Introduction
Atrial fibrillation (AF) is the most common arrhythmia. It is estimated that there are 43 million people affected worldwide, and its prevalence is 600 to 900 cases per 100,000 persons; thus, the risk of having AF in people older than 55 years is about 1 in 3.1 The current numbers suggest that there will be 5 million new cases every year in the world.2,3
There have been significant advances in the prevention of embolic events and rhythm control strategies. Nonetheless, few efforts have been made to reduce AF’s prevalence, progression and impact.1-3 Although AF can be present in young individuals without apparent heart disease, it has been acknowledged that its prevalence increases with aging and is related to multiple common risk factors such as obesity, sedentarism,1 stress, long working hours,4 alcohol intake and tobacco use.3 Atrial fibrillation is also associated with other co-morbidities such as high blood pressure (HBP), diabetes mellitus (DM), heart failure (HF), sleep apnea, cardio-metabolic syndrome (CMS),3 chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), ischemic heart disease (IHD), stroke,1 fatty liver,5 dyslipidemia, hypothyroidism,6 hypertrophic cardiomyopathy1 and peripheral artery disease (PAD).7
Aging and added risk factors and co-morbidities increase the chances of having AF.3,6 The significant association of AF with multiple risk factors has been proven, especially with HF in both men and women.8 Atrial fibrillation is a high-impact condition because it has higher morbidity rates. Its mortality rates go from 8 to 19.5% yearly from cardiac and non-cardiac causes.9 Figure 1 shows a proposed sequence of risk factors, co-morbidities and mortality in AF patients. Both risk factors and co-morbidities share common pathophysiologic mechanisms, such as atrial endothelial dysfunction, inflammation and fibrosis, that induce atrial cardiomyopathy with anatomic, electric, mechano-electric and autonomic remodeling that will promote AF,10 as shown in Figure 2. Different forms of AF (paroxysmal, persistent and permanent) have different clinical implications, slight differences in the risk profile and variable responses to treatment. Nonetheless, in every form of AF, there is a benefit from the prevention measures and risk factors control.
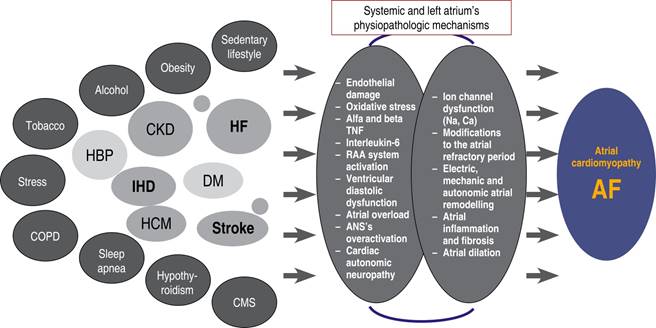
Figure 2: Risk factors and co-morbidities’ common physiopathologic mechanisms that induce atrial cardiomyopathy and AF.
Most of those risk factors are reversible when they are properly controlled and there is a positive impact on AF behavior.1-3 The most recent European and American guidelines rank risk factors and comorbidities control as a class I indication with evidence level B along with rhythm or rate control and anticoagulation.10-13 Because of the great number of elements related to AF, its management is more complex and might be suboptimal if only directed towards a fraction of them.1,13 A joint approach treatment to achieve rhythm control, anticoagulation, and derive the patient to a multidisciplinary team for adequate risk factor control might facilitate an intensive and complete treatment with better results.2,3 The acronym HEAD-2-TOES (heart failure, exercise, arterial hypertension, DM2, tobacco use, Obesity, Ethanol consumption, sleep apnea) refers to several modifiable risk factors, lifestyle and comorbidities that are related to the development, persistence and progression of AF.14 These and other elements that we show in the present work are targets for primary and secondary AF prevention. Several services and specialists might be needed to achieve an integrated multidisciplinary approach to AF, shown in Figure 3. Some recent controlled and randomized clinical studies have shown a positive impact on the arrhythmia burden when lifestyle modifications such as weight reduction (Table 1), exercise and modifiable risk factors control are implemented, showing up to a 35% reduction in re-hospitalizations and mortality,3 as well as a reduced progression from paroxysmal to permanent forms of AF and lower recurrence rates after ablation.3 Those benefits regarding AF behavior are associated with lesser atrial damage.10-12
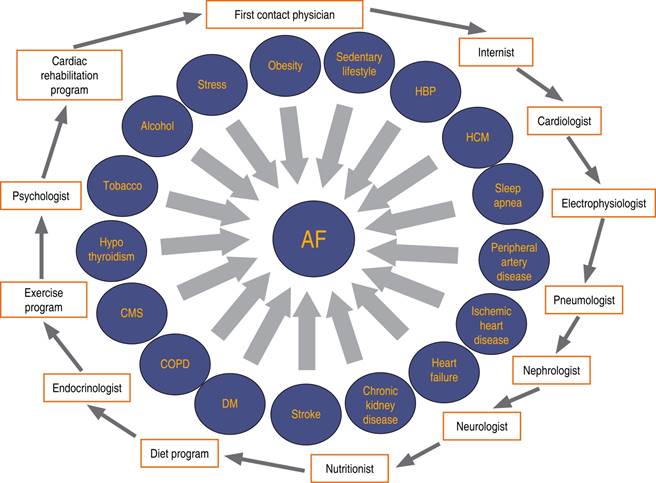
Figure 3: Necessary interactions between physicians and services to achieve an integral and multidisciplinary attention in the atrial fibrillation patients.
Table 1: Randomized clinical studies about risk factors control and their results in patients with atrial fibrillation.
| Study | Objective | Impact on AF’s behavior | Other findings |
|---|---|---|---|
| Legacy(11) 355 patients with AF Follow-up 48.4 ± 18.2 months |
Body weight reduction (> 10 vs 3-9 vs < 3% reduction) and risk factor’s management | Reduction of the frequency, duration and severity of AF’s
symptoms Higher AF-free survival (86.2 vs 65.5 vs 39.6%) |
Reduction of the left atrium’s indexed volume Reduction of interventricular septum’s thickness Reduction of the left ventricle’s end-diastolic diameter Reduction of high sensitivity C-reactive protein |
| Reverse-AF(12) Same patients as in the Legacy study(11) Follow-up 48 4 ± 18.2 months |
Body weight reduction (> 10 vs 3-9% vs < 3% reduction) and risk factor’s management | Lower progression to AF’s persistent forms (3 vs 32 vs
41%) Higher AF’s reversion from persistent to paroxismal forms (88 vs 49 vs 26%) |
Reduction of the left atrium’s indexed volume Reduction of interventricular septum’s thickness Reduction of the left ventricle’s end-diastolic diameter Reduction of high sensitivity C-reactive protein |
| Weight reduction in cardiometabolic risk(27) 150 patients with paroxismal or persistent AF Follow-up 15 months |
Body weight reduction and risk factors control | Reduction in frequency, severity, impact and duration of AF’s symptoms | Reduction of interventricular septum’s thickness Left atrium’s area reduction |
| Race 3(28) 250 patients with AF and HF 119 multiple combined therapies, 126 conventional therapy Follow-up 12 months |
Risk factors control vs conventional therapy | Sinus rhythm in the risk factors control group 75 vs 63% with
conventional therapy group Improvement of AF’s symptoms |
Improvement in systolic and diastolic blood pressure
control Weight reduction, body mass index reduction. Lower total cholesterol levels, LDL, NT-proBNP and urinary sodium |
| Arrest-AF(29) Patients submitted to AF ablation 149 Ablation + risk factor’s control 88 control group (ablation only) Follow-up 41.6 ± 14.2 months |
Risk factors control HBP, lipid, glycemic and sleep apnea control along with weight reduction, reduction of tobacco and alcohol use | Reduction in the frequency, duration and severity of AF’s
symptoms Higher AF-free survival after one or several procedures (87 vs 17.8%) |
Reduction of interventricular septum’s thickness Left atrium’s volume reduction |
| Cardio-Fit(38) 308 patients with FA Follow-up 49 ± 19 months |
High physical conditioning vs adequate vs low Physical conditioning gain during follow-up |
Reduction in the frequency, duration and severity of AF Higher AF-free survival in the high physical conditioning group (84 vs 76 vs 17%) |
Higher physical performance: higher weight reduction, lower blood pressure, LDL and triglyceride reduction, better glycemic control, lower high-sensitivity C reactive protein levels, left atrium’s volumen reduction |
AF = atrial fibrillation. HF = heart failure. LDL = low density lipoprotein. HBP = High blood pressure.
In the following pages, we will detail the risk factors and co-morbidities most frequently associated with atrial cardiomyopathy and AF development, as well as the recommended actions to control them and reduce their progression. In every case, it is recommended to adopt the most recent AF treatment guidelines that emphasize the permanence in sinus rhythm as the best option to delay the arrhythmia and atrial cardiomyopathy’s progression, along with lifestyle modifications and risk factors control, which is the main focus of the present work.
Obesity and AF
There is a strong correlation between obesity and AF. Patients with BMI > 35 kg/m2 have a 3 to 4-fold increase in the risk of AF.15 Several mechanisms are implicated in this phenomenon: the increase in pressure and size of left atrium’s favors greater release of transforming factor β1 and platelet derived growth factor (PDGF) concentrations.16 Increased pericardial and epicardial fat have arrhythmogenic effect through increased release of stearic acid and by induction of changes in the electric currents of a left atrium with stretched myocardial fibers.17 Sympathetic activation, the effect of the renin angiotensin aldosterone system (RAAS), leptin excess and insulin resistance induce cardiac remodeling and atrial cardiomyopathy.10,18 The association of obesity with cardio-metabolic syndrome,19 sleep apnea, HBP, DM,20 HF21 and IHD22 increase both the prevalence and impact of AF. Non-alcoholic fatty liver, present in 75-92% of patients with obesity,5 is associated with higher cardiovascular risk,23 AF24,25 and recurrence risk among patients that undergo a radiofrequency ablation procedure to control AF.26
Weight control and AF
Some studies have shown a positive impact of weight loss on AF control. People that achieve weight reductions above 10% or a BMI < 27 kg/m2 have a 6/fold reduction in AF events when compared to those that did not lose it or with reductions lesser than 3% of the initial total body weight.11,27 Weight loss is also related with less symptoms and higher sinus rhythm conversion rate (75 vs 63%).12,28 Weight reduction in patients that receive an ablation procedure has shown better AF-free survival.29 A Mediterranean diet, rich in vegetables, grains, fruit, coffee, tea and virgin olive oil has antioxidant and anti-inflammatory properties that reduce the overall AF risk as well as its progression and recurrences.30
Key points and recommendations:
Obesity increases the risk of AF.
Reduction of at least 10% of baseline weight or reach a BMI < 27 kg/m2 is a 1-B recommendation to reduce the progression and impact of AF.
In candidates for AF ablation, weight reduction increases AF-free survival rate.
A Mediterranean diet (rich in vegetables, grains, fruit, coffee, tea, and olive oil) is recommended because of its antioxidant and anti-inflammatory properties that reduce the risk of AF and its complications.
Physical activity and AF
Physical inactivity is associated with cardiovascular disease. Inactivity periods are associated with AF appearance: the longer the sedentary period, the higher the AF risk.31,32 Physical inactivity promotes electrical and structural remodeling of the atrial wall through an increase in systemic inflammatory processes and sympathetic activity that favors autonomic de-regulation processes that will eventually lead to AF.32 On the other hand, excessive exercising can also increase the risk of AF, as has been noted in marathoners, 33 that have a 5-fold increase in AF risk.34 That phenomenon among high-end athletes is somehow explained by dilation and fibrosis of the atrial muscle secondary to elevated hemodynamic loads, an increase in atrial ectopic activity and abrupt shifts in the sympathetic-parasympathetic balance.35
Exercise and AF control
Better physical capacity as a result of regular aerobic training is associated with lesser AF impact in middle age and older adults.36 Atrial fibrillation’s incidence is higher in subjects with low training (capable of less than 5 METs), but it is reduced by every MET (1 kcal/kg/h) increase. Regular exercise reduces the risk of AF by 7%37 and by 9% the risk of AF recurrence after ablation procedures.38 A two-month regular exercise program in subjects with permanent AF reduces symptoms and ameliorates quality of life (QoL).39 A meta-analysis shows that an increase in exercise capacity also increases left ventricular ejection fraction, QoL and vitality.40 Even if regular physical activity is beneficial to prevent and treat AF, in daily clinical practice, physicians recommend exercise in less than 10% of patients.14 Short intervals-high intensity exercise can improve physiologic parameters such as cardiorespiratory capacity,41 ejection fraction,42 diastolic function,43 as well as endothelial and general vascular function.43
Body-mind exercises as Yoga, Tai-chi and Chi-kung can also have beneficial effects because they improve elasticity, flexibility, equilibrium, general health conditions and autonomic cardiac functioning,44 that has an important role in AF’s genesis.45 The study YOGA My Heart46 showed that a three-month yoga training program reduces AF’s impact and improves symptoms and QoL. When comparing yoga against short Interval-high intensity exercise, it was found that yoga does not have adverse effects on left atrial remodeling, so it could further reduce AF’s recurrences.47
Key points and recommendations:
A sedentary lifestyle is associated with increased risk of AF.
A regular exercise program must be suggested in every AF patient as a class 1-B recommendation.
An increase in the physical capacity and cardiovascular endurance reduces the impact and recurrences of AF, and improves QoL and symptoms among AF patients.
Alcohol intake and AF
Regular alcohol intake is widely accepted in occidental cultures, with a prevalence of 57% in the US population.48 Some observational studies have observed a dose-dependent relationship between alcohol intake and AF: drinking of a weekly average of 7 to 14 alcoholic beverages significantly increases the risk of AF.49 Acute alcohol intake has also been reported as a trigger in up to 35% of AF new cases50 and is known to increase recurrences after ablation procedures.51 Alcohol intake induces both sympathetic and para-sympathetic stimulation that reduce heart rate variability, increases pericardial fat, inflammation, electrical and mechanical remodeling of the left atrium and thus, increases AF inducibility.52 It is also related to AF-favouring comorbidities such as obesity53 and HBP.54
Alcohol consumption control and AF behavior.
Reduction of alcohol intake to less than 3 drinks per week reduce atrial remodeling, AF impact and has become a class 1-B recommendation in the European guidelines for AF treatment and control.1 Complete abstinence in usual alcohol users further reduces AF recurrences.55
Key points and recommendations:
Alcohol intake is related with higher prevalence and recurrence of AF.
Restrict alcohol intake is a class 1-B recommendation to reduce AF’s progression and impact.
Alcohol abstinence further reduces AF recurrences.
High blood pressure (HBP) and AF
Epidemiological and clinical studies have found a close relation between HBP and AF since they share many risk factors.56 Because of its high prevalence, HBP is a major independent risk factor to develop AF. In the same way, poor HBP control is associated with higher AF and mortality risk.56 It has been reported that 21.6% of hypertensive subjects will have AF.57 High blood pressure might induce atrial cardiomyopathy by promoting architectural, contractile and electrophysiologic changes that affect both atria and produce arrhythmogenic foci.10 A hypertrophic left ventricle that is less compliant promotes a pressure increase and muscle fiber stretching of the atria, predisposing towards AF development.58,59 Higher levels of aldosterone and angiotensin II, in the context of HBP, induce inflammation as well as fibroblastic and extra-cellular proliferation, that in turn will promote more myocyte hypertrophy and atrial remodelling.56,57 Angiotensin II favors inward calcium currents and reduces potassium outward currents. These changes prolong the action potential plateau (phase 2), increase inotropism but favors anisotropism, thus increasing the risk of AF.10,58,59
Arterial hypertension control and AF
Arterial hypertension control is a class 1-B recommendation in the 2020 AF European1 and 2019 American guidelines.13 In order to achieve an adequate BP control, international guidelines must be followed.60 A target blood pressure between 120-129/< 80 shows better outcomes than > 130/80 mmHg or < 120/80 records, that have been related with a higher risk of adverse cardiovascular events.58,60 Stroke risk in patients with AF significantly increases with every 10 mmHg elevation above 120 mmHg or with systolic BP values lower than 115 mmHg.60 Treatment with Angiotensin Converting Enzyme Inhibitors (ACEI) or with Angiotensin II Receptor Blockers (ARB’s) reduce RAAS activation and has beneficial effects delaying atrial damage progression and AF prevalence.61,62 Hypertension treatment with mineralocorticoid receptors antagonists has also been noted to reduce FA risk and recurrence both in observational studies and meta-analyses.62,63 Patients with severe hypertension and AF treated with AF ablation and renal artery denervation have shown better BP control and less AF recurrences after a 12 month follow-up.64
Key points and recommendations:
Diabetes mellitus (DM) and AF
Diabetes is a major risk factor related with an increase in cardiovascular events and mortality.65 Patients with DM have more chances to develop AF with a 1.4 and 1.6-fold increase respectively in women and men.65 Atrial fibrillation incidence is 14.9% among diabetic patients versus 10.3% in non-diabetic ones.66 A metanalysis that included 1,6 million subjects showed that DM is an independent risk factor that increases 40% the chances of having AF.67 Diabetes increases AF symptoms and reduces QoL,68 it increases the hospitalization rate, the risk of acute coronary events, Stroke, HF, and all-cause mortality.65,69 The mechanisms involved in the genesis of AF in patients with DM include an increase in oxidative stress,70 atrial cardiomyopathy with electro-mechanic and autonomic remodeling of the left atrium,10,71 and release of advanced glycation products that favor more inflammation, dilation and atrial fibrosis.72 All these changes reduce the atrial functional reserve and impair electrical conduction through the atria, inducing a marked intra and inter-atrial electro-mechanical conduction delay.73 The longer DM has been present, and the worse its control, the higher the risk of having AF.65 Elevated levels of glycated hemoglobin, are associated with a 10% increased risk of having AF. For each 1% increase in glycated hemoglobin HbA1c, there is a 1% increase in the chances of having AF.74,75 DM is associated with a higher prevalence of obesity, hypertension, HF and CKD, which in turn increase the prevalence of AF.76 Figure 4 shows the mechanisms implicated in AF among DM patients.
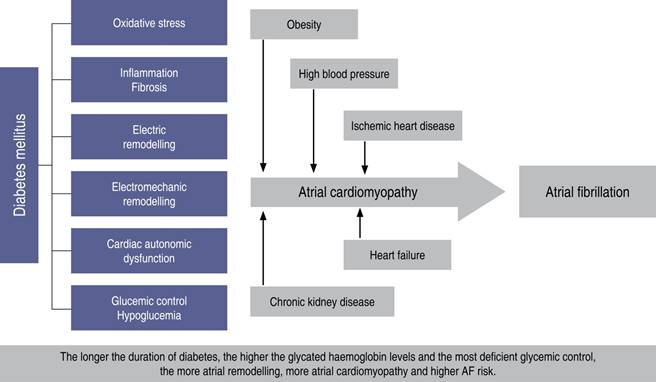
Figure 4: Physiopathologic mechanisms that induce atrial fibrillation in patients with diabetes mellitus.
Diabetes control and AF
Adequate control of blood glucose is important to reduce AF’s impact and recurrences.76,77 Metformin use has shown a 19% reduction in the risk of AF during a 13-year follow-up period.78 A metanalysis showed that pioglitazone was associated with a 27% risk reduction to develop AF, apparently because it reduces atrial inflammation and fibrosis.79 Drugs as SGLT2 inhibitors, seem to reduce the incidence of atrial arrhythmias and sudden death.80 The DPP-4 inhibitors and the GLP-1 inhibitors have not shown, so far, any protective effect against AF.81
Key points and recommendations:
Diabetes mellitus is associated with higher risk of AF.
Diabetes control is a class1-B recommendation to reduce AF’s recurrence rates, progression and impact.
So far, only metformin, pioglitazone and SLGT2 inhibitors (empaglifozin, dapaglifozin and canaglifozin) have proved to reduce AF’s prevalence and impact.
Heart failure and AF
Heart failure’s incidence has globally increased as well as its care costs. Atrial fibrillation is the most common arrhythmia related to HF, present in approximately 25% of HF patients.82 The combination of HF and AF has a negative synergistic effect that reduce QoL and functional capacity while they increase stroke risk, hospitalizations and all-cause mortality.83 Heart failure and AF share risk factors such as obesity, DM, HBP, IHD, tobacco and alcohol use and male gender.84 The higher HF prevalence, higher AF prevalence and vice versa.83 Atrial fibrillation can be present in patients with reduced or preserved ejection fraction HF.85 Among the different mechanisms that lead to AF in HF patients, there is atrial cardiomyopathy,10 blood pressure overloads, atrial myocardial stretching, abnormal myocardial conduction velocity, structural remodeling and atrial mal-adaptation gene expression.86 Acute increases of intra-atrial pressure promote dilation and fibrosis of the atrial myocardium, that in turn will induce electro-mechanical remodeling, shortening of atrial refractory periods, triggered activity and AF.87 On the other hand, fast ventricular response AF will reduce cardiac output, loss of atrial contraction and irregular ventricular filling intervals.10,85
A chronic inflammatory state prevalent in HF88 favors, as well, development of atrial cardiomyopathy10 alongside with the left’s atrium structural and anatomic remodeling that triggers and perpetuates AF.89 Figure 2 shows the physiopathologic features shared by HF and other co-morbidities that will lead to AF.88,89
Heart failure control and AF
The treatment for patients with HF and AF must be installed according to the international guidelines recommendations.1,90 Treatment with ARB’s, ACEI’s and neprilisin inhibitors in patients with HF and AF might improve ventricular function and reduce the prevalence and impact of AF.91-92 In patients with AF and rapid mean ventricular response, hemodynamic impairment or worsening HF, immediate external electrical cardioversion is a reasonable choice.90,92 Among subjects with AF and preserved, moderately reduced or reduced LVEF, early rhythm control with antiarrhythmic drugs or ablation has shown better results in hospitalization, stroke prevention and mortality when compared to conventional treatment.93
Beta-blockers are preferred over digoxin to achieve rate control since they improve prognosis. Only if the mean ventricular rate persists high with beta-blocker therapy or when these drugs are not tolerated or are contra-indicated, then digoxin may be used.92,93 Pulmonary vein isolation by a catheter ablation procedure in selected patients with AF and HF might be superior to both pharmacologic rate and rhythm control94 since it improves QoL, exercise capacity, LVEF and reduces mortality.95 Unfortunately, less than 10% of patients with HF and AF are eligible for pulmonary vein isolation.94-96
Key points and recommendations:
Heart failure increases the risk of AF, in the same way that AF increases the risk of HF.
Heart failure control is a class 1-B recommendation in patients with AF.
Early rhythm control has shown better results concerning hospitalization, stroke prevention and mortality in patients with preserved, moderately reduced and reduced LVEF.
Sleep apnea and AF
Sleep apnea is a common problem with strong correlation to cardiovascular disease.97 The prevalence of moderate to severe sleep apnea (SA) is 10 and 7% among men and women in the 30-49 years old range respectively and in 17 and 9% of men and women in the 50 to 70 years-old range respectively.98 Sleep apnea induces hypoxia and hypercapnia as well as repetitive events of increased intrathoracic negative pressure and atrial overdistension thus increasing sympathetic activity that activates ion channels that promote atrial cardiomyopathy10 and AF.99 Sleep apnea and AF share risk factors as obesity, older age, male gender, HBP, HF, genetic factors, IHD, stroke and alcohol intake.100 Patients with SA show a 2-fold increase in the risk of having AF and a 5.2-fold increase in cardiovascular death risk.101 It has been reported that AF is present in 21 to 87% of SA patients,102 according to the severity of the later, that is, the more severe the SA, the higher the risk of AF103 and the lower the response to antiarrhythmic drugs.104
Sleep apnea control and AF
Patients with SA and AF treated with continuous positive airway pressure (CPAP) have slower AF progression to permanent forms and show less recurrences after cardioversion or ablation.1,2,105 It is recommended to include every patient with SA in exercise, nutrition and body weight reduction programs in order to improve the results of other AF control interventions.1,2,3,105
Key points and recommendations:
Sleep apnea increases the risk of AF in a directly proportional way: The more severe the AS, the higher the risk of AF.
Sleep apnea diagnosis and treatment is a relevant component among the lifestyle modifications in AF patients and is a class 1-B recommendation.
Patients with SA treated with CPAP show slower AF progression and less recurrences after ablation or cardioversion.
Chronic kidney disease and AF
Chronic kidney disease (CKD) and AF are closely related. They both share risk factors and co-morbidities such as advanced age, obesity, sedentary lifestyle, tobacco use, HBP, HF, stroke and both show global increasing prevalence with a reciprocal relationship.1,106 Almost 20% of patients with CKD have AF and nearly 50% of patients with AF show some degree of kidney damage.107 Patients with both conditions are at greater risk of thromboembolism, cardiovascular morbidity, hemorrhage and all-cause mortality.108 Kidney disease promotes atrial cardiomyopathy10 through several mechanisms: it increases the release of B1 growth factor that has a pro-inflammatory effect, as well as cellular infiltration, interstitial fibrosis and atrial conduction velocity heterogeneity. Kidney disease also activates RAAS, sympathetic nervous system and induces water and salt retention that in turn will stretch atrial walls and induce atrial remodeling, inflammation and necrosis.106,109 On the other hand, AF promotes CKD progression to terminal phases.1,110
Chronic kidney disease control and AF
Several drugs reduce CKD progression and might be useful to reduce AF.as Angiotensin converting enzyme inhibitors and ARB’s, reduce RAAS’s activity and CKD progression as well as AF prevalence.110 It is mandatory to control risk factors and co-morbidities in the early stages of renal damage in order to reduce the prevalence of CKD and AF.111 Medications that increase renal damage such as non-steroidal anti-inflammatory drugs (NSAID’s) should be avoided, as well as vitamin K antagonists such as warfarin or acenocoumarin, which also increase bleeding and embolism risk when compared to direct anticoagulants as rivaroxaban, apixaban, dabigatran or edoxaban, that are preferred in those patients.1,112
Key points and recommendations:
Chronic kidney disease and AF are linked and show a reciprocal relationship: the more significant the kidney damage, the higher the risk of AF, and vice versa.
An adequate control of risk factors and specific measures to reduce kidney damage along with measures to prevent AF and reduce its impact are a class 1-B recommendation.
Direct oral anticoagulants are preferred over vitamin K antagonists since the later increase renal damage.
Chronic obstructive pulmonary disease and AF
Chronic obstructive pulmonary disease (COPD) is a major health problem: approximately 10% of the general population suffers from it.113,114 Up to 23% of patients with AF have COPD.115 The greater the COPD’s severity, the greater its association with HBP, IHD, HF and AF.116,117 Pulmonary disease increases the progression and recurrences of AF after pharmacological treatment,1 cardioversion118 or ablation.113 Atrial fibrillation in patients with COPD, in turn, will promote lung damage and increases the rates of cardiovascular events and long-term mortality.119 Several factors might promote AF in subjects with COPD: there is a systemic inflammatory state with increased oxidative stress.114,116 Hypoxia induces the production of factor 1-alfa, that will promote atrial remodeling, atrial cardiomyopathy and AF.116 Pulmonary artery hypertension and ventricular diastolic dysfunction induce atrial overload that may trigger arrhythmogenic automatic foci in the right atrium and favor AF.120
Chronic obstructive pulmonary disease control and AF
Patients with COPD and cardiovascular disease must be carefully monitored to timely detect AF.119,120 Some drugs used to control COPD, especially short-acting beta-2 agonists, might increase AF’s prevalence and exacerbate symptoms because they have positive chronotropic action and favor depolarization-repolarization heterogeneity.119 It is thus recommended to use long-acting beta-2 agonists, that do not increase AF’s prevalence.117 Anticholinergic drugs, theophylline and glucocorticoids can precipitate AF, either by a direct action or by modifying other risk factors.121 It is recommended to correct hypoxia and hypercapnia with oxygen and non-invasive ventilation systems in acutely decompensated patients, an intervention that can help to reduce AF’s impact.122
Key points and recommendations:
Chronic Obstructive Pulmonary Disease increases AF’s prevalence and impact.
Interventions to control COPD risk factors and reduce chronic lung damage in order to avoid AF and lessen its impact are a class 1-B recommendation.
It is advised to reduce short-acting beta-2 agonists, anticholinergic drugs, theophylline and steroids since they increase the prevalence and impact of AF.
Ischemic heart disease and AF
Ischemic heart disease (IHD) and AF coexist in an important number of cases: It is estimated that 15% of patients with IHD will have AF and 30% of the patients with AF will have IHD.123,124 Ischemia increases the prevalence of AF, especially recent onset AF after myocardial infarction.124 Patients with IHD and AF have worse outcomes.125 In the context of acute myocardial infarction and cardiogenic shock, AF is an independent one-year mortality predictor.126 Several mechanisms have been implied in the genesis of AF in subjects with IHD. They include shortening of refractory periods in the atria along with myocardial fibrosis and slow conduction areas, that along with post-depolarizations (early and delayed) will lead to triggered activity, abnormal automaticity and reentry circuits.124 Increased sympathetic activity will also induce autonomic, electrophysiologic and anatomical remodeling that promotes atrial cardiomyopathy.127
Ischemic heart disease control and AF
Ischemic heart disease and AF have common risk factors that must be controlled. Beta-blocker treatment, as well as ACEI’s or ARBS’s, might be useful in both conditions.1,3 In patients with acute coronary syndrome that will be submitted to angioplasty, a short period of triple combination therapy with a direct oral anticoagulant and dual anti-platelet treatment is recommended.1 Suggested drugs are rivaroxaban, apixaban, dabigatran or edoxaban along with clopidogrel and aspirin.123 After the initial treatment period, it is advised to shift to a combination of clopidogrel and direct oral anticoagulant, that has shown less hemorrhagic complications and more effectiveness.13
Key points and recommendations:
Ischemic heart disease increases the risk of AF.
Control IHD and its risk factors is a class 1-B recommendation in patients with AF.
Drugs as beta-blockers and ACEI’s or ARB’s are beneficial in subjects with both conditions (IHD and AF)
A combination of a direct oral anticoagulant and an antiplatelet agent offers better results in patients with AF and acute IHD.
Other risk factors and their control in order to reduce AF’s prevalence
Tobacco use increases the risk of AF. When compared against non-smokers,2 it also increases the risk of stroke and mortality in AF patients.2,128 Smoking aggravates endothelial dysfunction, accelerates atherosclerosis and causes arrhythmia because of the combined effects of nicotine, carbon monoxide and polycyclic aromatic hidrocarbons contained in the smoke.2 Patients that quit smoking show a 30% reduction in the risk of stroke and 16% in total mortality.128
Dislipidemia is another AF risk factor.129 Their association is limited and inconsistent, nonetheless, it is considered that elevated total cholesterol, triglycerides and LDL along with low levels of HDL are associated with increased AF risk.130 Current international guidelines consider lipid control as a necessary intervention.1,2
Hypothyroidism is present in 9% of the people with AF.131 It can be associated with chronic amiodarone use or other antiarrhythmic drugs, but it is unknown at the moment if it increases AF risk by itself or if it is associated with other conditions such as obesity, HBP, dyslipidemia, atherosclerosis and IHD that have proved to increase AF’s prevalence.132
Cardiometabolic syndrome (CMS) is a combination of three or more of the following conditions: obesity, HBP, dysglucemia or DM, high LDL levels and low HDL. Each one of the CMS components increases the risk of AF by itself, although HBP seems to be the most significant one. It is clear that the higher the number of risk factors, the higher the risk of AF and its complications.19
Atrial fibrillation is present in 22 to 32% of patients with hypertrophic cardiomyopathy (HCM), with an annual incidence rate of 2%.133 Major predictors of AF in HCM patients are: older age, high body mass index (BMI), moderate to severe mitral regurgitation, atrial volume increase and reduced left atrial contraction index.134 Early recognition of AF in these patients is of outmost importance, since it is a bad prognostic feature associated with systemic embolism and heart failure.1 Early anticoagulation is recommended135 along with rhythm control through radiofrequency pulmonary vein isolation or cryoablation in subjects with symptomatic paroxysmal or persistent AF that does not respond to medications.136
Stress is a newly recognized cardiovascular disease risk factor. It induces over-activation of the autonomic nervous system that in turn, will stimulate atrial and pulmonary vein automatic foci and induce reentry circuits to increase AF risk.137 It is advised to reduce stress levels, and it has been demonstrated that beta-blocker use reduces the probability of stress-related AF.138 More than 55 work hours per week increase AF risk through a combination of stress and fatigue.4
Peripheral artery disease (PAD) is a mortality predictor in AF patients and an independent stroke risk factor in patients who are not receiving anticoagulant.7 Stroke and AF share risk factors and show similar epidemiologic features.1 Atrial fibrillation in patients with PAD is usually the final part of a pathway that starts with risk factors, atherosclerosis, atrial cardiomyopathy and atrial remodeling. It is recommended thus, to control all risk factors and co-morbidities in order to reduce AF’s prevalence and impact.1,139
Atrial fibrillation might increase up to 10-fold the risk of a stroke.140 On the other hand, stroke can favor AF appearance through autonomic nervous system lesions and systemic inflammatory response triggered by brain necrosis.141 Atrial fibrillation also impairs cognition and increases dementia risk by 10%.142 Early oral anticoagulation, especially with direct oral anticoagulants, reduces stroke risk, neurological impairment and dementia.142 Direct anticoagulants are preferred over vitamin K inhibitors. Recent observational studies suggest that direct oral anticoagulants in subjects with AF might reduce dementia risk by 10 to 39% when compared to antiplatelet drugs or no antithrombotic therapy.142
Strategies that help risk factor control and lifestyle changes
Every person in the medical staff should promote lifestyle modifications.1-3 Initial goals can be set at a 10% body-weight loss and a 2 MET increase in exercise capacity. These changes reduce AF’s impact.1,13 It is recommended to participate in successive weight reduction programs until a BMI < 27 kg/m2,1,11 is attained. Patients should also be advised to reach 200 minutes of exercise per week or 10,000 daily steps.12,38 Podometers or smartphone applications might be useful to support and stimulate them to increase daily activity. Other applications designed to help choosing healthier meals such as vegetables, fruits and grains with anti-inflammatory properties,2 instead of pro-inflammatory nutrients as animal fats, processed or canned foods, might also be useful.143,144
The 2020 AF management guidelines of the European Society of Cardiology1 suggest the creation of multidisciplinary programs for an integral management of AF patients. Those programs must consider the needs and preferences of each individual as well as the active participation of the subject and relatives to achieve better results. Available resources such as cardiac rehabilitation programs can be used, or specific multidisciplinary programs «AF clinics» might be created to offer integral treatment and at the same time, educate patients, relatives and health personnel to achieve better treatment adherence and better overall results (Figure 5).
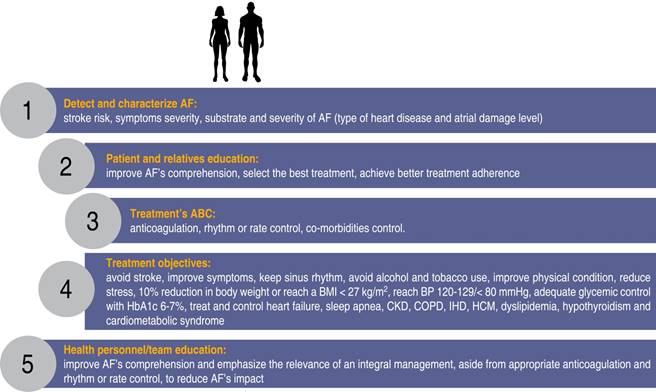
Figure 5: Integral approach to the patient with atrial fibrillation with emphasis on patient’s, relatives and health professionals education to achieve a better comprehension of the arrhythmia and select better treatments and improve adherence.
Atrial fibrillation patients should participate at least for six months in these programs in order to improve their lifestyle habits, treat co-morbidities, improve symptoms and reduce the impact and progression of AF.1,3 Aside from anticoagulation and rhythm or rate control, guidelines suggest as a class 1-B recommendation, to focus on lifestyle modifications, risk factors treatment and co-morbidities control, as shown in Figure 6. Adherence to the international AF diagnostic and treatment guidelines is advised since not doing so has been reported to increase morbidity and mortality.145 Table 2 shows the key messages for an integral and multidisciplinary treatment of AF patients.
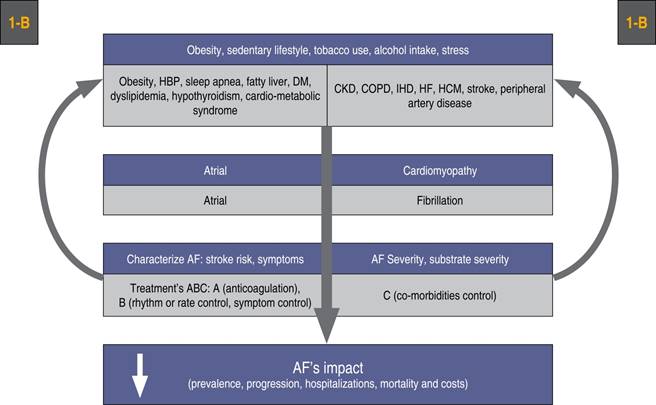
Figure 6: Integral treatment sequence in patients with atrial fibrillation. Aside from anticoagulation and rhythm or rate control, efforts must be made to control risk factors and co-morbidities as a class 1-B indication to reduce AF’s impact.
Table 2: Key messages to achieve an integral-multidisciplinary management of patients with atrial fibrillation.
| Key messages |
|---|
| 1. Atrial fibrillation is the most common arrhythmia. It has a high impact because it is associated with higher morbidity, mortality and care costs |
| 2. Atrial fibrillation has a close relation with sedentary lifestyles, alcohol intake, tobacco use and stress. It is also related to obesity, high blood pressure, diabetes mellitus, Ischemic heart disease, chronic kidney disease, sleep apnea, chronic obstructive pulmonary disease, peripheral artery disease, hypothyroidism, cardiometabolic syndrome, stroke, hypertrophic cardiomyopathy and heart failure |
| 3. Lifestyle modifications such as weight loss, regular exercise, avoid alcohol and tobacco use, as well as controlling other modifiable risk factors and co-morbidities, reduce the prevalence, recurrence rate and progression of AF and improve these patient’s prognosis |
| 4. Lifestyle modifications along with a joint aggressive management of risk factors and co-morbidities must be done at the same time as anticoagulant therapy and rhythm or rate control in order to achieve the best possible results |
| 5. It is recommended to create multidisciplinary programs for the management of patients with AF, cardiac rehabilitation programs can be used or create AF clinics to provide comprehensive treatment, promote greater education to patients, family members and health personnel, achieve greater adherence to treatment and best results |
AF = atrial fibrillation.
Conclusions
Atrial fibrillation is the most common arrhythmia and has a high impact on the general population. Its prevalence increases with age, habits and risk factors common in the general population such as obesity, sedentary lifestyle, stress, alcohol intake, tobacco use and stress as well as HBP, DM, IHD, HF. It is also related to dyslipidemia, hypothyroidism, stroke, HCM, CKD and peripheral artery disease. The current evidence shows that lifestyle modifications, adequate control of risk factors and co-morbidities management, are effective interventions in AF treatment. Risk factors control and co-morbidities treatment must be done in conjunction with other AF therapies such as anticoagulation and rhythm or rate control. It is advisable to consider the creation of specific AF services or the optimization of cardiac rehabilitation units to offer a multidisciplinary integral treatment for AF patients.











 text new page (beta)
text new page (beta)



