Introduction
Prostate cancer is one of the most common cancers in men in the world and in Latin America1. In Mexico, approximately one-third of patients present with de novo metastatic disease2. Androgen deprivation therapy (ADT) is the mainstay of treatment for advanced prostate cancer, with either medical or surgical castration3.
Surgical castration achieved with bilateral orchiectomy and estrogen-based medical therapy was the cornerstones of treatment for metastatic prostate cancer for several decades. Diethylstilbestrol (DES) is an orally administered synthetic estrogen with established activity against prostate cancer since the 1940s4. DES administration lowers testosterone, dehydroepiandrosterone, and estrone levels, and suppresses the hypothalamus-pituitary axis as well. In addition, DES has a direct cytotoxic and proapoptotic effect on prostate cancer cells, independent of the estrogen receptor pathway5.
In the 1980s, medical castration using gonadotropin-releasing hormone (GnRH) agonists was first reported as a treatment of advanced prostate cancer, with a similar response rate and 1-year survival similar to DES, but with a better safety profile6. Treatment with DES was associated with a higher rate of gynecomastia, nausea, vomiting, edema, and thrombotic events. Thus, once GnRH agonists were available in subcutaneous deposit presentations, the use of DES at conventional dosing was phased out.
Nevertheless, treatment with DES continued to be studied at lower doses: a dose of 1 mg/day offered similar efficacy in overall survival (OS) when compared to the higher 5 mg/day dose. In addition, there were fewer cardiovascular-related deaths in the lower dose group7. Moreover, given that most patients develop resistance to initial ADT, interest in DES as a later line treatment for patients with castration-resistant disease was maintained, using the safer lower dose of 1 mg/day8.
In the past 20 years, several therapeutic strategies have improved OS in metastatic castration-resistant prostate cancer (mCRPC), including taxane-based chemotherapy, newer androgen receptor-directed therapies, radiopharmaceuticals, and immunotherapy9-14. However, many of these options are not readily available for patients, due to limitations in health-care coverage and high out-of-pocket costs, even more so for patients living in low-and-middle-income countries (LMICs). Therefore, there is a need to search additional cost-effective therapies to include in treatment sequencing in this setting.
The aim of this study was to describe the efficacy and tolerability of treatment with low-dose DES in patients with mCRPC. There is no information from Latin America on treatment with DES for mCRPC in the context of contemporarily available therapies.
Materials and Methods
After obtaining local Institutional Review Board approval (reference number 3502), we performed a single-center retrospective review of data for patients ≥ 18 years diagnosed with mCRPC who received DES as a treatment for this diagnosis between January 2005 and September 2020. We excluded patients with treatment or follow-up outside of our institution and those without follow-up since the beginning of treatment with DES. We obtained demographic, clinical, laboratory, pathology, and treatment information from the medical records.
We used descriptive statistics for the demographic characteristics of the population, with parametric and non-parametric tests according to the distribution of the data. We compared patients who had received chemotherapy before treatment with DES to those who had not had prior chemotherapy. We evaluated prostate-specific antigen (PSA) biochemical response, defined as a 50% or greater decrease in PSA measurement at least 4 weeks apart. Progression-free survival (PFS) was defined as the time from DES initiation to disease progression (biochemical, radiographic, or clinical) or death from any cause. OS was defined as the time from DES initiation to death from any cause. PFS and OS were calculated using Kaplan–Meier curves and compared using the log-rank test. Patients lost to follow-up were censored at the date of last follow-up.
Results
We identified 34 patients who met the inclusion criteria, with a median follow-up of 7.9 months.
The median age at treatment initiation was 74 years (range 56-94 years). Twenty-three patients (67.6%) presented with de novo metastatic disease. The median time from initial diagnosis of prostate cancer to the start of DES was 33 months. The median number of previous lines of treatment was 3 (range 1-7), and 24 (64.7%) had received at least one line of chemotherapy before treatment with DES. Previous use of abiraterone and enzalutamide was 32.4% and 8.8%, respectively. Other baseline population characteristics are reported in table 1.
Table 1 Baseline characteristics
| Characteristic | n (%) or median (interquartile range) |
|---|---|
| Age at start of DES | 74 years (64-81) |
| Comorbidities | |
| Type 2 diabetes mellitus | 13/34 (38.2%) |
| Arterial hypertension | 10/34 (29.4%) |
| Dyslipidemia | 11/34 (32.4%) |
| Any cardiac comorbidity | 5/34 (14.7%) |
| Prior major cardiovascular eventa | 3/34 (8.8%) |
| Overweight or obesity | 14/34 (41.2%) |
| Time of sensitivity to initial ADT | 15 months (10-25) |
| Time from prostate cancer diagnosis to start of DES | 33 months (20-99) |
| Gleason score | 8 (7-9) |
| De novo metastatic disease | 23/34 (67.6%) |
| Sites of metastases | |
| Bone | 30/34 (88.2%) |
| Lymph node | 22/34 (64.7%) |
| Visceral | 6/34 (17.6%) |
| Previous systemic treatments | |
| Androgen deprivation therapy | 34/34 (100%) |
| Chemotherapy | 22/34 (64.7%) |
| Docetaxel | 21/34 (61.8%) |
| Cabazitaxel | 0/34 (0%) |
| Platinum derivatives | 4/34 (11.8%) |
| Bicalutamide or flutamide | 19/34 (55.9%) |
| Enzalutamide | 3/34 (8.8%) |
| Abiraterone | 11/34 (32.4%) |
| Ketoconazole | 3/34 (8.8%) |
| Radium-223 | 2/34 (5.9%) |
| Lutetium-177 | 4/34 (11.8%) |
| Total of previous lines | 3 (1-7) |
| Performance status | |
| ECOG 0 | 5/34 (14.7%) |
| ECOG 1 | 11/34 (32.4%) |
| ECOG 2 | 6/34 (17.6%) |
| ECOG 3 | 2/34 (5.9%) |
| Missing | 10/34 (29.4%) |
| Laboratory values | |
| PSA (ng/ml) | 80.3 (23-277) |
| Hemoglobin (g/dl) | 13.1 (9.7-15.8) |
| Alkaline phosphatase (U/L) | 141 (62-1573) |
| Albumin (g/dl) | 4.06 (3.19-5.4) |
| 25-hydroxyvitamin D (ng/ml) | 32.9 (13.9-62.5) |
aMajor cardiovascular event: acute coronary syndrome or stroke.
ADT: androgen deprivation therapy; DES: diethylstilbestrol; ECOG: Eastern Cooperative Oncology Group performance scale; PSA: prostate-specific antigen.
The majority of patients were treated with an initial dose of DES 1 mg/day (26 patients, 76.5%). Seventeen patients (50%) deemed to have an insufficient response or disease progression while having adequate treatment tolerance received a dose increase. DES was combined with low-dose aspirin in 76.5% and with oral anticoagulants in 14.7% (Table 2).
Table 2 Treatment characteristics
| Characteristic | n (%) |
|---|---|
| Initial dose of DES | |
| 1 mg | 26/34 (76.5%) |
| 2 mg | 6/34 (17.6%) |
| Missing | 2/34 (5.9%) |
| Dose increased | 17/34 (50.0%) |
| Maximum dose of DES | |
| 1 mg | 9/34 (26.5%) |
| 2 mg | 20/34 (58.8%) |
| 3 mg | 3/34 (8.8%) |
| Missing | 2/34 (5.9%) |
| Type of thromboprophylaxis | |
| Aspirin | 26/34 (76.5%) |
| Oral anticoagulant | 5/34 (14.7%) |
| None | 3/34 (8.8%) |
DES: diethylstilbestrol.
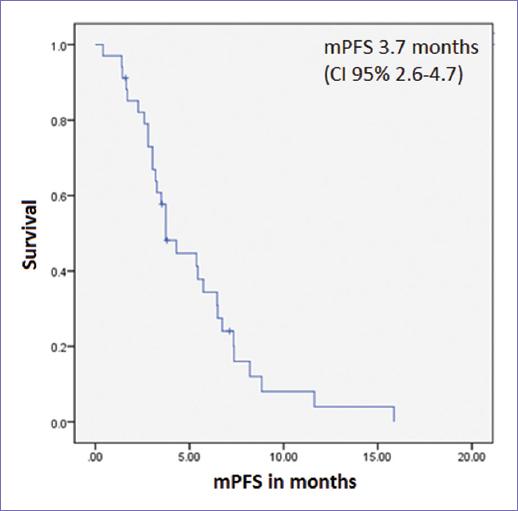
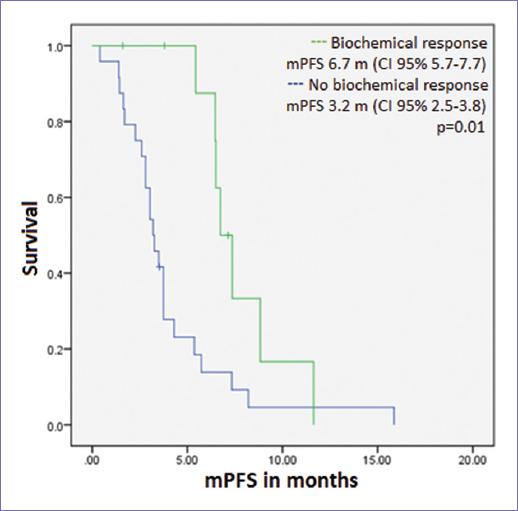
A biochemical response was observed in 10 of the 32 patients evaluable for response (31.3%). Biochemical response rate in patients with prior chemotherapy was 33.3% and 27.3% in those without prior chemotherapy use, without a statistically significant difference (p = 0.72). Median PFS (mPFS) was 3.7 months (CI 95% 2.6-4.7, Fig. 1). There was no difference in mPFS according to age or stage at diagnosis, number of previous lines of treatment, prior use of chemotherapy, or maximum dose of DES. mPFS was longer in those with a biochemical response to DES: 6.7 versus 3.2 months (p = 0.01, Fig. 2).
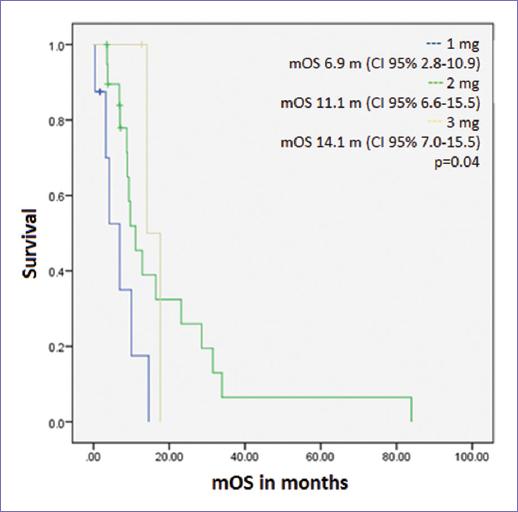
Median OS (mOS) was 9.7 months (CI 95% 7.0-12.3, Fig. 3). There was no difference in mOS according to age or stage at diagnosis, number of previous lines of treatment, prior use of chemotherapy, or biochemical response to DES. mOS differed according to the maximum dose of DES received: 1 mg 6.9 months, 2 mg 11.1 months, and 3 mg 14.1 months (p = 0.04, Fig. 4). Fourteen patients (41.2%) received at least one subsequent line of treatment for prostate cancer.
Thirty patients were evaluable for toxicity. The most common adverse events were fatigue (66.6%), gynecomastia (56.6%), and nausea (36.6%), all Grades 1-2 (Table 3). Five patients received prophylactic breast radiotherapy for gynecomastia. One patient suspended treatment due to adverse events (non-specified bleeding). There were two deaths potentially related to treatment with DES: one due to arterial mesenteric thrombosis and one due to a myocardial infarction. The former occurred in a patient who did not receive any form of thromboprophylaxis, and the latter occurred in a patient under total anticoagulation with a history of a cardiac valve replacement. There were no other arterial or thrombotic events.
Table 3 Treatment-related adverse events
| Adverse event | Total | Grade 1 | Grade 2 | Grades 3-5 |
|---|---|---|---|---|
| Gynecomastia | 17/30 (56.7%) | 11/30 (36.7%) | 6/30 (20.0%) | 0/30 (0%) |
| Arterial thrombosis | 2/30 (6.7%) | 0/30 (0%) | 0/30 (0%) | 2/30 (6.7%) |
| Venous thrombosis | 0/30 (0%) | 0/30 (0%) | 0/30 (0%) | 0/30 (0%) |
| Fatigue | 20/30 (66.7%) | 8/30 (26.7%) | 12/30 (40%) | 0/30 (0%) |
| Edema | 8/30 (26.7%) | 8/30 (26.7%) | 0/30 (0%) | 0/30 (0%) |
| Nausea | 11/30 (36.7%) | 9/30 (30.0%) | 0/30 (0%) | 0/30 (0%) |
| Vomiting | 3/30 (10.0%) | 3/30 (10.0%) | 0/30 (0%) | 0/30 (0%) |
Discussion
Despite the fact that treatment with DES was mostly ceased to be used due to the reasons exposed in the introduction, lower doses of DES have continued to be studied as a treatment for patients with progressive CRPC. In this series, we observed activity of this treatment, with a biochemical response rate of 31.3% in patients with several previous lines of therapy.
In a retrospective study15, 63 patients with CRPC (30 with metastatic disease) were treated with 1 mg of DES daily in combination with aspirin. The biochemical response rate in patients without prior chemotherapy use was 38.8%, with a median time to progression of 30 weeks. In patients who had received chemotherapy, there were 3/9 biochemical responses, with a median time to progression of 11.9 weeks.
Another retrospective study from the Gustave Roussy Institute16 reported results from 20 patients treated with DES 1 mg daily, all previously treated with docetaxel. There was a PSA reduction of ≥ 30% in 5 (25%) patients. mPFS was 3.7 months and mOS was 20.7 months. Biochemical response and mPFS are similar to those in our series, however, these studies report longer OS. As possible explanations for a shorter OS in our study, in this series, we describe heavily pretreated patients, with a median of three lines of treatment before the use of DES; in addition, the use of subsequent therapies after suspension of DES was relatively low (41.2%).
In comparison, other approved therapies for mCRPC after administration of docetaxel achieve mPFS of 2.8-8.3 months and OS of 14-18 months. These include cabazitaxel, abiraterone, enzalutamide, and radium-22310-13.
Regarding the observed association of an increasing dose of DES with improved survival, due to the small sample size, our analyses are merely hypothesis generating. No other previous series have reported on increasing the dose of DES for patients with progressive disease while maintaining adequate treatment tolerance. This association perhaps could also be explained by those patients having less aggressive disease being considered as maintaining some clinical benefit from DES and therefore selected to maintain the same treatment at a higher dose rather than continuing on to other lines of therapy.
As for adverse events, in the previously mentioned study by the University of Colorado, 59% of patients presented gynecomastia, which was managed with mammary gland irradiation in half of patients. There were two venous thrombotic events (lower extremity deep vein thrombosis) which required initiation of anticoagulant treatment. In that study, there was no Grade 4 or 5 adverse events. In our study, the two potentially treatment-related deaths were due to arterial thrombotic events, and one occurred in a patient with cardiovascular risk factors. Even if the use of lower doses of DES has a lower toxicity compared to the doses initially described for the treatment of advanced prostate cancer, clinicians should administer this treatment in patients eligible for thromboprophylactic medications, and keep in mind that prostate cancer occurs in a population with a higher cardiovascular risk. Besides, in the context of mCRPC, all patients have been previously exposed to some form of ADT, which has been associated with major cardiovascular events in population-based studies17-19. This emphasizes the importance of adequate patient selection for treatment with DES.
Although there are no specific recommendations for the follow-up of patients treated with estrogens for prostate cancer, it is important to note the incidence of cardiovascular risk factors in our series (diabetes in 38.2%, arterial hypertension in 29.4%, dyslipidemia in 32.4%, and overweight/obesity in 41.2%). Therefore, it seems reasonable to extrapolate recommendations to manage cardiovascular risk from those for patients under other forms of ADT. Another potential risk mitigating strategy is the use of transdermal estradiol, since the production of prothrombotic proteins is avoided through bypassing the hepatic metabolism of estrogen. Transdermal estradiol is currently under study as a first line of treatment for patients with advanced prostate cancer (PATCH [NCT00303784] and arm L of the STAMPEDE trial [NCT00268476])20.
The cost of treatments is also of great importance due of its impact for patient access and adherence to treatment for mCRPC. There are no cost-effectivity analyses performed in this context in Mexico, however, the daily cost of treatment with 1 mg DES is approximately 23 Mexican pesos (around 1 USD)21, whereas the daily cost of abiraterone or enzalutamide is approximately 2800 Mexican pesos (around 136 USD)22. A retrospective study performed through the United States Medicare system, patients who received low-income subsidies had lower rates of adherence to treatment with abiraterone or enzalutamide23.
This study has limitations associated to its retrospective nature. However, as future directions, low-dose DES, starting from a 1 mg dose, could be studied in prospective randomized studies in comparison to already available later-line treatments, with the intention of finding active therapies that are cost effective for patients and health-care systems, particularly in resource-limited settings such as Mexico and many other LMICs24.
Conclusions
Low-dose DES, starting from a 1 mg daily dosing, showed a promising biochemical response rate in heavily pretreated patients with mCRPC, with similar a mPFS to that of other available treatments that may not be accessible in resource-limited settings. Patient selection and administration with thromboprophylaxis are of utmost importance to ensure treatment safety.











 text new page (beta)
text new page (beta)