Introduction
Coarctation of the Aorta (CoA) is a congenital disease that affects the aorta, aortic and mitral valves together with intracerebral arteries.1 Once clinical suspicion has arisen, various paraclinical tests should be done in order to confirm and evaluate disease extension and possible complications. Besides, systemic hypertension is part of the natural history of disease and consistently leads to deleterious consequences;2 adequate treatment, therefore, is imperative. Stenotic correction is the treatment of choice, stenting angioplasty over surgical technique, depending on the presence or absence of cardiovascular comorbidities, age, and other aortic segments compromise, particularly if aortic disease is present,3 such as aortic dissection (AD) which constitutes alone a life-threatening cardiovascular condition with a high mortality risk at presentation; however, uncommonly some patients might have a subclinical course leading to a delayed diagnosis.
Case presentation
A 28-year-old patient was diagnosed with systemic arterial hypertension six years ago, receiving a medical prescription and no further evaluation until he presented to an emergency department after suffering sudden left hemiparesis and dysarthria. The immediate evaluation revealed systemic blood pressure of 220/110 mmHg, and subsequent non-contrast cranial tomography displayed right basal ganglia hemorrhage. The patient refused medical admission, alleging personal reasons; henceforth, home rest and effective hypertensive treatment consistent with enalapril and amlodipine were prescribed, besides the appropriate diagnostic approach. Two months later, a first thorough evaluation at a consulting room found a harsh, holosystolic murmur along the left parasternal border irradiated to the ipsilateral dorsum. Blood pressure in both arms differed by more than 30 mmHg compared to lower extremities (220/120 versus 140/90 mmHg) and the absence of femoral and distal pedal pulses. Twelve leads electrocardiogram showed only diastolic overload and a chest radiograph revealed inferior costal notching (Figure 1, black arrows) and the inverted number three sign (Figure 1, white arrow), ascending aorta (AscAo) dilation and discrete cardiomegaly; blood tests were unremarkable. Secondary systemic hypertension due to CoA was suspected. Therefore, he was admitted to a tertiary care center. A Transthoracic echocardiogram confirmed left ventricle hypertrophy and also revealed sinus of Valsalva dilation (50 mm, 27.7 mm/m2) extended to AscAo along a presumptive dissection anterior flap (Figure 2A) bicuspid aortic valve (BAV) (Figure 2B) with no significant aortic insufficiency (AI). Surprisingly, the patient did not recall any previous chest pain episodes.

Figure 1: Bilateral inferior costal notching is seen (black arrows) due to collateral vessel formation, and also, the inverted three sign is seen (white arrow), characteristic of coarctation of the aorta.

Figure 2: Enlargement of the aortic root and sinus of Valsalva, an anterior dissection flap is seen (A), and the aortic valve is seen with a bivalve configuration at the end-diastolic view (B).
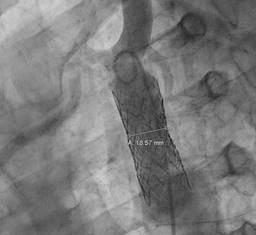
Nevertheless, expediting both lowering blood pressure and heart rate treatment was instated. A computed aortic tomography (CTA) confirmed CoA, the narrowest site up to 4 millimeters, post-stenotic dilation up to 34 mm (Figure 3) and aortic aneurysm (AA) and AD secondary to a dissection flap just above right coronary ostia extending just before the aortic arch, with a maximal diameter at the sinus of Valsalva level up to 56 mm (Figures 4 and 5), consistent with Stanford A classification of unknown evolution. After the heart team meeting, a very specific sequential treatment was established: firstly, CoA was successfully corrected after angioplasty with a 50 × 14 mm covered stent placement, with appropriate positioning and reducing medium gradient from 50 mmHg to six mmHg (Figures 6 and 7) and four weeks later, surgical procedure took place: Sievers type one BAV was replaced with bileaflet St. Jude mechanical prosthesis sized 25 mm along concomitant proximal AscAo replacement with 24 mm sized Woven-Dacron graft (wheat procedure). After complete recovery, he received a medical discharge, long-term beta blockade, angiotensin enzyme inhibitor and mid-term aspirin therapy. An evaluation six months later revealed a satisfactory evolution.

Figure 3: Maximal stenosis of the aorta due to coarctation and post-ductal aortic dilatation are seen.

Figure 4: A dissection flap extending from the aortic root traversing through the ascending aorta just before the aortic arch.

Figure 6: Depiction of the coarctation side at the greatest narrowing point and post-coarctation dilation.
Discussion
Secondary hypertension (SH) comprises those forms of systemic hypertension related to a specific etiology, and thus, a particular management strategy. It is recommended to screen SH in certain patients, such as those with younger age (< 40 years), acute worsening of a previously controlled hypertensive patient, severe (grade 3) or drug-resistant hypertension and the presence of extensive hypertensive mediated organ damage.3 Multiple conditions have been reckoned as secondary causes of SH, including congenital conditions like CoA. CoA might correspond to about one percent of congenital heart defects in live births and up to one out of 1,550 patients in necropsies.1,4 The traditional classification relies on an anatomical relationship with the ductus arteriosus: preductal CoA (aortic arch and isthmus) is typically found in newborns2 and, conversely, post ductal stenosis in children and adults.2,4 CoA has a male-to-female ratio of 1.5:1; when BAV coexist, this might increase to a 4:1 ratio.5 Although CoA can be found on a solitary basis, most cases feature several associated congenital conditions such as BAV (nearly 85% of cases),3,6 Willis circle aneurysms (5-10%), persistent ductus arteriosus, subaortic stenosis, parachute mitral valve, supravalvular stenosis; the last three altogether known as Shonen complex.4 Besides cardiovascular conditions, different perturbations have been described, i.e., neurological, hemangiomas and even ocular congenital syndromes.1
CoA does not only imply a local narrowing of the aorta but rather a generalized vasculopathy that compromises central and peripheral vasculature as well as heart valves. If untreated, significant CoA consistently leads to SH characterized by upper-lower extremities systolic difference > 20 mmHg,7 along with collateral circulation developing, left-side heart failure and cerebrovascular disease; therefore, the mortality risk can be as high as 90% by the age of 50.1 Besides narrowing severity, CoA affectation will also depend on its relationship with arch vessel disease and adequacy of vessel formation along collateral and concomitant diseases.1,4 Treatment consists of appropriate SH control along stenosis correction, indicated when either hemodynamic significant CoA exists or hypertensive illness.7 While surgical correction may be preferred in newborns and children, angioplasty is often favored in older populations, with stenting generally preferred over balloon techniques. Balloon techniques are typically reserved for cases of post-stenting restenosis.4
Despite the most common complication of BAV being aortic stenosis, several situations might arise since the interaction between BAV and CoA is complex and interdependent, i.e., both diseases involve a certain degree of aortopathy, along with the fact that CoA consistently leads to systemic hypertension. Several pathophysiological mechanisms can coexist, such as persistent shear stress and, possibly, chronic localized inflammation, leading to aortic intimal laceration and underlying media tunica,1,4 endothelial dysfunction, vascular remodeling, and even cystic medial degeneration.8 These mechanisms can lead to atherosclerosis, AA, and, in some cases, even aortic syndromes, including AD. AD is a condition characterized by the separation of the wall of the aorta at the outer media or media-adventitia border, a process that starts with an intimal tear. Blood subsequently passes through the tear, then moves in an anterograde or retrograde fashion, and later dissects the lumen of the aorta into both true and false lumens.9 AD is usually classified according to location and acuity. The most commonly known classification is the Stanford system,10 which separates AD according to whether the AscAo (type A) is involved or only the descending aorta (type B). The De Bakey system considers the origin site and extension of the intimal flap: type I involves the AscAo, aortic arch, and descending aorta, type II is confined to the AscAo, and type III only the descending aorta; distal to the left subclavian artery. Aortic syndromes are also classified into acute (≤ 14 days) and chronic (> 14 days), depending on the time of onset of initial symptoms and the time of presentation. Another described classification system recommended by the European Society of Cardiology (ESC) but less frequently used establishes three categories: acute (<14 days), subacute (15-90 days) and chronic (>90 days).11
Classically, type A AD is associated with a high mortality risk, which increases by one-to-two percent per hour until reaching up to 50% in the first 48 hours.11 However, there is a small group of patients who present minimal or no symptoms, making it difficult to determine the duration of the condition.12,13 Compared to those with acute presentation, these patients are associated with a history of cardiac surgery, BAV, wide AscAo diameter, severe AI and typically do not extend beyond the aortic arch.14 The approximate incidence of chronic AD is estimated at four to six cases per year with an average survival of seven years, with a prevalence of 28-42 cases per 100,000 people per year, consistent with the data for acute AD, which is three cases per 100,000 people per year.15 Besides, approximately 50% of patients with AD have been observed to develop aneurysms.11,15 Despite the fact that the first imaging study usually involves an echocardiogram, due to its low sensitivity, CTA or magnetic resonance imaging (MRI) is preferred; both offer optimal imaging, allowing diagnosis and management decisions.1,13 Features like a thick, immobile intimal flap, aneurysmal dilatation or thrombus in the false lumen suggest dissection chronicity.13 Treatment options include open surgery, which is the gold standard, and endovascular repair, hybrid methods, or even medical treatment alone. Surgical reparation is recommended in uncomplicated AA if the diameter exceeds 55 mm, but if complicated, such as ours, the decision should be individualized. However, if CoA coexists, expedited surgical treatment is advised to increase survival prognosis.16 In patients with chronic AD undergoing surgery, mortality can reach up to six percent at 30 days, much lower than in acute cases (30% at one month).11,17 Pavlou et al.11 along with Abugroun et al.12 reported the cases of patients with chronic AD, who had a favorable evolution after surgical repair, consistent with our very patient’s situation. Similarly, Jiang et al.17 described a case of a middle-aged man with the coexistence of CoA, BAV, AA and AD, with a successful surgical repair. Therefore, a multidisciplinary approach is fundamental to ensure long-term success with fewer complications when such atypical confluence is found. A two-staged approach was favored in our patient with relatively stable clinical status. In addition, the correction of CoA on the first time would lead to a better hemodynamic condition besides decreasing aortic clamping time during the latter surgical procedure, reducing its morbidity and mortality risk.
Conclusions
The simultaneous presence of CoA, BAV and Chronic AD complicated with AA is a medical trilemma. Therefore, multiple considerations should be considered before any definitive intervention is done. Since conservative management may lead to high morbidity and mortality, a combination of surgical and endovascular approaches should be preferred in most cases if it is consistent with care goals.











 text new page (beta)
text new page (beta)