Introduction
Colorectal cancer (CRC) is among the three most frequent cancers in Central and South America. Representing 8% and 7.7% of all cancer cases among males and females, respectively, trends in most countries in the region reveal rising age-standardized mortality rates1. In addition, more than 50% of diagnosed patients with CRC will eventually develop metastatic disease which is inoperable in the majority of patients2.
Metastatic CRC (mCRC) is characterized by molecular heterogeneity and particularly dismal outcomes3. Current options for mCRC include cytotoxic chemotherapy, targeted therapies, and, most recently, immune checkpoint inhibitors. Decision on treatment selection is based on the patient’s clinical characteristics with biomarkers playing an important role given his diagnostic, predictive, or prognostic features4. Current mCRC biomarkers include mutations in proteins downstream the epidermal growth factor receptor (EGFR) signaling pathway, including K-RAS, N-RAS, and BRAF; mismatch repair proficiency or microsatellite instability (MSI); and HER2 expression (human EGFR2). The frequency of RAS mutation in CRC is 48.5%5. With KRAS mutation representing around 36% and NRAS 3%6, other reference reported specifically for mCRC that KRAS mutation prevalence is approximately 40%3. BRAF mutations are found in the tumors of between 8% and 12% of patients with mCRC and are almost exclusively non-overlapping with RAS mutations while tumors with MSI represent only 4-8% of tumors in patients with mCRC7.
Clinical guidelines (ESMO and NCCN) recommend testing for RAS (KRAS and NRAS) and BRAF at the time of mCRC diagnosis and before first-line therapy4, universal MSI testing is also recommended given its predictive value for the use of immune check points inhibitors in the treatment of mCRC patients7-9. RAS mutational status is a negative predictive marker for therapeutic choices involving EGFR antibody therapies (cetuximab or panitumumab) in the metastatic setting. Anti-EGFR agents have shown benefits for patients with RAS wild-type (RASwt) tumors in several trials, with better clinical outcomes in the left-sided tumors in which EGFR expression is enriched in comparison with the right-sided tumors6. The latter should be considered along with other clinical factors to decide on first-line therapy. BRAF mutations are a significant negative prognostic marker for patients with mCRC7. The typical continuum of care for mCRC patients involves a first-line induction therapy followed by maintenance therapy, second- and third-line therapy, and best supportive care. In the first-line setting, a chemotherapy backbone that includes a fluoropyrimidine plus oxaliplatin or irinotecan accompanied by a targeted agent, such as an antibody targeting receptors for vascular endothelial growth factor (VEGF) or EGF, is recommended based on the clinical characteristics of patients. NCCN and ESMO guidelines recommend using cetuximab or panitumumab in confirmed RASwt and BRAFwt patients6. Primary tumor location is a prognostic factor in patients with mCRC: RASwt left-sided tumors gain a clear benefit from initial treatment with CT combined with an anti-EGFR drug, and anti-VEGF agents could be considered as an alternative choice. The selection of treatment in second and further line therapies relies on the patient’s clinical status and, more importantly, on the systemic therapies provided upfront. Anti-angiogenic agents (e.g., bevacizumab, ramucirumab, and aflibercept) with chemotherapeutic agents are indicated for most patients in the second-line treatment. For patients receiving third-line treatment, ESMO guidelines consider cetuximab or panitumumab in RASwt and BRAFwt patients not previously treated with EGFR antibodies and regorafenib (a multi-kinase inhibitor) or TAS-102 (trifluridine/tipiracil) if they have already been treated with EGFR antibodies7. Thus, in-depth analysis of the tumor characteristics, including molecular profiling, maximizes the probability of success when treating mCRC patients. Nevertheless, there is little evidence about the use of these tests and of the guided selection of therapy in mCRC patients in Latin American countries. In general, the region shows relevant inequalities regarding cancer data collection, population coverage, and quality of registers. It has been reported that population-based cancer registries (PBCRs) cover only 30%, 25%, and 10% of the population in Argentina, Brazil, and Mexico, respectively. Furthermore, when high-quality PBCRs are available, these proportions are reduced to <10%, about 5% and 0%, for these countries, respectively1.
Considering the lack of data from public sources regarding treatment options for mCRC patients in Latin America, we used private data datasets to provide elements for discussion in the region’s medical community regarding treatment decision-making among mCRC patients. As an example, we analyzed the cases of Argentina, Brazil, and Mexico and performed descriptive analyses of the trends in the use of biomarkers and their relationship with the use of targeted therapies among mCRC patients.
Material and methods
This descriptive study leverages two non-public data sources to describe and outline a general landscape of the clinical characteristics and management of mCRC on patients with RASwt tumors in Argentina, Brazil, and Mexico, throughout a 3-year time span (2017-2019). Both data sources are designed and managed by a global human data science company with extensive background and capabilities in developing data collection instruments for research purposes. The supervision of data collection and data analysis was in charge of a professional team with strong experience on these tasks.
For Argentina and Brazil, the data source was a quantitative study designed ad hoc for the market of oncological products indicated for the treatment of mCRC. The study consists of online interviews applied to a sample of medical specialists who are requested to fill in a questionnaire (“Patient Diary”) that, in a strictly anonymized way, collects data on the clinical condition of up to six patients per physician. This encompasses demographic characteristics (age and gender), baseline clinical data at the time of diagnosis, as well as monitoring, treatment, follow-up, and health outcomes along the evolution process of patients. The inclusion criteria for patients are clearly defined and are shown to the physician at the beginning of the survey. The data collection instrument only includes closed questions and specifies instructions to be answered based on the medical records of each patient. Along the questionnaire, there are several electronic quality controls, such as confirmation messages (to go on) and cell blocking in case of inconsistent data. Thus, the data obtained come from real experience and reflect the achieved results in the context of everyday medical practice.
The sample of physicians was predetermined for each country according to the following inclusion criteria: to be a certified specialist in oncology and to be providing medical care and/or active follow-up to a minimum of six patients diagnosed with mCRC at the time of the interview. Inclusion criteria for patients were the following: to be at least 18 years old, to have an mCRC diagnosis, to be receiving pharmacological treatment for cancer, or having received it recently, and being under active medical surveillance by an oncologist, including a consultation visit within the past 3 months before the survey. During the data collection period, a team of specialists from the interviewing company reviewed each of the filled-in questionnaires to verify that the answers were complete and searched for possible inconsistencies. If any inconsistencies or missing data were found, the physicians were asked to make the necessary modifications. In addition, a search was carried out specifically aiming at identifying possible adverse events that were subject to generation of a pharmacovigilance report. The answers to the survey were collected quarterly (Q1–Q4). Selected physicians could vary in the different quarters, making this a dynamic panel of consulting oncologists. To avoid patient clustering, the number of patients per physician was limited to six per quarter. Results are presented as a percentage of all answers for each question.
For Mexico, data were obtained from a private validated oncology database. This instrument is a cross-sectional, syndicated, and anonymized survey that collects unbiased patient-level data provided at one specific point in time from a sample of cancer-treating physicians. The survey captures quarterly current treatment patterns in the cancer population also covering treatment modalities, most recent treatment, and patient profile as well as biomarkers tests, tumor localization, and stage for 15 types of cancer. Only mCRC cases were considered for the study. All the patients who were included in the survey had been personally treated by the oncologist during the most recent quarter. Database management included high-level quality control standards for the data collection, coding, processing, and creation of the final dataset.
For both databases, data from Q2 and Q4 of three consecutive years (2017, 2018, and 2019) were extracted from the analysis. The analysis of tumor variables includes the conduction of biomarker tests (RAS, BRAF, and MSI) and localization (right, left, and transversal); also, the preferred treatment schemes per line of treatment for RASwt tumors are depicted. Each variable is described per quarter and by country.
Results
The number of oncologists included in the survey varied from 40 to 96 across the three countries and quarters with an average of 48, 83, and 78 oncologists per quarter assessment for Argentina, Brazil, and Mexico, respectively. The average number of patients included per quarter was 286 for Argentina, 498 for Brazil, and 167 for Mexico (Table 1). In Argentina, the proportion of oncologists working in the private and public health-care system was similar across all periods while private practice was highly predominant in Brazil. On the other hand, public practice oncologists were predominant in Mexico (Table 2).
Table 1 Number of oncologists per country and number of patients included
| Q2-2017 | Q4-2017 | Q2-2018 | Q4-2018 | Q2-2019 | Q4-2019 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOs | n | TOs | n | TOs | n | TOs | n | TOs | n | TOs | n | |
| Argentina | 40 | 238 | 49 | 292 | 50 | 299 | 50 | 297 | 50 | 293 | 50 | 295 |
| Brazil | 81 | 481 | 99 | 591 | 80 | 477 | 80 | 478 | 80 | 480 | 81 | 483 |
| Mexico | NA | NA | NA | NA | 96 | 173 | 84 | 153 | 70 | 160 | 62 | 183 |
NA: Not available, n: Number of patients, TOs: Treating oncologists.
Table 2 Proportion of oncologists in public/private health care institutions
| Q2-2017 | Q4-2017 | Q2-2018 | Q4-2018 | Q2-2019 | Q4-2019 | |
|---|---|---|---|---|---|---|
| Argentina | ||||||
| Private | 60% | 49% | 44% | 54% | 60% | 60% |
| Public | 40% | 51% | 56% | 46% | 40% | 40% |
| Brazil | ||||||
| Private | 68% | 81% | 100% | 100% | 100% | 100% |
| Public | 32% | 19% | 0 | 0 | 0 | 0 |
| Mexico | ||||||
| Private | NA | NA | 27% | 26% | 24% | 26% |
| Public | NA | NA | 73% | 74% | 76% | 74% |
NA: Not available.
Regarding the molecular profiling of mCRC tumors, the proportion of patients tested for different biomarkers increased across quarterly assessments in the three countries with RAS testing being the most frequent in Argentina and Brazil. The proportion of patients tested by oncologist for RAS increased by 15% from Q2-2017 to Q4-2019 in Argentina, notably this increase was of 29% for Brazil in the same period. In Mexico, the proportion of RAS testing was fairly stable, only increasing 3% from Q2-2018 to Q4-2019. These values are lower when compared to the other two countries (Fig. 1A). In the RASwt patient subgroup, tumor localization was characterized (Fig. 1B). The majority of patients had tumors on the left side of the colon followed by the right-sided tumors and a smaller fraction showed tumors on the transverse colon. These trends were observed regardless of the country analyzed and the time period. The left-sided RASwt mCRC tumors, in Argentina, range from 57 to 63%, and while Brazil shows similar results (54-57%), in Mexico, the proportion is around 50% in the last three quarters reported (Q4-2018 to Q4-2019). Furthermore, in RASwt patients, BRAF testing showed a similar increasing trend in Argentina and Brazil with less than 20% tested in Q2-2017 and 48% in Q4-2019 in both countries. However, in Mexico, BRAF testing requests were higher in general, starting at 35% of RASwt patients in Q2-2018 and peaking at 71% in Q2-2019 (Fig. 1C). The least analyzed biomarker was MSI, with low numbers in the three countries. However, for Argentina and Brazil, there is an increasing trend toward the Q-4 in 2019, with 19% and 27% more patients being tested in Q4-2019 compared to Q2-2017, respectively. In Mexico, testing rates among RASwt patients were below 20% in all quarters assessed (Fig. 1D).
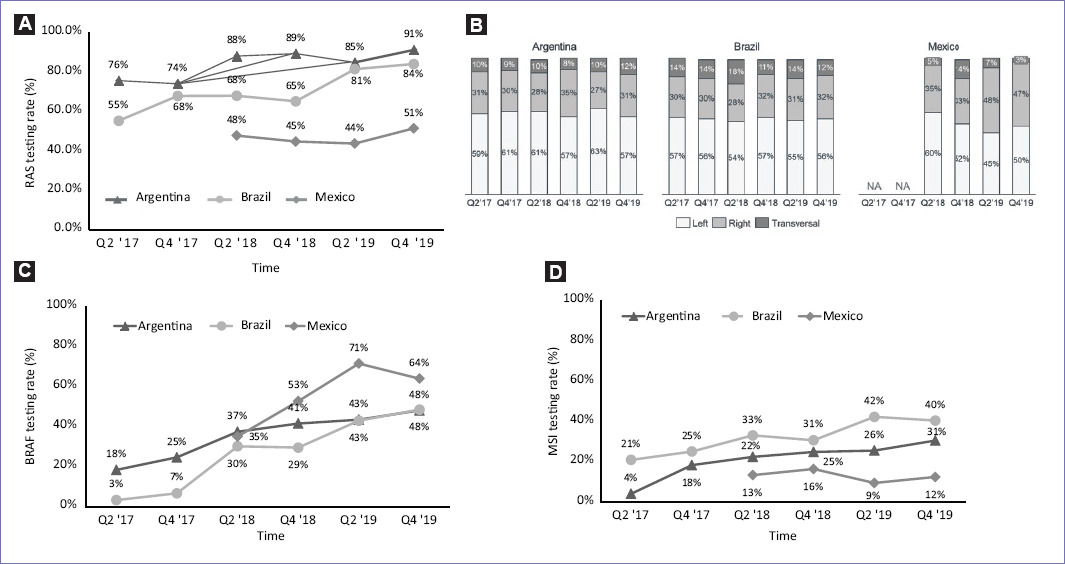
Figure 1 Access to metastatic colorectal cancer genetic testing in Argentina, Brazil, and Mexico from 2017 to 2019 and tumor localization in RASwt patients. RAS testing rate (%) per period (A); tumor localization in RASwt patients per period (B); BRAF testing rate (%) per period (C); MSI testing rate (%) per period (D), NA: Not available.
As we have already mentioned, the molecular characteristics of the tumors in mCRC patients are a key feature to be considered before initiating first line chemotherapy with or without the addition of targeted therapy. The possible treatment schemes for the first and second line were grouped as follows: chemotherapy alone, chemotherapy plus an anti-EGFR agent (cetuximab or panitumumab), chemotherapy plus an anti-VEGF agent (bevacizumab) immunotherapy (pembrolizumab), and other therapies that were not specified.
In RASwt patients, regardless of tumor localization, the use of chemotherapy alone was slightly higher in Brazil (20-37%) compared to Argentina (14-22%) and Mexico (12-24%). Conversely, the addition of an anti-EGFR agent to the chemotherapy backbone was the preferred choice of therapy in all three countries across all time points analyzed. In Mexico, the usage of anti-EGFR agents was the highest, reaching 76% in 2018 (Q2), while Brazil showed a stable increase over time and Argentina had the most stable proportion ranging from 53% to 62% (Fig. 2A). When the left-sided RASwt tumors were considered, the addition of anti-EGFR agents in the first line was largely the therapy of choice in all three countries. This therapy was selected by over 50% of all physicians regardless the time point analyzed (Fig. 2B). There was a fraction of patients with RASwt tumors that received a chemotherapy backbone plus an antibody targeting VEGF (bevacizumab), this varied from country to country yet never surpassed 26% (Fig. 2A). When focusing on RASwt left-sided tumors, this percentage dropped in all cases ranging from 12% to 22%.

Figure 2 Chemotherapy and therapies used in metastatic colorectal cancer metastatic colorectal cancer RASwt patients in first line. The first-line therapy in all mCRC RASwt patients per period in Argentina, Brazil, and Mexico (A); the first-line therapy in patients with the left-sided RASwt mCRC per period in Argentina, Brazil, and Mexico (B); CT, Chemotherapy alone; NA, Not available; anti-EGFRs (cetuximab and panitumumab), anti-VEGFs (bevacizumab).
As a part of the continuum of care, mCRC patients can be treated with subsequent therapy on progression. In the second-line setting, chemotherapy alone is used in Argentina and Brazil in the same proportion as in the first line, while in Mexico, physicians do not favor this regimen. Interestingly, there is a trend of increased use of anti-angiogenic therapy accompanying chemotherapy in detriment of the addition of anti-EGFR agents in the second line in all countries. This is likely due to the use of anti EGFR agents in the first line for these patients. Furthermore, immunotherapy became a choice for Brazil and Mexico for mCRC patients although in a low proportion (Fig. 3A). In the third-line setting, other treatment options become available for refractory mCRC, such as TAS-102 (trifluridine/tipiracil hydrochloride), a drug that prevents DNA synthesis, and regorafenib, a multi-kinase inhibitor with an angiogenic activity. Thus, the landscape of treatment appears more complex and heterogeneous as fewer patients are suited for these later line therapies. Given the limited number of patients reported in Mexico for third-line treatment, only data from Argentina and Brazil are presented (Fig. 3B).
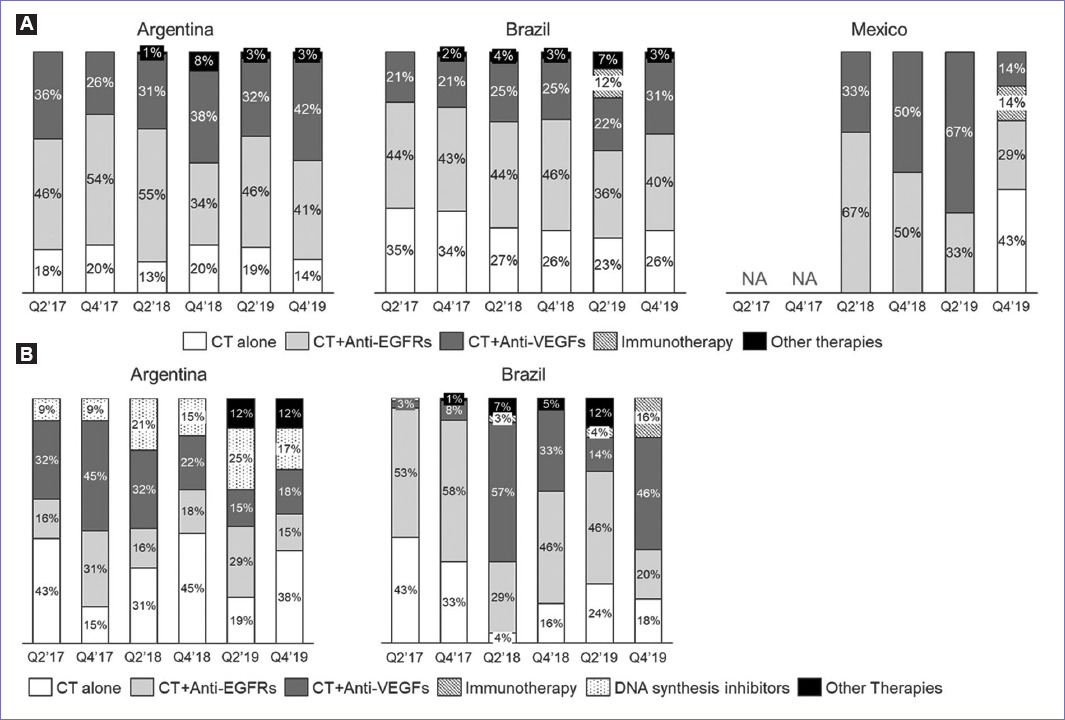
Figure 3 Chemotherapy and therapies used in metastatic colorectal cancer (mCRC) RASwt patients in second (A) and third line (B). The second-line therapy in mCRC RASwt patients per period in Argentina, Brazil, and Mexico (A); the third-line therapy in mCRC RASwt patients per period in Argentina and Brazil (B); CT, Chemotherapy alone; NA, Not available; anti-EGFRs (cetuximab and panitumumab), regorafenib, anti-VEGFs (bevacizumab), immunotherapy (pembrolizumab), and DNA synthesis inhibitors (TAS-102).
Chemotherapy alone is widespread as a treatment choice but when comparing between countries and over time, there is not a clear trend. In Argentina, the use of TAS-102 in 3L steadily increased in 2018 and 2019, while the proportion of anti-EGFRs use was also lower. In Brazil, anti-EGFRs were quite frequently used in 2017 with over 50% dropping in Q4 2019 to just 20%, while anti-angiogenic therapy showed dispersed results over time (3-57%). Interestingly, immunotherapy as a third-line agent was only seen in Brazil in Q4 2019 (16%). BRAF mutations may occur in mCRC patients who are wild type for K-RAS and N-RAS proteins (RASwt); this subset is shown in the three countries analyzed as a percentage of all mCRC cases in the database analysis. The prevalence of BRAF mutations in Argentina increased from 8% to 13% in 2 years (Fig. 4A), it should be kept in mind that the rates of BRAF testing also increased during this period (Fig. 1C). Similar rates are seen in Brazil with 13-14% in 2019 (the only available data). However, Mexico shows distinct results with a prevalence of only 4-7% in spite of having higher rates of BRAF testing.
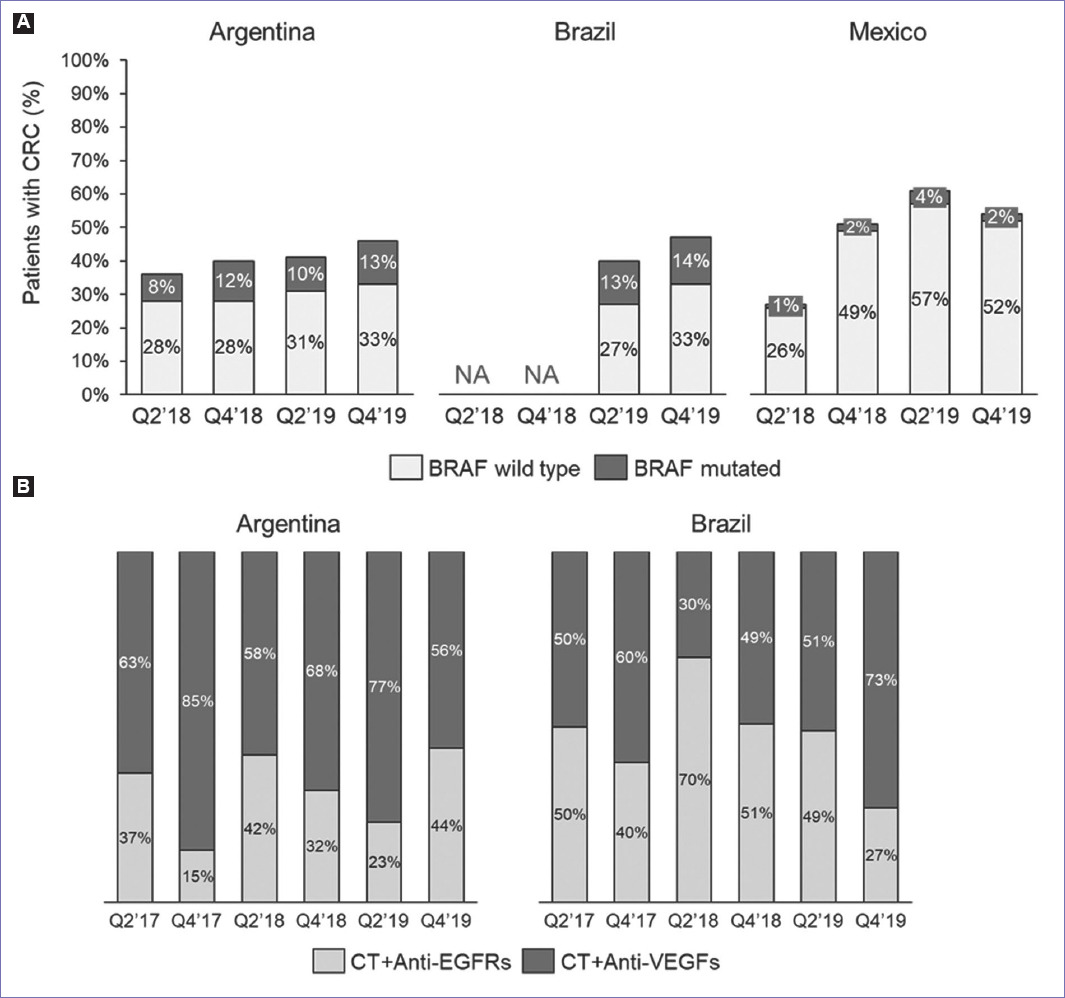
Figure 4 BRAF prevalence in all reported metastatic colorectal cancer (mCRC) patients and first-line treatment in BRAF-mutated mCRC patients. Prevalence of BRAF mutation per period in Argentina, Brazil, and Mexico (A); the first-line therapy in BRAF-mutated mCRC patients per period in Argentina and Brazil; NA, not available; anti-EGFRs (cetuximab and, panitumumab) and anti-VEGFs (bevacizumab).
Treatment of BRAFmt patients always included targeted therapy plus chemotherapy in all countries and across all time points (Fig. 4B). Antibodies targeting VEGF (bevacizumab) dominated in Argentina (56-85%), while in Brazil, this varied over time showing an increasing trend toward the end of 2019. Treatment data for Mexico are scarce given the low percentage of mutated patients and thus not presented.
Discussion
Cancer-related public data systems in Latin American countries have certain limitations. One way to address the lack of data is to leverage private databases developed for commercial purposes, which can help complement or expand the knowledge related to these health conditions at the population level. In this study, we aimed to describe the landscape of CRC medical care in 3 Latin American countries. Our intention was to provide elements for discussion and to generate research questions and hypotheses to be tested, to stimulate further research work.
During the study period, the use of biomarker tests (RAS, BRAF, and MSI) increased in the three countries, as suggested by the most recent international guidelines, but in different proportions. RAS testing grew by 29% points in Brazil (from 55% to 84%), 15 in Argentina (from 76% to 91%), and in 3% points in Mexico (from 48% to 51%). The use of the BRAF test increased in a more evident way: in Brazil, it went from only 3% of patients to 48%, while in Argentina, it went from 18% to 64%. In the case of Mexico, the first available data in Q2-2018 were 35%, which increased up to 71% and then decreased to 64% in the last measurement. The high rate in Brazil is likely due to the predominance of specialists with private practice, while Mexico, on the contrary, mostly has a public clinical practice and the testing rates are low. In the case of Argentina, the reason behind this increase may be related to the incorporation of BRAF testing into the gene panel supported by the pharmaceutical industry; this may also be explaining the rising trends depicted here.
The trends for MSI testing rates were different. Although there was an increase in its use in Brazil (from 21% to 40%) and particularly in Argentina (from 3% to 31%), Mexico showed a remarkably low rate with only 12%. Considering this test is recommended in patients diagnosed with CRC regardless the stage of disease (6), this could explain the lower rates observed in the metastatic setting. Yet, other factors may come into play, such as limited testing which is the case in Mexico and Argentina. In addition to its high cost, the current non-approval by regulatory agencies for the use of immunotherapy in patients with mCRC with MSI may also explain the lower testing index. The assessment of MSI is key to rule-out familial forms of CRC (6) and it also guides treatment with immunotherapy in the metastatic setting (10). In general, tumor localization in RASwt patients showed a similar distribution in Argentina and Brazil, with more than half located on the left side, about a third on the right side and around 10% within the transverse colon. The measurements in Mexico showed more variability, with the left side tumors moving from 60% to 45% with the last report in 50%. Similarly, the right side tumors had the highest value with 48% and the lowest with 33%; while the transversal ranged from 14% to 3%.
With regard to first-line treatment for RASwt patients, chemotherapy plus an anti-EGFR agent was the preferred treatment for both, left- and right-sided tumors in the three countries. When the left-sided RASwt tumors were considered, there was a higher use of this combination. This is in agreement with the enriched EGFR expression, reported for the left-sided tumors compared with the right-sided tumors6. For the second option of therapy, there is a variation among the measurements across countries as well as variability within the three countries, which involve chemotherapy plus anti-VEGFs and chemotherapy alone. Although it is not possible to outline a defined trend in the analyzed periods or by country, in the case of Mexico, many of the patients are from public medical institutions where the lack of resources limits the use of anti-EGFR agents in the first-line treatment of patients with RASwt mCRC and other treatment options must be chosen.
The usage of anti-EGFR agents in second-line therapy can be considered if the tumor is RAS and BRAF wild type (and left primary tumor) but the patients who received a first-line EGFR-based therapy should be switched to a VEGF-targeted agent4. In our analysis, the proportion of patients receiving anti-EGFR agents in the second line is between 34% and 55% in Argentina and Brazil, while in Mexico, this proportion is more variable (29-67%). This could be the case for patients who did not receive it in first line. Other factors that might also play a role are access to biomarker testing and/or to targeted therapy.
As expected, we found a wider range of options for third-line therapies, with drugs such as TAS-102 and regorafenib becoming available in this setting. Factors guiding treatment selection include not only the molecular profile of the tumor but also importantly the characteristics of the patient, potential severe adverse events, and agents used in previous lines10. Furthermore, as of the end of 2020, immunotherapy should be considered for the treatment of mCRC MSI-H patients. Nevertheless, this option is not registered in Latin America for this specific indication in the first line but in subsequent therapies11. Clinical trial enrolment is suggested, especially for BRAF- and HER2-directed combinations4. Furthermore, there is evidence that including an anti-EGFR drug (such as cetuximab) for the second time in the third line in patients with RASwt mCRC that had a good response in the first line to this agent, can be a valid option of treatment12.
The proportion of mCRC patients with BRAF mutation was approximately 10% in Argentina and Brazil and notoriously lowers in Mexico (2%). These proportions are in agreement with the frequency of patients with BRAF-mutated mCRC reported in the region, 7.8% for Latin America13 and 10.6% in Canada14. BRAF-mutated mCRC has a poor prognosis14 and the presence of the most common pathogenic variant (p.V600E) is associated with a low response to anti-EGFR therapy6. Current first-line chemotherapy for these patients includes the combination of a fluoropyrimidine and either irinotecan or oxaliplatin15. As we report here, physicians in Brazil and Argentina also add biological agents, anti-VEGFRs, or anti-EGFRs to chemotherapy.
Analysis of population-level health data provides the opportunity for improving the patient care process, through the identification of uncovered needs, by assessing trends in clinical practice, or by thoroughly planning the acquisition of the necessary resources for health care. In this paper, we provided meaningful results that describe the landscape of mCRC diagnosis and management in 3 Latin American countries using data from private sources. These data add value given the scarcity of official information and allow for better understanding of the current situation to improve the diagnosis and treatment of the disease in the region.
Conclusions
Within Latin American countries, cancer-related data availability is limited. Alternative data sources, such as market research or private databases, can be a good complement to help identify improvement opportunities in the care process, either at the clinical level or at the resource planning stage.











 text new page (beta)
text new page (beta)


