Introduction
Myocarditis is an inflammatory disease of the myocardium diagnosed by histological, immunological and immunohistochemical criteria. The World Health Organization and the working group of the Society and International Federation of Cardiology on the definition and classification of cardiomyopathies histologically define myocarditis as the presence of inflammatory infiltrates in the myocardium with degeneration and necrosis of myocytes of non-ischemic origin (Dallas criteria). Regarding immunohistochemical criteria the diagnosis would be made in the presence in the myocardium of ≥ 14 leukocytes/mm2, including up to 4 monocytes/mm2 and ≥ 7 CD3-positive T lymphocytes/mm2. The working group defines inflammatory dilated cardiomyopathy (DCM) as myocarditis associated with cardiac dysfunction.1,2 Myocardial damage leads to humoral and cellular response initiated in an attempt to eliminate the causal agent accompanied by edema, necrosis and regional or global alterations of myocardial contractility. Complete recovery of tissue and function can be seen in uncomplicated cases, whereas in severe cases an autoimmune reaction is triggered in the heart with myocardial necrosis and the release of antigens that perpetuate the damage. Progression to DCM occurs predominantly in patients with persistent inflammation who cannot eliminate the causal agent or develop antibodies against myocardial structures.3-6
Myocarditis has several different etiologies as infections, immune disorders, toxic agents, etc. The most common viruses implicated in myocarditis are coxsackie B, adenovirus, hepatitis C, cytomegalovirus (CMV), echovirus, influenza, Epstein-Barr, parvovirus B19 and herpes virus. In the past few years, myocarditis has been recognized as a complication of severe COVID-19.2,4-6 With the emerging use of the vaccine against COVID-19, post-vaccination-related acute myocarditis has been described.7-9 COVID-19 vaccine related myocarditis diagnosis is based on an appropriate clinical data scenario, cardiac biomarkers, and imaging studies, including echocardiogram, AngioCT and CMR. CMR allows the characterization of tissue and cardiac function and has become the non-invasive diagnostic gold standard in patients with suspected acute myocarditis.10-12
Case presentation
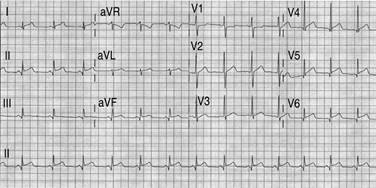
The authors describe a 16-year-old male case with no past relevant medical history. The patient had full immunization against SARS-CoV-2 with the BNT162B2 (Pfizer-BioNTech) vaccine; the second dose was administered in May 2021. On July 7, 2021, the patient complained of stabbing chest pain and was evaluated at another institution. A transthoracic echocardiogram was performed with global and segmental hypokinesia of the posteroinferior and mid-apical region. Left ventricular ejection fraction (LVEF) was normal with no pericardial effusion. Anti-inflammatory treatment was prescribed. The patient was admitted to our institution on July 10, 2021, with an improvement in symptoms. On emergency physical examination, vital signs were normal. An electrocardiogram (ECG) showed sinus rhythm, 81 bpm, normal QRS and elevation of ST-segment (Figure 1). Chest-X Ray was normal. Blood tests revealed elevated myocardial injury biomarkers: ultrasensitive troponin T 2123 ng /L, creatine phosphokinase (CPK) 1297 IU /L, MB fraction of creatine phosphokinase (CPK-MB) 105 ng /mL, brain natriuretic peptide (proBNP ) 493 pg /mL, D-dimer 618 ng /mL and C-reactive protein (CRP) of 14.97 mg /L. Coronary AngioCT was performed on July 11, 2021 with normal coronary arteries (Figure 2A). Slightly depressed left ventricular function with an ejection fraction of 47%, hypokinesia in non-contiguous segments and lower attenuation of inferolateral myocardium were observed (Figure 2B and 2C). Post-process was made, and inferolateral wall hypoperfusion was demonstrated (Figure 2D to 2F).

Figure 2: AngioCT with normal coronary arteries (A, B and C). Inferolateral hypodensity of myocardial wall (D) with hypoperfusion demonstrated after the post-process (E and F).
CMR (Optima™ MR450W GEM 1.5T, GE Healthcare, Chicago, IL, USA) with endovenous gadobutrol (Gadovist®) (0.1 mmol / kg) was performed to identify global hypokinesis of the LV with LVEF of 41%. The STIR sequence showed areas of increased myocardial signal intensity in the inferolateral, basal and medial, lateral-apical and anterior-apical segments, compatible with edema (Figure 3A). After administration of gadolinium, areas of the myocardium with early enhancement were identified in the anteroseptal basal, inferolateral basal, inferolateral medial, lateral-apical and anterior-apical segments compatible with hyperemia (Figure 3B). Non-ischemic pattern LGE in lateral subepicardial and midwall at the apical septal level was observed (Figure 3C). Pericardium was normal. The findings represent markers of non-ischemic intramyocardial inflammatory lesion. The viral serology was negative (Cytomegalovirus, Epstein Barr, Coxsackie A and B, Echovirus, as well as anti-Salmonella typhi and paratyphi, Brucella and Proteus). Due to the temporal relationship between vaccination and the development of signs and symptoms and having excluded other etiologies, this presentation of acute myocarditis is proposed to be an adverse reaction associated with the BNT162b2 vaccine against COVID-19. Beta-blocker, antihypertensive and anti-inflammatory treatment was prescribed. Due to the low-risk profile and favorable clinical evolution, an endomyocardial biopsy was not considered. The patient was discharged after five days without complications.

Figure 3: The CMR short axis STIR sequence showed increased myocardium signal (edema) in the inferolateral, basal and medial, lateral-apical, and anterior-apical segments (A). Early gadolinium enhancement (B) is compatible with hyperemia. Non-ischemic pattern late enhancement in lateral subepicardial and midwall is shown (C).
Discussion
Acute myocarditis has been recognized as an adverse event in patients vaccinated with Pfizer-BioNTech and Moderna (mRNA vaccines), mainly in adolescents and young adults. The Vaccine Adverse Event Reporting System (VAERS) had received 1,783 reports of myocarditis or pericarditis in the 12 and 29-year-old age group who received COVID-19 vaccines (November 4, 2021). Most cases in adolescents or young men after the second dose. The Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) confirmed 1,031 reports of myocarditis or pericarditis. The Clalit Health Services database in Israel reports its highest incidence in male patients aged 16-29 years, up to 10.69 cases per 100,000 persons.7-9,13
The Pfizer-BioNTech vaccine has been shown to be 94% to 95% effective in preventing COVID-19 infection in the 16- to 55-year-old population and 100% effective in the 12-to-15-year age group. To date, the FDA has authorized its emergent use in the population aged 5 to 15 years and full approval in 16 years and older. The long-term risks are still unknown. The adverse reactions described in different vaccines within the cardiovascular sphere are isolated cases of the acute coronary syndrome, atrial fibrillation, ventricular extrasystole and cardiac arrest.14-17
Under the clinical suspicion of acute myocarditis, the diagnostic approach includes a 12-lead ECG (changes in ST-segment, T wave, Q waves, AV and bundle branch block, arrhythmias; limited use), biomarkers of cardiac injury (high sensitivity, although low specificity), transthoracic echocardiography, CMR and endomyocardial biopsy in selected cases. Echocardiography helps to rule out other entities and to monitor changes in cavity size, myocardial thickness, ventricular function and pericardial effusion. Some alterations such as global ventricular dysfunction, alterations in segmental contractility or diastolic dysfunction are nonspecific. Myocardial deformation analysis by speckle tracking shows greater sensitivity for the detection of myocardial damage in patients with preserved LVEF and its prognostic evaluation.18,19
In patients who undergo AngioCT, hypodensity of the myocardium has been described. Post-process demonstrated hypoperfusion could be an indicator of edema, although further investigation on this topic is needed.20
CMR modified Lake Louise criteria (sensitivity 87.5%, specificity 96.2%) consists in identifying three diagnostic targets for myocarditis: edema, hyperemia and LGE. A positive case is defined as the presence of at least one T2 criterion (sensitivity 84.6%, specificity 88.5, precision 86.2%) with at least one additional T1 criterion (sensitivity 90.0%, specificity 76.9%, accuracy 84.8%). Pericardial effusion and LV wall abnormality are considered supporting criteria.10-12
Conclusions
Acute myocarditis is a multifactorial inflammatory condition of the heart that can be assessed non-invasively by using CMR-modified Lake Louise criteria. Post COVID-19 vaccine related myocarditis is a rare adverse event most likely seen in children, adolescents and young male adults. However, the authors believe that future research is needed to provide more evidence that can establish recommendations for immunization based on the well-known benefits of SARS-CoV-2 vaccination and better identification of patients with increased vaccine-related risk of adverse effects.











 text new page (beta)
text new page (beta)