Introduction
Otitis media (OM) are the inflammation of the middle ear and mastoid cells. This inflammation can be due to various factors such as foreign bodies, chemical substances, or infection. If the inflammation lasts longer than 6 weeks, accompanied by ear discharges (otorrhea), without fever or pain, then OM is considered chronic suppurative OM (CSOM). CSOM is characterized by an insidious, progressive onset and in case of potential complications; it can lead to sequelae1.
Complications secondary to CSOM are more frequent in adult patients. These are divided into intracranial and extracranial complications. As for intracranial complications, the most common is meningitis. It can present itself as hematogenous dissemination from the subarachnoid space, by contiguity of the middle ear through Hyrtl's fissure or by bone erosion and, finally, by direct extension. Other intracranial complications include brain abscess, thrombosis of the lateral sinus, or sigmoid sinus1.
Extracranial complications are subdivided into extratemporal and intratemporal complications. Extratemporal complications include subperiosteal abscess, Bezold's abscess, and deep neck abscess. Intratemporal complications include cholesteatoma, labyrinthine fistula, cochlear fistula, mastoiditis, petrous apicitis, and facial paralysis. Labyrinthine fistula is primarily associated with cholesteatoma, in affecting the horizontal semicircular canal 90% of the time2.
Cholesteatoma is the main intratemporal complication2. Cholesteatoma is a non-neoplastic lesion with destructive properties. It is characterized by the migration of keratinized squamous epithelium with ciliated pseudostratified columnar epithelium lining, in a fibrous stroma within the middle ear and the mastoid cavity. The existence of molecular dysregulation involving inflammatory mediators and growth factors has been detected in these cases3. The incidence of cholesteatoma in adults varies according to the population, ranging from 4.2 to 9.2/100,000 adults and is 1.4 times more common in men than in women4.
Cholesteatoma can be primary when it occurs in children with intact tympanic membrane. Primary acquired cholesteatoma is the presence of a secondary diverticulum in the pars flaccida and secondary acquired cholesteatoma occurs when it is associated with perforation of the tympanic membrane. In this article, acquired cholesteatoma is studied.
There are multiple theories that attempt to explain the physiopathogenesis of acquired cholesteatoma, theories related to metaplasia, migration, basal hyperplasia, iatrogenesis, and retraction pocket, to name a few5. However, none of these theories has been proven until today or is deemed to have a multifactorial origin6. It has been proven that the degree of inflammation responds to the aggression to which the middle ear is subjected, as in the case of a repetitive bacterial superinfection7.
The immune system responds to lesions or irritations that occur in the body through an inflammation cascade. As part of this inflammation, cascade, epithelial cells, endothelial cells, and, mainly, inflammatory cells (mononuclear cells, macrophages, lymphocytes, and mast cells)3 produce inflammatory mediators. Molecularly, inflammatory mediators can be proteins, glycoproteins, peptides, cytokines, and others. Although the primary objective of inflammatory mediators is to protect the host, it can also cause damage, such as cholesteatoma in OM.
The etiopathogenesis of cholesteatoma is not yet clear; however, the participation of different inflammatory mediators as a central mechanism for its appearance has been studied3. Cytokines are among the main inflammatory mediators. Cytokines are low-molecular-weight glycoproteins that allow intercellular communication, through which immune responses are stimulated or suppressed and participate in the healing of wounds and tissue remodeling.
The cytokines involved in acquired cholesteatoma, which are mentioned in literature, are interleukin (IL)-1, IL-5, IL-6, IL-8, interferon-gamma (IFN-g) and tumor necrosis factor-alpha, tissue growth factor alpha and beta, and nitric oxide3,8,9. Another element present in the cholesteatoma is cytokeratins (CKs), due to their hyperproliferative properties. CKs 1, 2, 5, 10, and 14 have been found in cholesteatoma8.
IL-2
IL-2 is a chain of 133 polypeptides produced by T-lymphocytes; its main function is to initiate the proliferation and differentiation of T-lymphocytes. IL-2 is necessary to generate CD8+ T-cells and increases the function of natural killer (NK) cells3,10.
IL-9
IL-9 is produced by T-lymphocytes, stimulates the release of mast cell mediators, and promotes the expression of high-affinity IgE receptors. It also promotes mucosal hypersecretion and maturation of eosinophils in conjunction with IL-53.
At present, the use of imaging methods has allowed greater certainty in the diagnosis of cholesteatoma, especially computed axial tomography and magnetic resonance imaging, in T1 diffusion-weighted sequences11,12.
Few studies suggest the medical treatment of cholesteatoma in the reviewed literature. The medical treatment of choice is generally surgery.
Objectives
The objectives of this study were to compare the expression of IL-2 and IL-9 in patients with CSOM with or without cholesteatoma.
Justification
CSOM and its complications pose a great challenge in ENT consultations, being one of the most frequent reasons for medical appointments. The pathogenesis of cholesteatoma has been studied for more than 20 years and is still not fully understood, although it has been observed that a large number of inflammatory markers are involved, including IL. We consider that, when IL are detected in the inflammatory tissue at an early stage, it can be treated in a timely manner by specific inhibitors to prevent complications.
Methodology
This is a cross-sectional, descriptive study conducted in surgical patients suffering from CSOM with or without cholesteatoma at the Otorhinolaryngology and Head and Neck Surgery service of the General Hospital of Mexico (Hospital General de México). Inclusion criteria were as follows: patients aged 18 years or older, of both sexes, having a computed tomography scan of the ear in axial and coronal planes in a simple phase with data compatible with the diagnosis, who agreed to participate in the study and signed the informed consent form. Exclusion criteria included patients with neoplastic diseases, comorbidities with systemic inflammatory diseases, and those who had medical contraindications for surgical treatment. Elimination criteria were for tissue sampling that was not performed systematically and appropriately, and for those whose biopsy results were not compatible with CSOM diagnosis.
Regarding the 16 patients studied, five patients had no cholesteatoma (Group A) and 11 of them presented cholesteatoma (Group B). In both groups, the expression of IL of two comparative tissue biopsies was determined, the first (problem tissue) of the mastoid antrum for Group A and cholesteatoma for Group B. Skin biopsy control of the retroauricular region was performed in all cases (control tissue).
The samples were sent to histopathological tissue analysis in 10% formalin buffer. For the analysis, paraffin was included and 3 µm cuts were made. For the macroscopic study, hematoxylin-eosin was stained and Masson's trichrome stain was used to determine fibrosis and changes related to chronicity. They were incubated with IL-2 and IL-9 antibodies and analyzed with conventional ABC immunohistochemistry technique.
Diaminobenzidine chromogen was used for ochre brown staining as positive and this was compared with a negative control incubated in normal sheep serum. In all samples, anti-CK 13 antibody was incubated to corroborate the presence of cholesteatoma.
For comparison of CSOM with or without cholesteatoma, cholesteatoma was evidenced histologically in the presence of continuous stratified squamous epithelium or fragments thereof that included keratin layers with a cystic center. Moreover, positive immunohistochemistry for CK-13 was confirmed. The diagnosis of mastoiditis was achieved histologically with the presence of acute and/or chronic inflammatory tissue, granulation tissue and/or necrosis without stratified squamous epithelium, and typical of cholesteatoma; diagnosis was confirmed with the immunohistochemical absence of CK-13.
The microscopic sections were digitized and standardized and later analyzed using an image processor, with Image-Pro Plus 5.1 software. The number of pixels of the positive samples was converted into numerical values. The differences in the analysis of the tissue image generated by software are shown in figures 1 and 2.

Figure 1 Group A micrograph analyzed with Image-Pro Plus 5.1 showing poor expression of interleukin-2.
The statistical analysis was conducted on a comparison test of independent groups with t-test.
Results
Paired samples from 16 patients were analyzed; nine women and seven men, with a mean age of 42.5 years (± 12.5 years), in a range of 18-58 years (median: 41.4 years).
Table 1 shows the measurements of the expression of IL-2 and IL-9, as well as CK-13 in the 16 patients (Table 1).
Table 1 Measurement of cytokine expression in individuals
| CK-13 | IL-2 | IL-9 | ||||
|---|---|---|---|---|---|---|
| C* | P** | C* | P** | C* | P** | |
| Group A | ||||||
| 1 | 1.08 | 0 | 0.00 | 0.52 | 0.17 | 0.23 |
| 2 | 1.22 | 0.14 | 0.00 | 0.48 | 0.17 | 0.27 |
| 3 | 1.43 | 0.1 | 0.00 | 0.60 | 0.17 | 0.35 |
| 4 | 0.88 | 0.1 | 0.00 | 1.07 | 0.14 | 0.14 |
| 5 | 1.06 | 0 | 0.00 | 0.56 | 0.10 | 0.34 |
| Group B | ||||||
| 1 | 1.23 | 0.72 | 0.00 | 0.87 | 0.23 | 0.64 |
| 2 | 1.47 | 0.85 | 0.00 | 1.07 | 0.20 | 0.75 |
| 3 | 1.2 | 0.79 | 0.00 | 1.04 | 0.17 | 0.62 |
| 4 | 0.94 | 0.4 | 0.00 | 1.02 | 0.20 | 0.73 |
| 5 | 1.43 | 0.73 | 0.00 | 0.97 | 0.20 | 0.83 |
| 6 | 1.17 | 0.62 | 0.00 | 1.27 | 0.00 | 0.85 |
| 7 | 1.37 | 0.61 | 0.00 | 0.97 | 0.00 | 0.59 |
| 8 | 1.32 | 0.54 | 0.00 | 0.84 | 0.10 | 0.54 |
| 9 | 1.47 | 0.59 | 0.00 | 1.16 | 0.10 | 0.59 |
| 10 | 1.23 | 0.71 | 0.00 | 1.15 | 0.17 | 0.51 |
| 11 | 1.07 | 0.48 | 0.10 | 1.08 | 0.10 | 0.45 |
*C: Control tissue. In both groups, it corresponds to retroauricular skin.
**P: Problem tissue: in Group A, it corresponds to inflammatory tissue of the middle ear. In Group B, it corresponds to cholesteatoma, IL-2: interleukin-2, IL-9: interleukin-9.
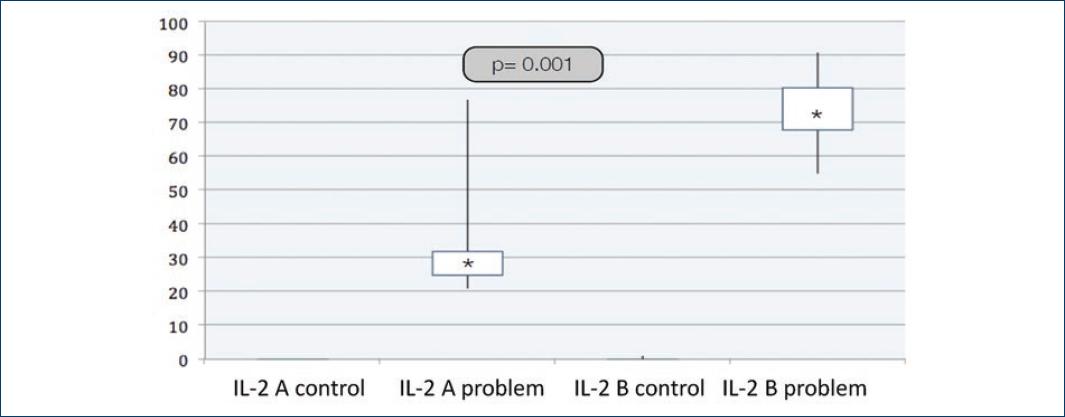
Table 2 shows the difference in cytokine expression in the problem tissue as statistically significant between both groups. For Group A, the average IL levels were as follows (the median is reported in parentheses): IL-2; 36.6 ± 22.94 (28) and IL-9, 7.4 ± 12.13 (7). In Group B, the average IL levels were IL-2; 73.5 ± 10.87 (274) and IL-9, 36.4 ± 12.13 (34) (Table 2).
Table 2 Expression of cytokines in both groups
| Group A | Group B | p | |||
|---|---|---|---|---|---|
| C* | P** | C* | P** | ||
| CK-13 | 79.8 | 0.8 | 88.9 | 35.9 | < 0.001 |
| IL-2 | 0 | 36.6 | 0.09 | 73.5 | 0.001 |
| IL-9 | 2.4 | 7.4 | 2.3 | 36.4 | < 0.001 |
*C: Control tissue. In both groups, it corresponds to retroauricular skin.
**P: Problem tissue: in Group A, it corresponds to inflammatory tissue of the middle ear. In Group B, it corresponds to cholesteatoma, IL-2: interleukin-2, IL-9: interleukin-9.
Figures 3 and 4 show that the expression of IL-2 and IL-9 is greater in Group B than in Group A, with greater expression in the problem tissue than in the control tissue. CK-13 is also statistically different in both problem groups (Fig. 5). IL are elevated in patients with cholesteatoma.
Discussion
Cholesteatoma is one of the multiple complications of CSOM, which is characterized by bone destruction and stratified squamous epithelium replacement. The pathogenesis and pathophysiology of cholesteatoma have not been fully clarified yet; however, inflammatory mediators play a central role in its development.
During the period of this study, no IL-2 or IL-9-focused studies were found in literature. IL-2 is a growth factor as well as being a factor of survival and differentiation originated in T-lymphocytes and for T-lymphocytes. It promotes the activation of NK cells and the proliferation of B-lymphocytes. IL-9 also originates in T-lymphocytes, promotes the expression of high-affinity IgE receptors, and promotes mucus hypersecretion.
The present study showed that IL-2 and IL-9 are elevated in cholesteatomatous tissue. IL-2 can explain the persistence of T-lymphocytes and their differentiation in tissue. The presence of IL-9 can explain otorrhea, as it promotes mucosal hypersecretion in conjunction with IL-5.
This study shows that the cholesteatomatous tissue has a high presence of cytokines. Further studies are necessary to determine the degree of association between IL-2 and IL-9 levels and the degree of T-lymphocyte proliferation and otorrhea that is observed in CSOM with cholesteatoma.
Conclusions
Advances in the study of molecular biology techniques and other techniques, such as digital image analysis, allow for the study of several pathologies, including cholesteatoma.
The present study showed that the association of IL-2 and IL-9 with cholesteatoma is statistically significant, being high in cholesteatomatous tissues compared with control tissues.
The morphometric study by digital analysis is useful in the diagnosis of cholesteatoma because it allows determining the presence of cytokines and other molecules in the tissue quantitatively.
More studies are required to prove the participation of IL-2 and IL-9 in cholesteatoma, as well as the degree to which these are factors for the persistence and progression of cholesteatoma.











 text new page (beta)
text new page (beta)