Introduction
Cervical cancer (CC) mortality rates have decreased due to optimal prevention, early detection programs, and access to adequate health services. However, CC is one of the leading causes of death from cancer in women, especially in countries with insufficient human and financial resources to control this disease, mainly least-developed countries, developing countries, and countries with medium human development1. According to the Human Development Report of the United Nations Development Program, Mexico belongs to a group of countries with a high human development index (HDI)2. However, the incidence and mortality rates due to CC are similar to those with low or intermediate HDI2. This contradictory phenomenon is related to social disparities in Mexico.
In Mexico, CC ranks second in incidence in women and persists as a public health problem, with 7869 new cases diagnosed and 4121 estimated deaths in 20203. The slight decrease in the mortality rate documented since 1990 shows that disease control and prevention have not been homogeneous. The most affected states are Chiapas, Colima, Quintana Roo, Morelos, Yucatán, Veracruz, and Oaxaca, where health services are limited4,5.
The complete and adequate control of this disease requires the coordinated effort of multiple specialists and health institutions in a consolidated health system that allows universal access6. Therefore, hospitals must be qualified to manage CC patients in a standardized way, with the necessary diagnostic and therapeutic tools.
In Mexico, Seguro Popular (Public Health Insurance) has covered CC since 2004, and there were 64 Accredited Medical Units of Seguro Popular for its care in 29 States7. In 2017, of the total national population (119,938,473)8, 98.2 million (81.2%) had public insurance coverage. It served 54.9 million (45.4%), of which 13.3 million (24.2%) belonged to indigenous people9.
Mexico has made substantial efforts regarding innovation and quality in health10. Initially, the National Crusade for the Quality of Health Services generated a series of guidelines that considered three qualities, adequate access to health services, good medical care, and prevention, with the generation of strategies to improve the countrys health services11.
With these new strategies, the advancements in prevention, early diagnosis, and novel treatment modalities, we expected a decrease in mortality; however, mortality rates have not significantly improved for CC patients in the past decade3. Hence, after investigating the resources available in the Mexican health-care system, a lack of information was identified. Therefore, this study aimed to describe the resources available to treat CC in Mexican public institutions. A survey was conducted for physicians to investigate the resources available for diagnosing and treating CC patients in public institutions in Mexico.
Methods
Study design
An observational, cross-sectional, and stratified study was carried out in 2018. We created a closed voluntary survey for medical specialists in a branch of oncology, which consisted of questions about the resources and strategies for managing CC patients. Questions were structured by the principal investigator and her panel of experts. Before the launch of the survey, the adaptations of the model were made through the digital server Survey Monkey® platform. Internal tests were carried out for 8 weeks to confirm the proper operation and security of the data obtained in the survey. Cookies were used and were set on the first page. The IP address was used for the filters.
The survey could be accessed from the same IP address but 24 h later. Users could return to the survey if they did not complete it. They could not go back to previous questions already responded to; if they returned to the survey, the next unanswered question was automatically displayed. No duplicate individuals were analyzed; only the last survey completed was included in the analysis. The Sociedad Mexicana de Oncología (SMeO) (Mexican society of oncology, SMeO) emailed each specialist personal invitations. It clearly stated that no personal information would be collected from the participant. If they accepted to participate and opened the link to the survey, the header specified the approximate duration of 10 min to answer it, and personal information or identification of the participant was not collected. Each participant had to accept the survey terms and confidentiality data and check a box if they agreed to do the survey. In the email invitation, the participants were told the length of the survey availability and purpose of the study. The survey link was emailed to answer the survey anonymously, thus ensuring the confidentiality of the information of all participants. Each participant responded in 80 days maximum.
The survey comprised three sections: (1) Demographic information of the physician; (2) resources of the institution (equipment, personnel, and materials for the care of patients with CC); and (3) information related to diagnosis and treatment (Annex 1).
The survey had an initial section with 26 questions that were applied to all respondents:
− Page 1: General information of the participants. Five questions (from 1 to 5).
− Page 2: Institutional information. Six questions (from 6 to 11).
− Page 3: Information on equipment and supplies available. Fifteen questions (from 12 to 26).
Subsequently, the second part of each survey was configured with questions specifically focused on the area of specialty of the respondent, being as follows:
− Medical Oncologists: 34 questions in total (from 1 to 34)
− Surgeon-oncologists and gynecologist-oncologists: 29 questions (1 to 26 and 35 to 37)
− Radiation oncologists: 29 questions (from 1 to 26 and 38 to 40).
Where pertinent, items provided a non-response option such as “not applicable” or “I do not have that information,” and the selection of one response option was enforced. Once page 1 was completed, it was possible to advance to the next page without the opportunity to return to the previous one; at the end, the information was saved automatically, and the link was closed, being able to generate a certificate of gratitude for having participated later. No summary was displayed.
To compare the interregional differences in the results, these were stratified into four geographic regions of the Mexican Republic, North, Center-North, Center, and South, following the classification of the Banco de México12 (Fig. 1).

Figure 1 Distribution of medical specialists according to region. Top. Map of the Mexican Republic divided into four geographic regions, North, North-Center, Center, and South, following the classification of the Banco de México12. The states that comprise each region are specified. The percentage of participation of each region is described for each specialty. Bottom. The proportion of participants from each public institution included in the survey is shown.
The Ethics and Research Committees approved this study (018/002/ICI)-(CEI/1192/17). Because this was a voluntary survey with no risk involved, the study was approved without informed consent.
Participants
A list of the members from the SMeO13 and the Sociedad Mexicana de Radio-oncología (Mexican Society of Radio-oncology, SOMERA)14 was obtained. The survey was sent to medical oncologists, gynecologic oncologists, surgical oncologists, and radiation oncologists, assigned to public institutions, such as Instituto Mexicano del Seguro Social (IMSS), Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado (ISSSTE), Secretaría de Salud (SS), Secretaría de la Defensa Nacional, Secretaría de Marina, Petróleos Mexicanos, and University Hospitals (UH). The inclusion criterion for the participation of specialist physicians was the care of CC patients in a Mexican public institution. Incomplete questionnaires were eliminated from the analysis.
Statistical analysis
Only completed surveys were analyzed. No duplicate individuals were analyzed. The descriptive analysis determined the frequencies and percentages of the responses to each question. Items were not weighed; all were analyzed equitably. The response rates were compared among public health services, specialists, and regions. The uncertainty coefficient was used for the nominal variables, and Kendalls Tau-C was used for the ordinal variables. The statistical significance was established at p < 0.05. The statistical analysis was performed using SPSS version 23 (IBM Corp, USA, 2014).
Results
Demographic characteristics of the respondents
The survey was sent to a total of 1810 specialists, of whom 653 responded (36%); 147 participants were excluded, and 506 (27.9%) were analyzed (Fig. 2). From the total surveys analyzed, according to the participants specialty, 20.9% were medical oncologists, 15.8% were gynecologic oncologists, 45.8% were surgical oncologists, and 17.4% were radiation oncologists (Fig. 2). Twenty-nine percentages were female; the most frequent age range was 35-45. Ninety-two percentages of the respondents kept their current Mexican Council of Oncology certification. Within the Mexican Republic, the Center region had the highest participation (Fig. 1). Sixty-eight percentages belonged to third-level hospitals, while 31.8% belonged to second-level hospitals. The public institution with the highest participation in this survey was the IMSS (40.6%), as described in figure 1.
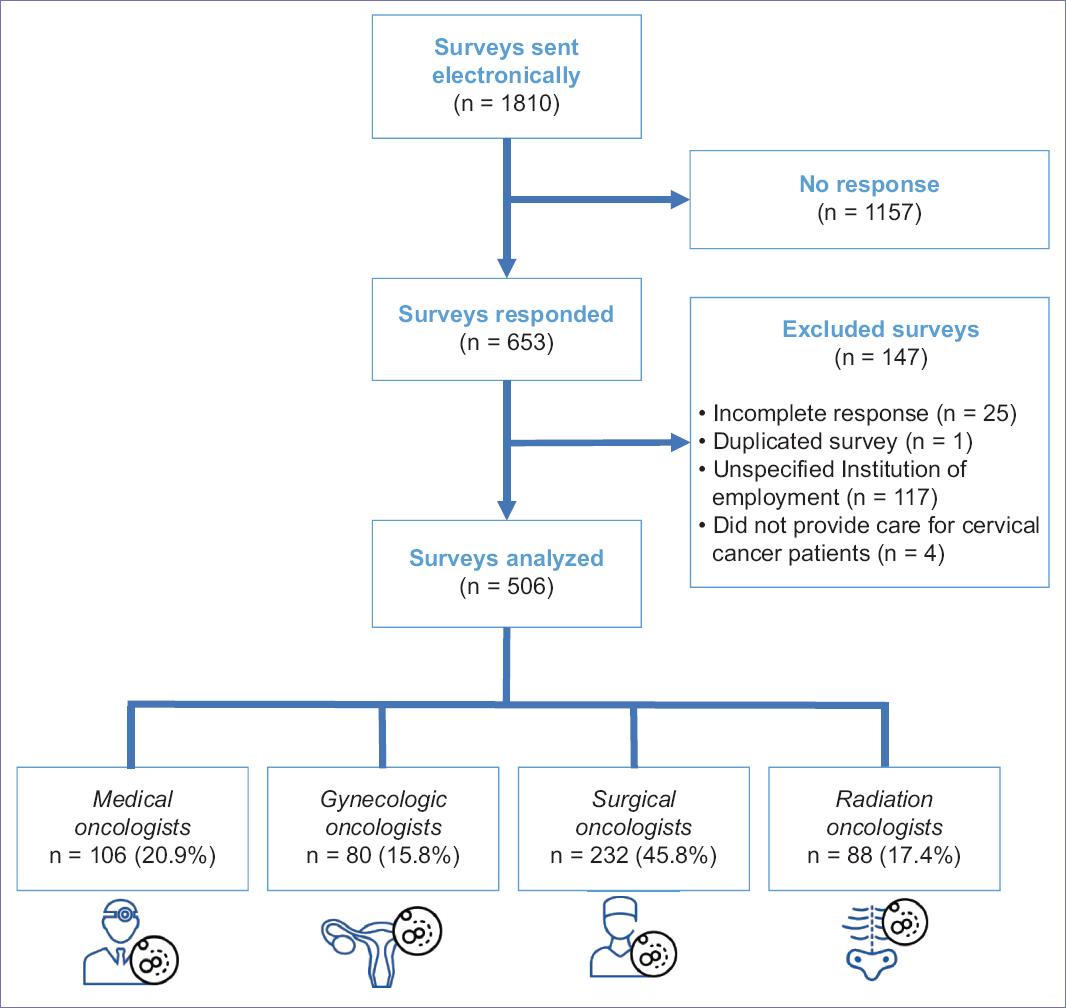
Figure 2 Flow diagram of survey study participants. Surveys were sent electronically to members of the Mexican Society of Oncology13 and the Mexican Society of Radio-oncology14. *Response rate was 36%, and 27.9% were analyzed. The surveys participants analyzed included medical oncologists, gynecologic oncologists, surgical oncologists, and radiation oncologists assigned to public institutions.
Care of CC patients
The specialists from the different institutions treated a different number of CC patients annually. About 35.4% of respondents attended up to 50 patients, 26.5% attended up to 100 patients, 21.1% up to 300 patients, and 17% cared for more than 300 patients. Regarding the time elapsed between diagnosis of the disease and the beginning of treatment, 50.1% of the respondents from the IMSS, Federal SS, and ISSSTE answered that starting treatment after diagnosis takes 5-8 weeks. On the other hand, 56% of specialists from State SS, PX-SD-SM, and UH began treatment after 1-4 weeks. About 69.4% of the respondents followed the National Comprehensive Cancer Network care guidelines for treating patients with CC.
Availability of equipment in public institutions
Regarding the resources for the diagnosis of CC patients, following the International Federation of Gynecology and Obstetrics (FIGO, 2018)15, 96.2% of the respondents answered that they had computed axial tomography (CAT), 2.2% had magnetic resonance imaging (MRI), and 1.5% had positron emission tomography (PET) or PET-CT (Table 1). From the participants with radiotherapy (RT) equipment in their institutions, 76.8% indicated that they had a linear accelerator, 7.8% had a cobalt pump, 13.7% had both, and 0.7% had TomoTherapy. The respondents that did not have RT equipment (39.5%) or brachytherapy (BT) equipment (44.7%) had to refer their patients to other institutions for treatment.
Table 1 Availability of equipment, services, and surgical resources for the care of CC patients
| Resources | Institutions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Available | All n (%) | IMSS n (%) | Federal-SS n (%) | State-SS n (%) | ISSSTE n (%) | PX, SD, SM n (%) | UH n (%) | pa | |
| Imaging | ✓ | 506 (100) | 207 (100) | 83 (100) | 84 (100) | 79 (100) | 26 (100) | 17 (100) | 1 |
| ✗ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Radiotherapy | ✓ | 307 (60.7) | 90 (43.5) | 74 (89.2) | 74 (78.7) | 34 (43) | 20 (76.9) | 15 (88.2) | < 0.001 |
| ✗ | 199 (39.3) | 117 (56.5) | 9 (10.8)b | 20 (21.3) | 45 (57) | 6 (23.1) | 2 (11.8) | ||
| Brachytherapy | ✓ | 280 (55.3) | 91 (44) | 69 (83.1) | 65 (69.1) | 23 (29.1) | 19 (73.1) | 13 (76.5) | < 0.001 |
| ✗ | 226 (44.7) | 116 (56) | 14 (16.9)c | 29 (30.1) | 56 (70.9) | 7 (26.9) | 4 (23.5)c | ||
| Pathology | ✓ | 479 (94.7) | 198 (95.7) | 81 (97.6) | 84 (89.4) | 75 (94.9) | 24 (92.3) | 17 (100) | 0.258 |
| ✗ | 27 (5.3) | 9 (4.3) | 2 (2.4)b | 10 (10.6) | 4 (5.1) | 2 (7.7)c | 0 | ||
| Algology | ✓ | 346 (68.4) | 112 (54.1) | 69 (83.1) | 67 (71.3) | 61 (77.2) | 24 (92.3) | 13 (76.5) | < 0.001 |
| ✗ | 160 (31.6) | 95 (45.9) | 14 (16.9) | 27 (28.7) | 18 (22.8) | 2 (7.7) | 4 (23.5) | ||
| Nutrition | ✓ | 341 (67.4) | 110 (53.1) | 73 (88) | 71 (75.5) | 54 (68.4) | 22 (84.6) | 11 (64.7) | < 0.001 |
| ✗ | 165 (32.6) | 97 (46.9) | 10 (12) | 23 (24.5) | 25 (31.6) | 4 (15.4) | 6 (35.3) | ||
| Psychology | ✓ | 360 (71.1) | 109 (52.7) | 71 (85.5) | 89 (94.7) | 55 (69.6) | 24 (92.3) | 12 (70.6) | < 0.001 |
| ✗ | 146 (28.9) | 98 (47.3) | 12 (14.5) | 5 (5.3) | 24 (30.4) | 2 (2.7) | 5 (29.4) | ||
| Psychiatry | ✓ | 134 (26.5) | 48 (23.2) | 36 (43.4) | 8 (8.5) | 26 (32.9) | 8 (30.8) | 8 (47.1) | < 0.0001 |
| ✗ | 372 (73.5) | 159 (76.8) | 47 (56.6) | 86 (91.5) | 53 (67.1) | 18 (69.2) | 9 (52.9) | ||
| Thanatology | ✓ | 136 (26.9) | 21 (10.1) | 44 (53) | 24 (25.5) | 29 (36.7) | 15 (57.7) | 3 (17.3) | < 0.001 |
| ✗ | 370 (73.1) | 186 (89.9)cd | 39 (47) | 70 (74.5) | 50 (63.3) | 11 (42.3) | 14 (82.4) | ||
| Operating room | ✓ | 130 (41.7) | 56 (43.4) | 16 (37.2) | 25 (39.7) | 24 (43.6) | 6 (42.9) | 3 (37.5) | 0.979 |
| ✗ | 182 (58.3) | 73 (56.6) | 27 (62.8) | 38 (60.3) | 31 (56.4) | 8 (57.1) | 5 (62.5) | ||
| Pre-operative studies | ✓ | 263 (84.3) | 98 (76) | 37 (86) | 57 (90.5) | 49 (89.1) | 14 (100) | 8 (100) | 0.005 |
| ✗ | 49 (15.7) | 31 (24) | 6 (14) | 6 (9.5) | 6 (10.9) | 0 | 0 | ||
| Consumables | ✓ | 254 (81.4) | 112 (86.8) | 30 (69.8) | 45 (71.4) | 50 (90.9) | 12 (85.7) | 5 (62.5) | 0.009 |
| ✗ | 58 (18.6) | 17 (13.2) | 13 (30.2) | 18 (28.6) | 5 (9.1) | 2 (14.3)e | 3 (37.5) | ||
| Surgical instruments | ✓ | 263 (84.3) | 114 (88.4) | 36 (83.7) | 47 (74.6) | 48 (87.3) | 13 (92.9) | 5 (62.5) | 0.101 |
| ✗ | 49 (15.7) | 15 (11.6) | 7 (16.3) | 16 (25.4) | 7 (12.7) | 1 (71) | 3 (37.5) | ||
| Surgical personnel | ✓ | 266 (85.3) | 110 (85.3) | 39 (90.7) | 50 (79.4) | 47 (85.5) | 14 (100) | 6 (75) | 0.170 |
| ✗ | 46 (14.7) | 19 (14.7) | 4 (9.3) | 13 (20.6) | 8 (14.5) | 0 | 2 (25) | ||
| Anesthesia | ✓ | 288 (92.3) | 121 (93.8) | 39 (90.7) | 59 (93.7) | 48 (87.3) | 14 (100) | 7 (87.5) | 0.431 |
| ✗ | 24 (7.7) | 8 (6.2) | 4 (9.3)d | 4 (6.3) | 7 (12.7) | 0 | 1 (12.5) | ||
| Post-operative recovery | ✓ | 290 (92.9) | 120 (93) | 40 (93) | 59 (93.7) | 49 (89.1) | 14 (100) | 8 (100) | 0.500 |
| ✗ | 22 (7.1) | 9 (7) | 3 (7) | 4 (6.3) | 6 (10.9)b | 0 | 0 | ||
✓Available, ✗Not available. IMSS: Instituto Mexicano del Seguro Social; SS: Secretaría de Salud; ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado; PX: PEMEX Hospital; SD: SEDENA Hospital; SM: SEMAR Hospital; UH: University Hospitals.
aUncertainty coefficient. Significantly affected regions were:
bSouth,
cNorth,
dNorth-Center, and
eCenter.
Availability of services in public institutions for the care of CC patients
Concerning other services necessary for the care of CC patients, 94.7% had a pathology service; the remaining 5.3% sent samples of patients tumors to other institutions. Psychology service was available for 71.1% of respondents, psychiatry service for 26.5%, algology service for 68.4%, nutrition service for 67.4%, and thanatology service for 26.9% (Table 1).
Early stages of CC treatment (FIGO IA-IIA)
Sixty-nine percentages of the specialists performed open surgery in their institutions, 19.9% performed laparoscopic surgery combined with open surgery, 7.1% used laparoscopic surgery, and 3.2% performed open and robotic laparoscopic surgery. Forty-seven percentages of the specialists indicated that 20% of the patients treated with radical hysterectomy receive adjuvant RT or chemo-RT (CT-RT) followed by BT. In contrast, 43.6% indicated that more than 40% of patients receive only adjuvant treatment with RT or CT-RT. The supplies for surgical treatment were sufficient in the institutions of 70.5% of the participants (Table 1). The main factor that affected the participants during surgical treatment was the lack of operating room equipment (58.3%) (Table 1).
Locally advanced stages of CC treatment (FIGO IB-IVA)
Most participants (93.5%) from the different public institutions responded that the treatment for locally advanced disease was concomitant CT-RT; the rest used neoadjuvant CT, followed by RT (4.5%), RT as single therapy (1.8%), and neoadjuvant CT with surgery (0.2%). Twenty-four percentages of the specialists referred more than 50% of their patients to another hospital to receive CT-RT. This situation was consistent in the four regions. Cisplatin was the most used radio sensitizer regardless of the institution or the region (98.1%). For bulky tumors (stages IB2 and IIA2), the most used treatment was RT or CT-RT (65.4%), followed by complementary hysterectomy (29.2%), surgery (4.5%), and other treatment modalities (0.8%).
Advanced (FIGO IVB), persistent, and recurrent stages of CC treatment
According to 89.6% of medical oncologists, first-line therapy for metastatic, persistent, or recurrent advanced disease consisted of cisplatin, carboplatin, or carboplatin in combination with paclitaxel (Table 2). The patients that received a second line of treatment, according to 55.6% of the oncologists, were < 40%, while patients that received three or more lines of treatment were < 20% (Table 2). Regarding the availability of drugs, most institutions had cisplatin, paclitaxel, carboplatin, and gemcitabine. Seventy-three percentages of respondents had vinorelbine, 26.4% had topotecan, and 41% had bevacizumab (Table 2). Regarding treatment adherence, 95.8% of the respondents stated that more than 20% of the patients abandoned the treatment. Oncologists believe that the reasons include financial problems (27.7%), patients personal decisions (27.7%), treatment toxicity (13.8%), and family decisions (3.2%).
Table 2 Treatment availability for CC patients in advanced disease (metastatic, persistent, or recurrent)
| Treatment availability | Institutions | ||||||
|---|---|---|---|---|---|---|---|
| IMSS (n = 39) (%) | Federal-SS (n = 29) (%) | State- SS (n = 10) (%) | ISSSTE (n = 18) (%) | PX, SD, SM (n = 6) (%) | UH (n = 4) (%) | p-value | |
| 1st line treatment | |||||||
| Cisplatin or carboplatin+Paclitaxel | 37 (94.9) | 28 (96.6) | 9 (90) | 14 (77.8) | 3 (50) | 4 (100) | |
| Cisplatin or carboplatin+Gemcitabine | 1 (2.6) | 1 (3.4) | 1 (10) | 0 | 1 (16.7) | 0 | |
| Cisplatin or carboplatin+Topotecan | 0 | 0 | 0 | 1 (5.6) | 0 | 0 | |
| Other | 1 (2.6) | 0 | 0 | 3 (16.7) | 2 (33.3) | 0 | 0.126a |
| Patients receiving 2nd line treatment | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.981b |
| 1-20% | 8 (20.5) | 3 (10.3) | 0 | 5 (27.8) | 2 (33.3) | 2 (50) | |
| 21-40% | 24 (61.5) | 16 (55.2) | 6 (60) | 9 (50) | 2 (33.3) | 2 (50) | |
| 41-99% | 7 (17.9) | 10 (34.5) | 4 (40) | 4 (22.2) | 2 (33.3) | 0 | |
| Patients receiving 3rd line treatment | |||||||
| 0 | 2 (5.1) | 0 | 0 | 0 | 0 | 1 (25) | 0.085b |
| 1-20% | 25 (64.1) | 19 (65.5) | 7 (70) | 15 (83.3) | 6 (100) | 3 (75) | |
| 21-40% | 10 (25.6) | 9 (31) | 3 (30) | 3 (16.7) | 0 | 0 | |
| 41-99% | 2 (5.1) | 1 (3.4) | 0 | 0 | 0 | 0 | |
| Availability of bevacizumab | |||||||
| ✓ | 15 (51.7) | 1 (10) | 17 (94.4) | 5 (83.3) | 1 (25) | < 0.001a | |
| ✗ | 35 (89.7) | 14 (48.3) | 9 (90) | 1 (5.6) | 1 (16.7) | 3 (75) | |
✓Available. ✗Not available. IMSS: Instituto Mexicano del Seguro Social; SS: Secretaría de Salud; ISSSTE: Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado; PX: PEMEX Hospital; SD: SEDENA Hospital; SM: SEMAR Hospital; UH: University Hospitals.
aUncertainty coefficient,
bKendalls Tau-C.
Discussion
In Mexico, access to cancer treatment, the segmentation of the health system, and the lack of information on resources for CC management make it difficult to obtain accurate data. This study allowed a comprehensive understanding of the characteristics of the infrastructure of the different public hospitals in the country, the resources, the specialized personnel, the supplies, and the equipment for diagnosing and treating women with CC.
The number of oncology specialists for CC care is limited
In this study, 106 Medical Oncologists participated, from 352 clinical oncologists reported nationwide (0.28/100,000 inhabitants for 2018)16. Globally, a median of 1.25 medical oncologists/100,000 population and 0.48/100 cancer patients is estimated17. Eighty-eight radio-oncologists participated, from 346 registered nationwide (0.26/100,000 inhabitants)18. Thus, we have insufficient oncology specialists. A limited number of oncology specialists generates more consultations for these medical professionals. The Global Survey of Clinical Oncology Workforce, which included 93 countries worldwide, identified that in 22 countries (24%), a medical oncologist provides care to < 150 newly diagnosed cancer patients; in 39 countries (42%), a medical oncologist offers medical care for more than 500 cancer patients. Of these, 26 countries were in Africa (81%), nine in Asia (47%), two in Europe (6%), and two in South America (29%). While in some countries, there is an extreme shortage of oncologists, where they attend to more than 1000 patients with this diagnosis, 25 countries are in Africa (78%), and two countries (11%) are in Asia16. This last piece of data is consistent with the results of our study, where 17% of those surveyed report attending more than 300 CC appointments per year, confirming the disparities in the workload of Mexican oncologists.
Deficiencies in patient care
For treatment success and a better prognosis of the life expectancy of patients with CC, the treatment must begin immediately19. In our study, half of the respondents answered that the treatment delay in their institutions was 5-8 weeks, and up to 5% reported more than 13 weeks delay. The impact caused by treatment delays in CC has been documented. A study suggests that waiting for pathology results to define the diagnosis can delay treatment20. Furthermore, patients with longer delays before RT have a reduction in overall and disease-specific survival21. Finally, another study found that patients who received treatment between 90 and 180 days, or more than 180 days after diagnosis, had an increased risk of death (1.33 and 1.36, respectively, p < 0.05) when compared with those who received treatment within 90 days after diagnosis22. In addition, participants reported that more than 20% of patients discontinued treatment after establishing the therapeutic regimen. Previous studies have identified multiple factors that influence treatment refusal or discontinuation by cancer patients23.
The adequacy of public hospitals
In our study, the most significant participation was carried out by IMSS physicians. Of the 246 IMSS second-level units, oncology patients are cared for in 111 (45.1%). In the third level of care, 19 hospitals (7 in Mexico City, 3 in Jalisco, 3 in Nuevo León, 2 in Guanajuato, 1 in Yucatán, 1 in Coahuila, 1 in Puebla, and 1 in Veracruz) offer services to cancer patients24. This data denotes a high urban concentration, which reflects the inequalities in hospital availability between localities and regions in the country. The states with the lowest levels of development have the highest number of public health units/100,000 inhabitants: Oaxaca, Nayarit, Chiapas, Guerrero, and Hidalgo. However, when it comes to resources specifically for cancer treatment, Mexico City concentrates most of the resources25. Thus, 96% of participants surveyed have access to CAT scanners, and only 2.2% have access to an MRI service, which is the method of choice recommended by FIGO for staging15.
Magos et al. surveyed to determine the countrys current infrastructure and RT capabilities. The total number of RT machines was 162, a median of three devices per state (ranging from 0 in Tlaxcala to 46 in Mexico City)18. These data are consistent with what was found in our study. The density of devices per million inhabitants in Mexico is the lowest among other OECD members26.
Regarding treatments
Surgical treatment is essential in the early stages of CC27. Respondents reported low equipment availability and insufficient surgical procedure supplies in this study. This lack of resources has been observed in low- and middle-income countries due to the cost of equipment, facilities, maintenance, and training time for qualified personnel and surgical specialists28.
Access to CT-RT treatment is essential for locally advanced CC since it constitutes 80% of the diseased population29. Several respondents reported that patients needed to move to another city or state to seek care, generating high costs, causing a lack of adherence and treatment abandonment, and conditioning a worse prognosis. Similar results were reported in the study by Torreglosa-Hernández et al. in the Mexican population with CC; 20.7% of women received medical care in a unit other than the habitual residence, with more significant displacement toward Mexico City30. In addition, the impact on prognosis has been demonstrated. For example, Gong et al. found that patients who lived in an urban area had a more favorable prognosis than those who lived in a rural area31. In addition, it has been established that patients who do not access this treatment have a poor prognosis and a 4-year loss in overall survival32.
Regarding the advanced stage of the disease, few specialists had the resources to indicate a second or third line of treatment. The combination of chemotherapy plus the monoclonal antibody bevacizumab has been the standard of care since it improves the overall survival of this group of patients27. However, the cost of this drug probably represents one of the main limitations of its use in Mexico. The survey reported that none of the patients treated in the participating institutions had access to this treatment in the countrys southern region. It is essential to add that Seguro Popular was replaced by the Instituto de Salud para el Bienestar (Institute of Health for Welfare, INSABI) in 2020. The results presented in this study correspond to the benefits provided by Seguro Popular, so the impact of the new policies established by INSABI on the current condition of CC care remains to be studied. We urge that guaranteeing womens access to CC treatment, particularly for those with significant social disadvantages regarding access to and coverage of highly specialized medical services, is essential.
Conclusion
Despite the investment in the health system in recent years, the resources and infrastructure for the care and treatment of CC patients in Mexico are limited. New public policies must be created and evaluated for this diseases proper diagnosis, prognosis, and treatment.
This study has some limitations. First of all, it was limited in scope. The response rate was 36%, and 27.9% were analyzed; also, most of the participants who completed the survey (52.3%) were from the central region, specifically Mexico City, which limits our findings in other areas of the country. Second, the opportunity to respond to the survey was only 80 days; therefore, we do not know if the non-responders were busy or not interested in the survey.











 text new page (beta)
text new page (beta)


