularly dramatic case of dam construction and flow modification in the past century in the La Laguna region of Coahuila and Durango was mentioned above. Although almost all major rivers in Mexico have dams (840 major dams, according to Martínez-Yrízar et al., 2012), there have been no studies on their impacts on gastropod communities.
According to Köhler et al. (2012), climatic change, including shifts in the amount and timing of precipitation, are superimposed on the 5 main threats they emphasized. Such climatic impacts may apply especially to the spring habitats of hotspots A and B that are in one of the hottest and driest regions of Mexico where increased evapotranspiration rates and smaller aquifer recharge are expected in the future (Burroughs et al., 2017). Heat waves with maximum temperature of 50 °C, which have frequently occurred in the last decade in northern Mexico, may also be important in raising water temperatures that affect species metabolism and ecosystem processes. These atmospheric-based phenomena are affecting many freshwater ecosystems in North America (Blunden et al., 2018).
Almost all endemic species of hotspots A and B are cochliopid and hydrobiid springsnails of the genera Pyrgulopsis and Tryonia that occur in springs or spring systems maintained by groundwater. Natural or anthropogenically caused fluctuations of the water flow, groundwater pumping and other spring modifications impact directly the occurrence and abundance of springsnails by changing important environmental variables such as water temperature, pH and dissolved oxygen and will be the main stressors over the next 50 years for springsnail’s habitats (Burroughs et al., 2017).
Severe negative impacts on native gastropods in at least 2 of the 3 hotspots, have resulted from invasive species, especially Melanoides tuberculata (Müller, 1774) (Thiaridae), which is distributed widely in freshwater sites in Mexico (Naranjo-García & Olivera-Carrasco, 2014). This species may be able to displace native species and affect the integrity of native ecosystems (Burroughs et al., 2017; das Chagas et al., 2018; Hershler, 1998; Murray, 1971). However, its impact was never proven definitely. Nevertheless, our observations since 2014 in rivers and springs of hotspot A demonstrate that the presence of M. tuberculata is positively correlated with the absence of native species, especially of hydrobioid snails. In contrast, M. tuberculata was first observed in the springs of Cuatro Ciénegas in 1994 (Contreras-Arquieta, 1998) but has not yet had any detectable negative impacts (Dinger, 2001; Dinger et al., 2006; our observations since 2014). However, the impact of the introduced African cichlid fish, Hemichromis guttatus Günther, 1862, in these small spring-fed habitats may perhaps affect native snails as well as native fishes (Marks et al., 2011).
Invasive aquatic plants may also have a detrimental effect on gastropod communities. For example, Eichhornia crassipes (Mart.) Solms, Pistia striatiotes L. and Salvinia natans L. can favor generalist species, especially planorbids and lymnaeids (Aravind et al., 2011). Eichhornia crassipes adversely affects native plant species in Cuatro Ciénegas (Vela-Coiffier et al., 2015), and an unusual growth of an invasive algae has negatively impacted the freshwater communities of Churince spring in Cuatro Ciénegas (De la Maza-Benignos, 2017).
A successful snail conservation program begins with understanding which taxa are present in the area (Lysne et al., 2008). Inconsistent taxonomy hinders conservation efforts and can lead to premature or incorrect decisions as well as inefficient use of scarce financial resources. The systematic revisions are incomplete for almost all Mexican gastropods, especially for those taxa described in the 19th century and at the beginning of the 20th century. While the Cochliopidae, Hydrobiidae and Ampullariidae are reasonably well studied, most Pachychilidae and almost all Planorbidae, Valvatidae and Lymnaeidae require further analysis using molecular genetic approaches.
Almost all snails from hotspots A and B share their habitat with endemic fishes, for which there has long been a conservation focus (Contreras-MacBeath et al., 2014). For example, in recent decades, great efforts have been made to protect endemic freshwater fishes of Cuatro Ciénegas (Carson et al., 2013; De la Maza-Benignos, 2017), which will, ultimately, also benefit the endemic snails.
Presently, there is not a single biological monitoring program or conservation plan for sustaining gastropod diversity in Mexico, not even in Cuatro Ciénegas. We do not know the population structure or population size of any species of Mexican freshwater snail. Only a small amount of basic biological information is available. There is almost no information about the impact of invasive species on the native malacofauna. Without this information and additional data on multiple environmental stressors, we do not know how the native snail species will withstand stochastic events, or if the species have sufficiently robust populations to withstand frequent catastrophic events (Burroughs et al., 2017).
De la Maza-Benignos (2017) also recommended active management both in situ and ex situ in some of the springs of Cuatro Ciénegas that are nearly in a state of ecological collapse (including the Churince spring, type locality of Mexipyrgus carranzae, one of the most emblematic endemic freshwater snails of Mexico (Fig. 14).

Photograph: J.L. Estrada-Rodríguez, May 2018
Figure 14 Churince spring, type locality of Mexipyrgus carranzae Taylor, 1966 (Cochliopidae). Cuatro Ciénegas Basin, Coahuila, Mexico.
Although translocation is one possible option to prevent impending extinction, especially in the face of complete destruction of the habitat, assisted dispersal of species at risk of extinction probably will not be effective if no ecological data are available to provide a sound basis for evaluating the potential for success. The benefits and potential risks must be carefully weighed for each species. Captive maintenance studies to increase population numbers and genetic variability as well as feeding relationships should precede any translocation experiments (Funkhouser, 2014). To carry out such studies, an area should be established for the conservation, protection, experimentation and reproduction of the endemic species, as already proposed by De la Maza-Benignos (2017) for fishes of Cuatro Cienégas.
Of the 193 reported freshwater gastropods, only 7 (3.6%) are currently listed officially as endangered (Semarnat, 2010). Three highly localized hotspots of endemicity are identified in the north and 2 potential hotspots in the southeast of Mexico. These hotspots include a high number of narrow endemic species with the highest conservation rank. These river, lake and spring habitats should be the highest priority in future conservation efforts. However, we consider, in addition to classical (passive) conservation measures, active management and experiments will be necessary to provide sufficient information for successful species translocation. Sustaining species and genetic diversity is especially important in the northern hotspots, because of the current degree of anthropogenic habitat destruction in this region. The information in the present paper can serve as a first step for the development of a program for in situ and ex situ management for the most threatened freshwater snails of Mexico.











 text new page (beta)
text new page (beta)