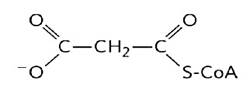Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.13 Tepic Jan./Dec. 2023 Epub Oct 27, 2023
https://doi.org/10.21929/abavet2023.10
Literature review
The goat udder: mechanism of milk secretion, and protein/fat synthesis
1Estudiante de la Maestría Interinstitucional en Producción Pecuaria (MIPPE), Universidad de Colima. México.
2Facultad de Medicina Veterinaria y Zootecnia, Universidad de Colima. México.
3Facultad de Ciencias Agropecuarias, Universidad Autónoma de Campeche. México.
In recent years, the shift in milk marketing towards a standardized price structure based on dairy components has placed greater emphasis on lipid and protein concentration over the quantity of L or kg of milk produced. The nutritional content of goat's milk exceeds the nutritional content of cow's milk, in terms of proteins and fats, and it is the lactocytes of the caprine mammary gland, which must replicate and synthesize these components dairy products. Therefore, this review initially considers the understanding of the anatomy and histology of the mammary gland as responsible for all the activities related to the milk ejection mechanism. The development of the mammary gland determines all aspects of cell behavior, so its development is reviewed through four stages: i) mammogenesis, ii) lactogenesis, iii) galactopoiesis, and iv) involution. The remainder of the review emphasizes milk lipogenesis and proteogenesis, due to their various roles within cellular metabolism and the production of the lipid fraction and protein fraction of milk.
Keywords: mammary gland; goat milk; lipogenesis; proteogenesis
En los últimos años, el cambio en la comercialización de la leche hacia una estructura de precios estandarizada a partir de los componentes lácteos ha puesto un mayor énfasis en la concentración lipídica y proteica sobre la cantidad de L o kg de leche producidos. En virtud de lo anterior, el contenido nutricional de la leche de cabra supera al contenido nutricional de la leche de vaca, en lo que respecta a proteínas y grasas, y son los lactocitos de la gandula mamaria caprina, quienes deben replicarse y sintetizar estos componentes lácteos. Por lo tanto, esta revisión considera de manera inicial la comprensión de la anatomía e histología de la glándula mamaria como responsable de todas las actividades vinculadas al mecanismo de eyección de la leche. El desarrollo de la glándula mamaria determina todos los aspectos del comportamiento celular, por lo que se revisa su desarrollo a través de cuatro etapas: i) mamogénesis, ii) lactogénesis, iii) galactopoyesis, y iv) involución. El resto de la revisión hace hincapié en la lipogénesis y la proteogenesis láctea, debido a sus diversas funciones dentro del metabolismo celular y la producción de la fracción lipídica y fracción proteica de la leche.
Palabras clave: glándula mamaria; leche de cabra; lipogénesis; proteogénesis
ABBREVIATIONS
A |
aminoacyl site |
aa |
amino acids |
ACAT |
acyl-CoA:cholesterol acyltransferase |
ADH |
antidiuretic hormone |
DNA |
deoxyribonucleic |
mRNA |
messenger ribonucleic acid |
tRNA |
transfer ribonucleic ribonucleic acid |
ATP |
adenosine triphosphate |
C3H3O3 |
pyruvate |
Ca3(PO4)2 |
tricalcium phosphate |
CCT CTP |
phosphocholine cytidylyl transferase |
CO2 |
carbon dioxide |
DAG |
diacylglycerol |
DGAT |
acyl-CoA:diacylglycerol acyltransferase |
E1 |
estrone |
E2 |
17 β-estradiol |
E3 |
estriol |
FAS I |
fatty acid synthase I |
GH |
growth hormone |
GPAT |
glycerol-3-phosphate acyltransferase |
HCO3 |
hydrogenocarbonate anion |
LFLAT |
lysophospholipid acyltransferase |
NADPH |
nicotinamide adenine dinucleotide phosphate |
O=C-N-H |
peptide bonding |
OXT |
oxytocin |
P |
peptidyl site |
p.p. |
postpartum |
P4 |
progesterone |
PAP |
phosphatidic acid phosphatase |
PLA |
phospholipase A |
Plin |
perilipin |
PRL |
prolactin |
TAG |
triacylglycerols |
UAA |
uracil-adenine-adenine |
UAG |
uracil-adenine-guanine |
UGA |
uracil-guanine-guanine-adenine |
INTRODUCTION
Goat milk has positioned itself as an important element in the human diet (Bauman et al., 2006). Its nutritional relevance lies mainly in two components: i) the lipid fraction, formed by fatty acids (Harvatine et al., 2009) and ii) the protein fraction, where caseins, whey proteins and fat globule membrane proteins are distinguished (Swaisgood, 2003). The nutritional content of goat milk exceeds that of cow milk in terms of protein (goat milk: 3.40 g/100 mL-1 vs cow milk: 3.30 g/100 mL-1) and fat (goat milk: 4.30 g/100 mL-1 vs cow milk: 3.95 g/100 mL-1) content (Davidson & Stabenfeldt, 2020). Current trends in milk production and consumption enhance lipid and protein concentration over the amount of L or kg of milk produced (Manterola, 2011), taking into account the eating habits of a growing urban population (Vidal, 2013). Goat milk collectors use this product mainly for cheese production (National Chamber of Industrial Milk, 2021). For this reason, it is necessary to increase our understanding of the metabolism involved in goat milk production and its lipid and protein contents (Heid & Keenan, 2005; Kumar et al., 2009). Therefore, this review discusses the anatomy and histology of the mammary gland. Its development as a milk-producing organ through four stages: i) mammogenesis, ii) lactogenesis, iii) galactopoiesis, and iv) involution. Continuing with milk ejection and its hormonal control, and to substantiate the basic biochemistry of milk lipid and protein synthesis, information on the processes of milk lipogenesis and proteogenesis is presented at the end.
I. Anatomy and Histology of the Mammary Gland
Goats have two independent mammary glands, their location in the body of the animal is inguinal and they are: i) pear or elongated type, ii) oval or Alpine type, and iii) globular or Saanen type (Menzies, 2021). Histologically, each mammary gland is composed of two tissues: i) the parenchyma whose origin is embryonic ectoderm, and which includes lactocytes or lacteal exocrineocytes and myoepithelial cells (Lawhead & Baker, 2017), and ii) the stroma whose origin is embryonic mesoderm, and which includes blood vessels, lymphatic vessels, adipose tissue, connective tissue and nervous tissue (Baljit, 2017).
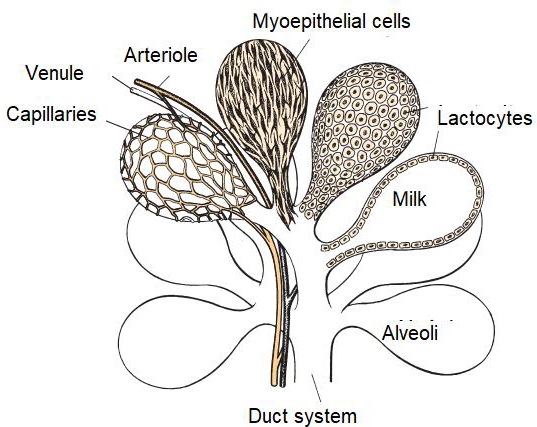
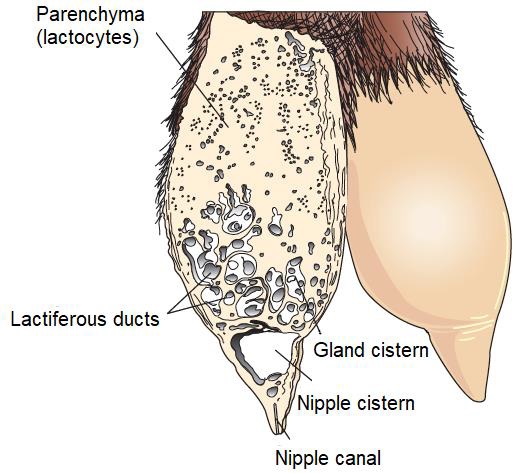
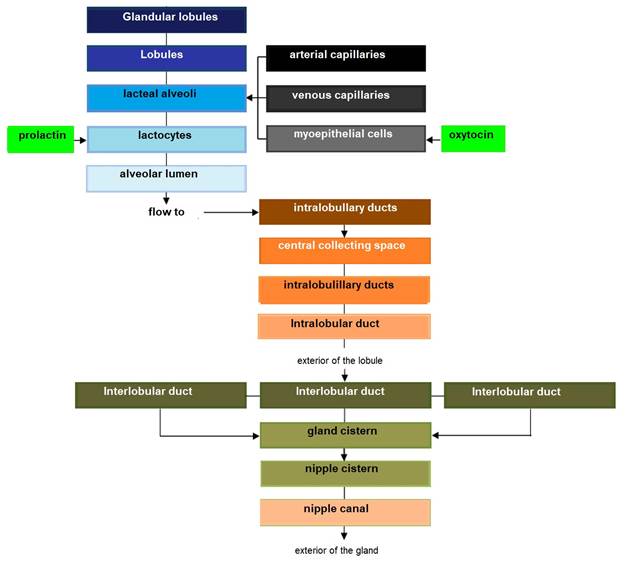
The parenchyma develops through the proliferation of lactocytes arising from the primary mammary cord (Menzies, 2021). Lactocytes present receptors for prolactin (PRL) (Baljit, 2017), and eventually form hollow circular structures with a length of 100 to 500 µm called alveoli (Lawhead & Baker, 2017). The outer wall of each alveolus is surrounded by arterial capillaries and venous capillaries along with a layer of myoepithelial cells with receptors for oxytocin (OXT) (Davidson & Stabenfeldt, 2020). The internal structure of the mammary gland consists of: i) parenchyma, ii) lactiferous ducts: intra and inter lobular and lobular depending on their connection within the mammary gland (Reese et al., 2020), iii) glandular lobules (Figure 1), formed by lobules with 150 to 220 lacteal alveoli, iv) myoepithelial cells, v) venules, vi) arterioles, vii) capillaries, viii) gland cisternae (Figure 2), ix) teat cistern and x) nipple canal (Davidson & Stabenfeldt, 2020).
The irrigation of the mammary gland is carried out by the external pudendal artery, which passes through the inguinal canal and divides into cranial and caudal branches (Davidson & Stabenfeldt, 2020). Venous circulation is mainly by the venous circle formed by the external pudendal vein, the caudal superficial epigastric vein and the perineal vein (Lérias et al., 2014). Innervation of the mammary gland is mainly carried out by sympathetic nerve fibers in the first and second lumbar nerves and the inguinal nerves, their function is the control of blood flow in the udder and innervation of the smooth muscle tissue surrounding the lactiferous ducts, gland cistern muscles, teat cistern muscles and teat canal (Dee & Magee, 2018). Milk contained within the alveolar lumen empties into small intralobulillar ducts (Figure 3) that empty into a central collecting space, from which the interlobular ducts emerge (Davidson & Stabenfeldt, 2020).
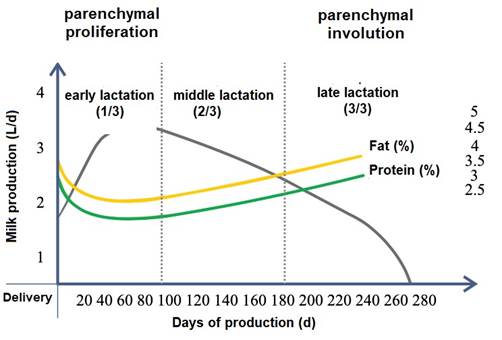
Within the lobule the interlobular ducts join to form an intralobular duct, which upon exiting the lobule acquires the name interlobular duct; these ducts may lead directly into the cistern of the gland or join other interlobular ducts before reaching it (Dee & Magee, 2018). Reece & Rowe (2017b). noted that the ductal system connects the gland cistern to the teat cistern, which allows milk to pass from the formation area to the delivery area or nipple canal. The gland cistern in goats is larger in volume compared to that of cattle, and allows for almost 70 % of the milk produced between each milking (Martínez & Suárez, 2018). The anatomy and histology of the mammary gland are modified throughout lactation, by changes associated mainly with the neuroendocrine system (Dee & Magee, 2018). Therefore, there are three stages in mammary biology, characterized by gestation/lactation cycles: i) proliferation, ii) secretion and iii) involution (Lawhead & Baker, 2017). Although most of the proliferation occurs during gestation and most of the involution occurs after milk production, both stages overlap: parenchymal proliferation continues during early lactation (1/3 lactation) and its involution begins during late lactation (3/3 lactation) (Lérias et al., 2014). Event that in goats is reached from 180 to 280 d, with peak production between 8 and 12 weeks postpartum (p.p.) (Menzies, 2021) (Figure 4).
Reese et al. (2020) indicated that when the mammary gland is in a resting state, the lactocytes have a cubic appearance, whereas when the mammary gland is in milk production, their shape is cylindrical (Davidson & Stabenfeldt, 2020). Furthermore, it is important to note that higher milk production is negatively correlated with milk fat and protein, i.e. a decrease in milk L is equivalent to a higher milk solids content and vice versa (Martínez & Suárez, 2018).
II. Mammary gland development
Lactation proceeds through a cycle consisting of four stages: i) mammogenesis, ii) lactogenesis, iii) galactopoiesis, and iv) involution (Baljit, 2017). Mammogenesis initiates during fetal life in the embryonic ectoderm, forming the mammary band in the inguinal region after 30 d of conception (Reese et al., 2020), the mammary gland at two months and the nipple cistern at three months of fetal life (Lawhead & Baker, 2017).
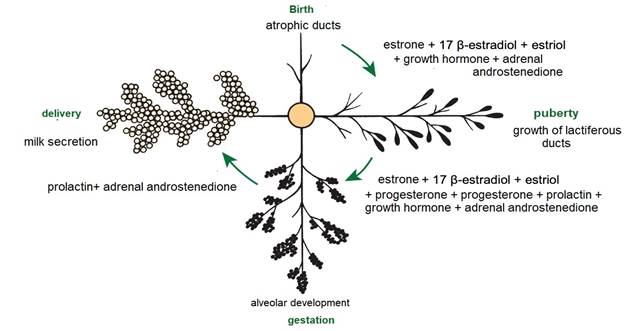
From birth to puberty, the mammary gland exhibits isometric growth with increased connective tissue and fat deposition (Dee & Magee, 2018). Cyclic ovarian activity results in the production of estrogens e.g., estrone (E 1), 17 β-estradiol (E 2) and estriol (E 3). E1, E2 and E3 (Reece & Rowe, 2017a) (Figure 5), together with growth hormone (GH) and adrenal androstenedione, are responsible for the growth of lactiferous ducts (Maldonado et al., 2018). In this regard Lérias et al. (2014) stated that the use of plastic implants with estrogens e.g., E1, E2 and E3, directly and locally stimulates the growth of the lactiferous ducts and on the contrary the application of implants with anti-estrogenic activity, inhibits the growth of the lactiferous ducts within circumscribed areas (Reese et al., 2020).
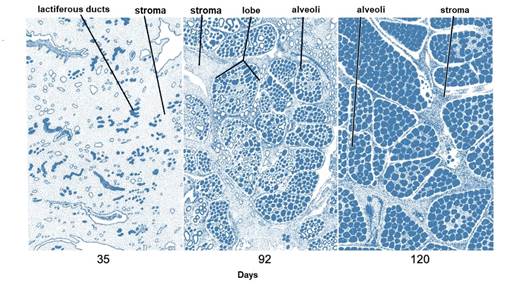
After puberty, the mammary gland shows allometric growth (Goff, 2015) and with each estrus there is a slight development of alveoli influenced by E1, E2 and E3, progesterone (P 4) (Reece & Rowe, 2017a), GH and adrenal androstenedione (Lawhead & Baker, 2017). Information consistent with that reported by Reece & Rowe (2017b) who noted a synergistic stimulation of PRL, androstenedione, E1, E2 and E3, and P4 on mammary gland growth and alveolar lobe development (Neville et al., 2002). Most parenchymal growth happens during gestation (Goff, 2015), induced by P4, PRL and adrenal androstenedione (Reese et al., 2020). By day 35 (Figure 6) stroma is abundant, by day 92 glandular lobules form with several lobes clustered together; milk secretion is present within the alveolar lumen in some lobules, and considerable stroma is still present, by day 120 the lobes of the alveoli are almost fully developed (Svennersten & Olsson, 2005); the alveoli are filled with milk secretion and stroma is reduced to thin bands (Lawhead & Baker, 2017).
After parturition and with the placenta expulsion, P4 levels fall, initiating lactogenesis (Saipin et al., 2020). The initial phase of lactation is characterized by the positive regulation of milk production, cell proliferation, and a decrease in the apoptosis process in the mammary gland (Henna et al., 2021). During this process the adenohypophyseal endocrine tissue intervenes with the secretion of PRL in its lactotrope cells (Dee & Magee, 2018). PRL is a peptide of 199 amino acids (aa) and atomic mass of 23,000 Da, which binds to its tyrosine kinase family receptors located on lactocytes and activates signal transducers and activators of transcription associated with proliferation, differentiation, and lactogenesis (Lawhead & Baker, 2017). Therefore, PRL is indispensable in milk production (Svennersten & Olsson, 2005). In relation to the topic, an investigation that aimed to evaluate the effect of long-term inhibition of PRL, reported that administration for nine weeks of a dopaminergic agonist called quinagolide decreased milk production, confirming the importance of PRL in the functioning of the mammary gland (Lacasse et al., 2011).
III. Milk ejection
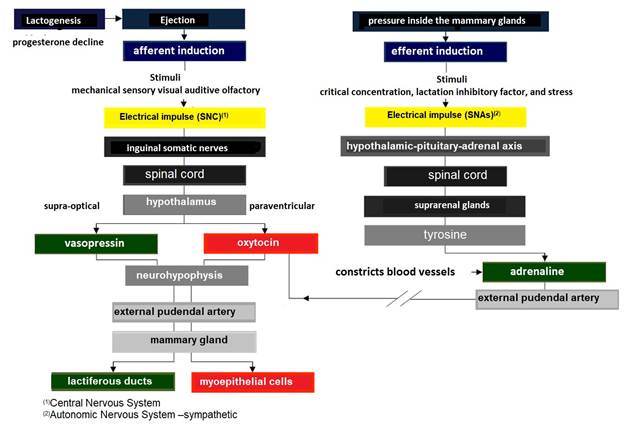
Milk from the cistern (70 % of the milk produced between each milking), can be extracted independent of hormonal processes, by a passive mechanism (only by gravity) (Menzies, 2021). For its part, alveolar milk ejection begins with afferent induction (Lérias et al., 2014), by sensory cells in the teat skin and udder base (Martínez & Suárez, 2018) and mechanical stimuli on the teat (Reece & Rowe, 2017b). It can also be triggered by visual stimuli e.g., milking from other females, auditory stimuli e.g., noise from buckets or the milking machine, olfactory stimuli e.g., the milk itself, and even become a conditioned reflex (Lawhead & Baker, 2017).
The ejecto-lacteal reflex, becomes an electrical impulse that travels up the inguinal somatic nerves to the spinal cord (Figure 7), reaching the paraventricular nucleus of the hypothalamus (Davidson & Stabenfeldt, 2020), where action potentials are produced in intermittent pulses, releasing 9 aa OXT [peptide (cysteine-tyrosine-isoleucine-glutamineasparagine-asparagine-cysteine-proline-leucin e-glycine)] (Svennersten & Olsson, 2005) stored in the neurohypophysis (Dee & Magee, 2018).
The electrical impulse that travels up the somatic inguinal nerves to the spinal cord also reaches the supraoptic nucleus of the hypothalamus (Reese et al., 2020),, where action potentials are produced in intermittent pulses, which release the antidiuretic hormone (ADH) also called vasopressin peptide of 9 aa (cysteine-tyrosine-tyrosine-phenylalanineglutamine-asparagine-asparagine-cysteine-proline-arginine-glycine) stored in the neurohypophysis (Thul et al., 2020).
Both hormones travel via the external pudendal artery to the mammary gland (Davidson & Stabenfeldt, 2020). Main functions of OXT in the mammary gland are: i) to cause contraction of the myoepithelial cells surrounding the alveoli, to empty milk from the lactocytes into the alveolar lumen (Belo & Bruckmaier, 2010), and ii) to cause contraction of the intra- and inter-lobular milk ducts, forcing the flow of milk into the cistern of the gland (Neville et al., 2002; Svennersten & Olsson, 2005).
For its part, ADH acts on vascular smooth muscle causing vasoconstriction and osmotic and oncotic pressure changes in the lactiferous ducts facilitating milk outflow (Goff, 2015). The increase in pressure within the mammary gland is evident at the minute of the ejection reflex, as milk is expelled from the alveoli and lactiferous ducts due to contraction of the myoepithelial cells (Lérias et al., 2014). The term used in mammals to describe this phenomenon is milk "let-down" (Davidson & Stabenfeldt, 2020).
Milk flow increases the gland cistern size, originating an increase in pressure (Lawhead & Baker, 2017). Thus, the ejection rate presents an autocrine control at the glandular level by lactation inhibitory factor (Dee & Magee, 2018). This protein is produced by the same lactocytes of the glandular parenchyma and it is secreted together with the milk into the lacteal alveoli (Davidson & Stabenfeldt, 2020). In this regard Bruckmaier & Wellnitz (2008) indicated that lactation inhibitory factor exhibits two modes of action: i) it accumulates in milk until it reaches a critical concentration that inhibits ejection, and ii) when milk accumulates within a lacteal alveolus, it extends its surface exposing potential receptors for lactation inhibitory factor, allowing its binding and triggering inhibition.
Simultaneously, the ejecto-lacteal reflex can be temporarily inhibited by the release into the bloodstream of adrenaline also called epinephrine (Svennersten & Olsson, 2005) (Figure 7), as a result of increased pressure generating stress (Reese et al., 2020). Adrenaline constricts blood vessels including the external pudendal artery, making it impossible for OXT to reach the myoepithelial cells surrounding the lacteal alveoli and indirectly inhibiting their contraction (Reece & Rowe, 2017b).
The maintenance period, or galactopoiesis, occurs when constant suckling at the teat continues to stimulate milk production (Bruckmaier & Wellnitz, 2008), main hormones controlling this physiological stage are PRL and GH (Lawhead & Baker, 2017). Both hormones are important for galactopoiesis, but one predominates in importance relative to the other depending on the species (Baljit, 2017). In rodents as in humans PRL is more important and in ruminants GH has a more active participation (Goff, 2015).
IV. Milk lipogenesis
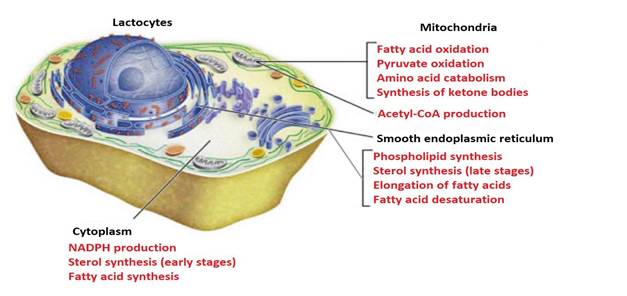
Milk lipogenesis takes place in different cellular compartments (Gartner, 2018). It starts in the mitochondria of lactocytes (Friedman & Nunnari, 2014), with the production of acetylCoA from fatty acid oxidation (Nelson & Cox, 2017b), pyruvate (C 3 H 3 O 3) oxidation (McDonald et al., 2011) and catabolism of aa carbonaceous skeletons (Rodwell, 2018) (Figure 8).
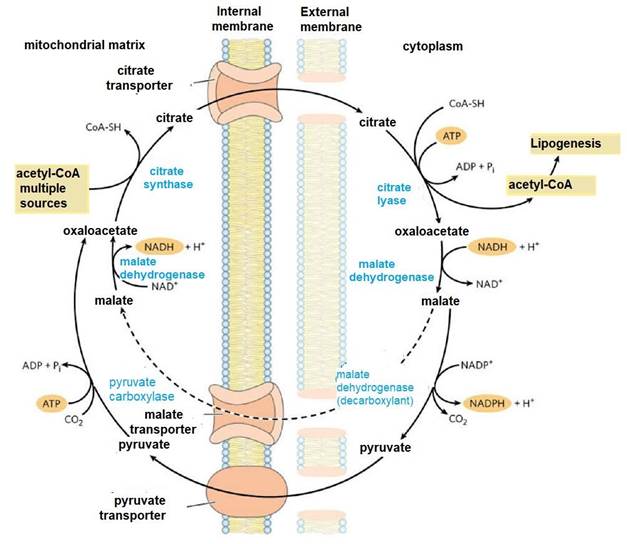
Like other metabolic pathways, fatty acid synthesis is endergonic and reductive (Botham & Mayes, 2018b). Therefore, the process uses adenosine triphosphate (ATP as an energy source (Botham & Mayes, 2018a) and nicotinamide adenine dinucleotide phosphate (NADPH) as a reduced electron carrier (Madigan et al., 2019a). Consequently, fatty acid synthesis continues in the cytoplasm (Appleton et al., 2013d), where NADPH is available for reductive synthesis [i.e., where the (NADPH)/(NADP+) ratio is high] (Cooper, 2019a). However, the inner mitochondrial membrane is impermeable to the passage of acetyl-CoA (Ellis et al., 2015; Mas, 2018), so a shuttle for transfer of acetyl-CoA (acetyl groups) from the mitochondrial matrix to the cytoplasm is required (Nunes et al., 2013) (Figure 9).
Intra-mitochondrial acetyl-CoA first reacts with oxaloacetate to form citrate (Nelson & Cox, 2017a), in the citric acid cycle reaction catalyzed by citrate synthase (Appleton et al., 2013b). Citrate passes through the mitochondrial membrane on its transporter (Nunes et al., 2013). In the cytoplasm, citrate cleavage catalyzed by citrate lyase regenerates acetylCoA and oxaloacetate in an ATP-dependent reaction (Ellis et al., 2015; Verschueren et al., 2019). Oxaloacetate cannot return directly to the mitochondrial matrix, as there is no transporter for it (Nelson & Cox, 2017a). So, malate dehydrogenase catalyzes its reduction to malate, and this passes through the mitochondrial membrane on its transporter (Nunes et al., 2013).
In the mitochondrial matrix, malate is re-oxidized to oxaloacetate catalyzed by malate dehydrogenase to complete the shuttle (Friedman & Nunnari, 2014). The pyruvate produced is sent to the mitochondrion by its transporter, and then converted back to oxaloacetate catalyzed by pyruvate carboxylase (Nelson & Cox, 2017a). In the resulting cycle, two molecules of ATP are consumed (by citrate lyase and pyruvate carboxylase) for every molecule of acetyl-CoA supplied for lactate lipogenesis (Appleton et al., 2013c).
In the cytoplasm, fatty acid biosynthesis begins with the participation of a three-carbon intermediate called malonyl-CoA (Mas, 2018) (Figure 10).
The formation of malonyl-CoA is from acetyl-CoA in an irreversible process catalyzed by biotin carboxylase (Nunes et al., 2013). In this two-step reaction a carboxyl group derived from the hydrogenocarbonate anion (HCO 3 -), binds to a nitrogen of the biotin ring in an ATP-dependent reaction (Botham & Mayes, 2018a), activating carbon dioxide (CO 2) (Mas, 2018). The biotinyl group serves as a temporary CO2 carrier (Nelson & Cox, 2017a), and part of the transporter protein and the long flexible biotin arm turn to transport activated CO2 from biotin to acetyl-CoA producing malonyl-CoA (Cooper, 2019a).
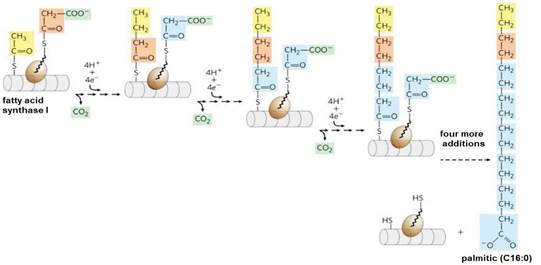
From malonyl-CoA lipogenesis is performed by the fatty acid synthase I (FAS I) protein complex (Suburu et al., 2014). This system performs synthesis, reduction, dehydration and again reduction by concentrating malonyl-CoA groups with acetyl-CoA, with loss of CO2 at each step (Belew et al., 2019). After each two-carbon addition, reductions convert the growing chain to a four-carbon fatty acid, then six, then eight carbons, and so on (Song et al., 2018) (Figure 11). Fatty acid synthesis in FAS I always reaches 16 carbons (palmitic, C16:0) and no intermediates are released (Chandel, 2021).
Palmitic, leaves FAS I and can enter the endoplasmic reticulum (Olarte et al., 2020), to be elongated (coupling new carbons to lengthen the chain) (Balla et al., 2019) and desaturated (introducing cis double bonds between its carbons) to form polyunsaturated fatty acids (Rowland & Voeltz, 2012). At this time the mammary gland parenchyma undergoes a functional and morphological differentiation termed lactogenesis I (Reese et al., 2020; Menzies, 2021), and initiates the accumulation of lipid microdroplets (Ashdown & Done, 2011). These droplets are obtained from NEFA, released from circulating lipoproteins by lipoprotein lipase located in the vascular bed of the mammary gland (Davidson & Stabenfeldt, 2020) or by circulating fatty acids derived from adipose tissue bound to ALB (Fox et al., 2015).
After parturition lactogenesis II begins (Baljit, 2017), this process requires: i) coordination and activation of fatty acids by acyl-CoA synthetases (Fernandez & Ellis, 2020), ii) de novo synthesis of medium-chain fatty acids from GLU (Jones, 2016; Cooper, 2019a), and iii) synthesis of neutral lipids e.g., triacylglycerol (TAG), COL and diacylglycerol (DAG) esters (Sanhueza et al., 2012; Nelson & Cox, 2017b), which provide binding for accessory proteins (Reece & Rowe, 2017b), e.g., CTP:phosphocholine cytidylyl transferase (CCT) and perilipins (Plin) (Henry et al., 2015).
In general, milk fat globules are thought to form from tubular microdomains of the smooth endoplasmic reticulum (Pol et al., 2014). However, unlike other lipogenic cells e.g., hepatocytes and adrenocortical cells (Saheki & De Camilli, 2017), mammary gland parenchyma is highly enriched in rough endoplasmic reticulum with enzymes for neutral lipid synthesis (Sturley & Hussain, 2012).
This process begins with the esterification of fatty acids to a glycerol molecule to form TAG (Tortora et al., 2019), in four reactions catalyzed by members of glycerol-3phosphate acyltransferase (GPAT), phosphatidic acid phosphatase (PAP) and acylCoA:diacylglycerol acyltransferase (DGAT) enzyme families (Monks et al., 2020). Monks et al., 2020). The final step in this pathway is the esterification of DAG to TAG (Chandel, 2021). In turn, the synthesis of COL esters is mediated by acyl-CoA:cholesterol acyltransferase (ACAT) (Sanhueza et al., 2012).
Once these elements are structured, they are incorporated into the fat globule (Figure 12), together with carotenoids, fat-soluble vitamins and phosphatidylcholine (Mas, 2018). Two main pathways contribute to phosphatidylcholine synthesis: i) the Kennedy pathway for de novo synthesis of phospholipids, a reaction catalyzed by CCT and ii) the Lands cycle (Appleton et al., 2013d; John et al., 2022). In the latter, the remodeling of phospholipids in the fat globule membrane takes place in deacylation/reacylation reactions (Henry et al., 2015; Guoyao, 2017a), catalyzed by phospholipase A (PLA) and lysophospholipid acyltransferase (LFLAT) (Seoane et al., 2018). Botham & Mayes (2018b) established that, thanks to these phospholipids, the apolar tails project to glycerides and the polar heads project to water.
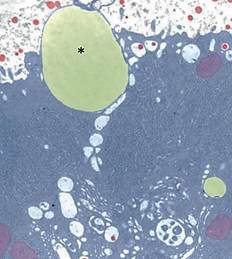
Fuente: (Dee & Magee, 2018)
Figure 12 Electron micrograph of a milk fat globule (asterisk) attached to the apical plasma membrane; casein micelles (red)
Regulation of lipolysis appears to be the main function of the five Plin proteins (Lundquist et al., 2020), which prevent access of lipases to the fat globule (Zhang & Liu, 2019). Unlike lipoproteins, milk lipids are not packaged into vesicles in the Golgi apparatus (Wilson et al., 2011), nor are they secreted by an exocytic mechanism (Lowe, 2011). Instead, they advance unidirectionally toward the apical pole of the lactocyte (Davidson & Stabenfeldt, 2020) and once there, they pass into the alveolar lumen via an apocrine mechanism (Figure 12), in the form of Plin-coated milk fat globules (Lundquist et al., 2020), to continue their transit into the intralobulillar ducts and flow into a central collecting space (Davidson & Stabenfeldt, 2020).
V. Milk proteogenesis
Usually a portion of the dietary protein resists bacterial proteolysis in the rumen and passes into the abomasum without being catabolized (Appleton et al., 2013a), along with ruminal bacteria attached to the fermented feed (Guoyao, 2017b). Pancreatic acinar cells translate hydrolases e.g., peptidase or protease, aminotransferase and nuclease (Philipps, 2018). In the duodenum, these enzymes soak the food bolus and its proteins lose their peptide bonds by hydrolysis (Lozano et al., 2005). This process, releases the aa from their polymeric structure to be absorbed at the intestinal level (Piña & Flores, 2018), transported to the liver and redirected to the cytoplasm of lactocytes (Ahern, 2019).
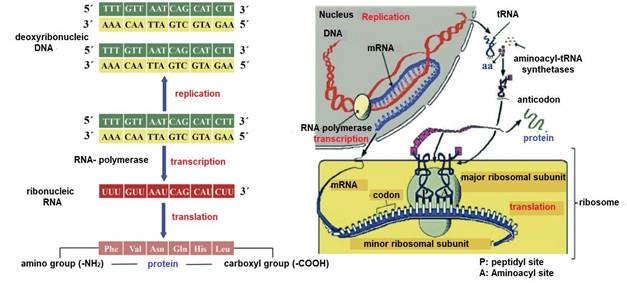
Milk proteogenesis begins in the lactocyte nucleus with transfer ribonucleic acid (tRNA) transcription (Madigan et al., 2019b). RNA polymerase carries out the transcription of messenger ribonucleic acid (mRNA) (Cooper, 2019b), starting from a segment of deoxyribonucleic acid (DNA) that serves as a template (Singh & Rajeev, 2020). This DNA segment contains exons (coding regions) and introns (non-coding regions) (Nelson & Cox, 2017c). Before leaving the nucleus, segments corresponding to introns are cut (Weil, 2018b) and segments corresponding to coding exons are spliced (Madigan et al., 2019b).
Next, tRNA and mRNA exit the nucleus and enter the cytoplasm (Weil, 2018a). At this point, protein translation is promoted on ribosomes with three main steps: i) initiation, ii) elongation and iii) termination (Nelson & Cox, 2017c) (Figure 13). 2
tRNA transports aa from the cytoplasm to the ribosomes (Weil, 2018b) and to ensure that the tRNA carries the correct aa, each tRNA contains a specific sequence of three nitrogenous bases called an anticodon (Lozano et al., 2005). In the case of mRNA its specific sequence of three nitrogenous bases is called a codon (Angov, 2011). Ribosomes contained in the lactocytes of the glandular parenchyma are the organelles responsible for the translation of different types of caseins e.g., αS1-CN, αS2-CN, β-CN, and κ-CN (Doherty & Doudna, 2000). A lactocyte can have thousands of ribosomes (Ingolia, 2014), and their number increases with the proliferation of the glandular parenchyma during early lactation (1/3 lactation) (Reese et al., 2020) and their involution begins during late lactation (3/3 lactation) along with the decrease in milk (Davidson & Stabenfeldt, 2020).
Each ribosome consists of two subunits the peptidyl (P) site and the aminoacyl (A) site (Ingolia, 2014), which associate for codon-anticodon base pairing (Weil, 2018a) and dissociate during translation termination (Swaisgood, 2003). The A site is where the first aa-loaded tRNA is docked (Madigan et al., 2019b). P site is where the growing polypeptide chain binds to the preceding tRNA (Piña & Flores, 2018). During peptide bond formation (O=C-N-H) the growing chain moves toward the tRNA at the A site (Nelson & Cox, 2017c). After elongation the tRNA containing the polypeptide translocates from the A site to the P site (Madigan et al., 2019b), thus freeing the A site for a new aa-loaded tRNA (Lozano et al., 2005) (Figure 13). In each translocation, the ribosome advances three nucleotides (one codon) along the mRNA (Appleton et al., 2013a), exposing a new codon at the A site (Weil, 2018a). Accuracy in translocation is essential for the accuracy of dairy proteogenesis (Weil, 2018a). That is, the ribosome must move exactly one codon at each step or the fidelity of translation would be compromised (Angov, 2011).
Protein translation ends when the ribosome reaches a termination codon e.g. uraciladenine-guanine (UAG), uracil-adenine-adenine (UAA) and uracil-guanine-adenine (UGA) (Weil, 2018a), as no tRNA binds to a codon of these (Piña & Flores, 2018). Instead, release factors recognize UAG, UAA and UGA (Nelson & Cox, 2017c) and cleave the attached polypeptide from the final tRNA (Lozano et al., 2005), releasing the finished caseins (Davidson & Stabenfeldt, 2020). mRNA is then released and can be re-read by other ribosomes (Madigan et al., 2019b), and the ribosomal subunits dissociate and become free to form new initiation complexes and repeat the process (Pacheco et al., 2021). Finally, in the Golgi apparatus, glycosylation of caseins (binding with lactose) takes place (Wilson et al., 2011) and during their movement through the cytoplasm tricalcium phosphate [Ca 3 (PO 4 )2] and other ions are coupled to form a structure called a micelle (Reese et al., 2020), which will be exported to the alveolar lumen (Dee & Magee, 2018), to continue its transit to the intralobulillar ducts and flow into a central collecting space (Davidson & Stabenfeldt, 2020).
The protein fraction is divided into 20 % for soluble or serum proteins where the following stand out: i) β-lactoglobulin (retinol and fatty acid binding and possible antioxidant) (McKerchar et al., 2023), ii) α-lactoalbumin (lactose production, calcium transport, immunomodulatory and anticancer) (Diao et al., 2022), iii) immunoglobulins IgA, IgM and IgG (immune protection) (Nayik et al., 2022) iv) lactoferrin (antibacterial, antioxidant, immunomodulatory, iron absorption and anticarcinogenic) (Sansi et al., 2022), and v) lactoperoxidase (antibacterial) (Lérias et al., 2014). All soluble proteins present a higher proportion of leucine, isoleucine and valine (Ahern, 2019).
Eighty percent of the protein fraction corresponds to insoluble proteins or caseins whose function is to transport and bind minerals, mainly calcium and phosphorus (Dhasmana et al., 2022). The concentration of αs1-casein and αs2-casein is lower in goat milk than in cow milk, the fraction of β-casein is higher and the amount of and κ-casein is equal to that of cow milk (Saikia et al., 2022). The αs-casein is the main protein found in cow's milk, whereas the main protein factor found in goat's milk is β-casein (Dhasmana et al., 2022). All insoluble proteins have a higher proportion of histidine, methionine and phenylalanine (Ahern, 2019).
CONCLUSIONS
In recent years, the shift in milk marketing towards a standardized price structure based on lipid and protein concentration requires a better understanding of the anatomical and physiological processes occurring in the mammary gland. Goats appear to be the least affected species with respect to emotional stress and milk ejection. Because of the morphological and physiological characteristics of the goat mammary gland, 70 % of the milk produced between milkings can be extracted independently of hormonal processes. The development of the mammary gland through its four stages: i) mammogenesis, ii) lactogenesis, iii) galactopoiesis, and iv) involution, determines all aspects of the behavior of the lacteal glandular parenchyma. Lactocytes possess a high metabolic complexity and a robust microscopic organization to develop their systems of extraction, utilization of polysaccharides, lipids and proteins and transformation into milk fat and protein. Knowledge of the anabolic and catabolic processes of these molecules will allow understanding the basic biochemistry of milk production.
ACKNOWLEDGMENTS
This work was supported by the National Council of Science and Technology (CONACyTMexico) and the project: Relationship between blood biochemical analytes and milk fat/protein in goat and cow (University of Colima)
REFERENCES
Ahern K. 2019. Amino acids: 20 building blocks of life. In: Ahern K, Biochemistry and Molecular Biology: How Life Works. Virginia, United States: The Teaching Company. Pp. 29-40. ISBN: 978-1-25-983793-7. https://www.thegreatcourses.com/courses/biochemistry-and-molecular-biology-how-lifeworks [ Links ]
Angov E. 2011. Codon usage: nature's roadmap to expression and folding of proteins. Biotechnology Journal. 6(6):650-659. ISSN: 1860-7314. https://doi.org/10.1002/biot.201000332 [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013a. Metabolismo de las proteínas. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 71-82. ISBN: 978-0-7234-3626-3. https://www.elsevier.com/books/lo-esencial-en-metabolismo-y-nutricion/978-84-9113537-1 [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013b. Metabolismo energético I: ciclo ATC. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 13-17. ISBN: 978-0-7234-3763-5. https://www.elsevier.com/books/lo-esencial-en-metabolismo-y-nutricion/978-84-9113537-1 [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013c. Metabolismo energético II: generación de ATP. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 17-23. ISBN: 978-0-7234-3763-5. https://www.elsevier.com/books/lo-esencial-en-metabolismo-y-nutricion/978-84-9113537-1 [ Links ]
Appleton A, Vanbergen O, Dominiczak MH. 2013d. Transporte y metabolismo de los lípidos. En: Horton-Szar D, Lo Esencial en Metabolismo y Nutrición. Barcelona, España: Elsevier Health Sciences. Pp. 45-70p. ISBN: 978-0-7234-3626-3. https://www.elsevier.com/books/lo-esencial-en-metabolismo-y-nutricion/978-84-9113537-1 [ Links ]
Ashdown RR, Done SH. 2011. La ubre. En: Ashdown RR, Atlas en color de anatomía veterinaria. Rumiantes. Barcelona, España: Elsevier Health Science Division. Pp. 219-230. ISBN: 9788480865418. https://catalogo.udes.edu.co/cgi-bin/koha/opacdetail.pl?biblionumber=31192 [ Links ]
Baljit S. 2017. Ruminants / the udder. In: Baljit S, Dyce, Sack and Wensing's Textbook of Veterinary Anatomy. New York, EE. UU.: Elsevier Health Science Division. Pp. 1252-1264. ISBN: 978-0323442640. https://www.elsevier.com/books/dyce-sack-andwensings-textbook-of-veterinary-anatomy/singh/978-0-323-44264-0 [ Links ]
Balla T, Kim YJ, Alvarez-Prats A, Pemberton J. 2019. Lipid dynamics at contact sites between the endoplasmic reticulum and other organelles. Annual Review of Cell and Developmental Biology. 35(1):85-109. ISSN: 1530-8995. https://doi.org/10.1146/annurevcellbio-100818-125251 [ Links ]
Bauman DE, Mather IH, Wall RJ, Lock AL. 2006. Major advances associated with the biosynthesis of milk. Journal of Dairy Science. 89(4):1235-1243. ISSN: 1525-3198. https://doi.org/10.3168/jds.S0022-0302(06)72192-0 [ Links ]
Belew GD, Silva J, Rito J, Tavares L, Viegas I, Teixeira J, Oliveira PJ, Macedo MP, Jones JG. 2019. Transfer of glucose hydrogens via acetyl-CoA, malonyl-CoA, and NADPH to fatty acids during de novo lipogenesis. Journal of Lipid Research. 60(12):2050-2056. ISSN: 1539-7262. https://doi.org/10.1194/jlr.RA119000354 [ Links ]
Belo CJ, Bruckmaier RM. 2010. Suitability of low-dosage oxytocin treatment to induce milk ejection in dairy cows. Journal of Dairy Science. 93(1):63-69. ISSN: 1525-3198. https://doi.org/10.3168/jds.2009-2084 [ Links ]
Botham MK, Mayes AP. 2018a. Bioenergetics: the role of ATP. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 277-291. ISBN: 978-1-25-983793-7. https://accessmedicine.mhmedical.com/book.aspx?bookID=2386 [ Links ]
Botham MK, Mayes AP. 2018b. Lipids of physiological significance. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 483-511. ISBN: 978-1-25-983793-7. https://accessmedicine.mhmedical.com/book.aspx?bookID=2386 [ Links ]
Bruckmaier RM, Wellnitz O. 2008. Induction of milk ejection and milk removal in different production systems. Journal of Animal Science. 86(13Suppl):15-20. ISSN: 15253163. https://doi.org/10.2527/jas.2007-0335 [ Links ]
Chandel NS. 2021. Lipid metabolism. Cold Spring Harbor Perspectives in Biology. 13(9):34-41. ISSN: 1943-0264https://doi.org/10.1101/cshperspect.a040576 [ Links ]
Cooper GM. 2019a. The biosynthesis of cell constituents. Carbohydrates, lipids, proteins, adn nucleic acids. In: Cooper GM, The Cell: A Molecular Approach. Oxford, New York: Oxford University Press. Pp. 102-111. ISBN: 978-1-60535-707-2. https://learninglink.oup.com/access/cooper8e [ Links ]
Cooper GM. 2019b. Eukaryotic RNA polymerases and general transcription factors. In: Cooper GM, The Cell: A Molecular Approach. Oxford, New York: Oxford University Press. Pp. 258-276. ISBN: 978-1-60535-707-2. https://learninglink.oup.com/access/cooper8e [ Links ]
Davidson PA, Stabenfeldt HG. 2020. The mammary gland and lactation. In: Klein BG, Cunningham’s Textbook of Veterinary Physiology. St. Louis, Missouri: Elsevier Health Science Division.Pp. 458-471. ISBN: 978-0-323-55227-1. https://www.elsevier.com/books/cunningham's-textbook-of-veterinary-physiology/978-0323-55227-1 [ Links ]
Dee FA, Magee C. 2018. Anatomy and physiology of the mammary gland. In: Iowa State University Press, Anatomy and Physiology of Farm Animals. Iowa, EE. UU.: Wiley Blackwell. Pp. 501-515. ISBN: 9780813813943. https://www.wiley.com/enie/Anatomy+and+Physiology+of+Farm+Animals%2C+8th+Edition-p-9781119239765 [ Links ]
Dhasmana S, Das S, Shrivastava S. 2022. Potential nutraceuticals from the casein fraction of goat's milk. Journal of Food Biochemistry. 46(6):e13982. ISSN: 1745-4514. https://doi.org/10.1111/jfbc.13982 [ Links ]
Diao M, Liang Y, Zhao J, Zhang J, Zhang T. 2022. Complexation of ellagic acid with αlactalbumin and its antioxidant property. Food Chemistry. 372(1):131307. ISSN: 1873-7072. https://doi.org/10.1016/j.foodchem.2021.131307 [ Links ]
Doherty EA, Doudna JA. 2000. Ribozyme structures and mechanisms. Annual Review of Biochemistry. 69:597-615. ISSN: 0066-4154. https://doi.org/10.1146/annurev.biochem.69.1.597 [ Links ]
Ellis JM, Bowman CE, Wolfgang MJ. 2015. Metabolic and tissue-specific regulation of acyl-CoA metabolism. PLOS ONE. 10(3):e0116587. ISSN: 1932-6203. https://doi.org/10.1371/journal.pone.0116587 [ Links ]
Fernandez RF, Ellis JM. 2020. Acyl-CoA synthetases as regulators of brain phospholipid acyl-chain diversity. Prostaglandins Leukot Essent Fatty Acids. 161(1):102-115. ISSN: 1532-2823. https://doi.org/10.1016/j.plefa.2020.102175 [ Links ]
Fox PF, Lowe UT, McSweeney PLH, O'Mahony JA. 2015. Structure and development of mammary tissue. In: Fox PF , McSweeney PLH, Dairy Chemistry and Biochemistry. New York, United States: Springer International Publishing. Pp.1-7. ISBN: 978-3-319-14892-2. https://link.springer.com/book/10.1007/978-3-319-14892-2 [ Links ]
Friedman JR, Nunnari J. 2014. Mitochondrial form and function. Nature. 505(7483):335343. ISSN: 1476-4687. https://doi.org/10.1038/nature12985 [ Links ]
Gartner LP. 2018. The cell and the organelles. In: Vosburgh A , Horvath K, Color atlas and text of histology. Philadelphia, United States: Lippincott Williams & Wilkins. Pp. 1726. ISBN: 9781496346735 https://meded.lwwhealthlibrary.com/book.aspx?bookid=2066 [ Links ]
Goff PJ. 2015. Endocrinology, reproduction, and lactation/The mammary gland and lactation. In: Reece OW, Erickson HH, Goff PJ , Uemura EE, Dukes' Physiology of Domestic Animals. New York, EE. UU.: John Wiley & Sons. Pp. 617-727. ISBN: 9780118501399/2015. https://www.wiley.com/enus/Dukes%27+Physiology+of+Domestic+Animals%2C+13th+Edition-p-9781118501399 [ Links ]
Guoyao W. 2017a. Chemistry of lipids. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 109-142. ISBN: 978-1-4987-2160-8. https://www.routledge.com/Principles-of-Animal-Nutrition/Wu/p/book/9781032095998 [ Links ]
Guoyao W. 2017b. Nutrition and metabolism of protein and amino acids. In: Guoyao W, Principles of Animal Nutrition. New York, United States: CRC Press. Pp. 349-411. ISBN: 978-1-4987-2160-8. https://www.routledge.com/Principles-of-AnimalNutrition/Wu/p/book/9781032095998 [ Links ]
Harvatine KJ, Boisclair YR, Bauman DE. 2009. Recent advances in the regulation of milk fat synthesis. Animal. 3(1):40-54. ISSN: 1751-732X. https://doi.org/10.1017/S1751731108003133 [ Links ]
Heid HW, Keenan TW. 2005. Intracellular origin and secretion of milk fat globules. European Journal of Cell Biology. 84(2-3):245-258. ISSN: 0171-9335. https://doi.org/10.1016/j.ejcb.2004.12.002 [ Links ]
Henna K, Boudjellaba S, Khammar F, Amirat Z, Chesneau D, Charallah S. 2021. Endocrine, energy, and lipid status during parturition and early lactation in indigenous goats native to the Algerian Sahara. Veterinary World. 14(9):2419-2426. ISSN: 0972-8988. https://doi.org/10.14202/vetworld.2021.2419-2426 [ Links ]
Henry C, Saadaoui B, Bouvier F, Cebo C. 2015. Phosphoproteomics of the goat milk fat globule membrane: New insights into lipid droplet secretion from the mammary epithelial cell. Proteomics. 15(13):2307-2317. ISSN: 1615-9861. https://doi.org/10.1002/pmic.201400245 [ Links ]
Ingolia NT. 2014. Ribosome profiling: new views of translation, from single codons to genome scale. Nature Reviews Genetics. 15(3):205-213. ISSN: 1471-0064. https://doi.org/10.1038/nrg3645 [ Links ]
John PAT, van Schie SNS, Cheung NJ, Michel AH, Peter M, Kornmann B. 2022. Rewiring phospholipid biosynthesis reveals resilience to membrane perturbations and uncovers regulators of lipid homeostasis. Journal of the European Molecular Biology Organization. 41(7):e109998. ISSN: 1460-2075. https://doi.org/10.15252/embj.2021109998 [ Links ]
Jones JG. 2016. Hepatic glucose and lipid metabolism. Diabetologia. 59(6):1098-1103. ISSN: 1432-0428 https://doi.org/10.1007/s00125-016-3940-5 [ Links ]
Kumar S, Puniya AK, Puniya M, Dagar SS, Sirohi SK, Singh K, Griffith GW. 2009. Factors affecting rumen methanogens and methane mitigation strategies. World Journal of Microbiology & Biotechnology. 25(9):1557-1566. ISSN: 0959-3993. https://doi.org/10.1007/s11274-009-0041-3 [ Links ]
Lacasse P, Lollivier V, Bruckmaier RM, Boisclair YR, Wagner GF, Boutinaud M. 2011. Effect of the prolactin-release inhibitor quinagolide on lactating dairy cows. Journal of Dairy Science. 94(3):1302-1309. ISSN: 1525-3198. https://doi.org/10.3168/jds.2010-3649 [ Links ]
Lawhead BJ, Baker M. 2017. The endocrine system/endocrine glands. In: Lawhead BJ, Baker M, Introduction to Veterinary Science. Wisconsin Madison, EE. UU.: Cengage Learning. Pp. 169-179. ISBN: 978-1-1115-4279-5. https://www.cengagebrain.com.mx/shop/isbn/9781111542795 [ Links ]
Lérias RJ, Hernández CLE, Suárez TA, Castro N, Pourlis A, Almeida AM. 2014. The mammary gland in small ruminants: major morphological and functional events underlying milk production-a review. Journal of Dairy Research. 81(3):304-318. ISSN: 1469-7629. https://doi.org/10.1017/S0022029914000235 [ Links ]
Lowe M. 2011. Structural organization of the Golgi apparatus. Current Opinion in Cell Biology. 23(1):85-93. ISSN: 1879-0410. https://doi.org/10.1016/j.ceb.2010.10.004 [ Links ]
Lozano JA, Galindo JD, García BJC, Martínez LJH, Peñafiel GR, Solano MF. 2005. Metabolismo nitrogenado. En: Lozano JA, Bioquímica y Biología Molecular para Ciencias de la Salud. Barcelona, España: McGraw-Hill Interamericana. Pp. 275-302. ISBN: 4-4860642-68. https://jabega.uma.es/discovery/fulldisplay/alma991000078319704986/34CBUA_UMA:VU1 [ Links ]
Lundquist PK, Shivaiah KK, Espinoza-Corral R. 2020. Lipid droplets throughout the evolutionary tree. Progress in Lipid Research. 78(1):101-109. ISSN: 1873-2194. https://doi.org/10.1016/j.plipres.2020.101029 [ Links ]
Madigan TM, Bender SK, Buckley HD, Sattley WM, Stahl AD. 2019a. Biosyntheses. Sugars and polysaccharides. Amino acids and nucleotides. Fatty acids and lipids. In: Madigan TM, Brock Biology of Microorganisms. New York, United States: Pearson. Pp. 130-137. ISBN: 978-1-292-23510-3. https://www.pearson.com/en-us/subjectcatalog/p/brock-biology-of-microorganisms/P200000006867/9780135860717 [ Links ]
Madigan TM, Bender SK, Buckley HD, Sattley WM, Stahl AD. 2019b. Protein synthesis: translation. In: Madigan TM, Brock Biology of Microorganisms. New York, United States: Pearson. Pp. 156-170. ISBN: 978-1-292-23510-3 https://www.pearson.com/enus/subject-catalog/p/brock-biology-of-microorganisms/P200000006867/9780135860717 [ Links ]
Maldonado JJA, Salinas GH, Torres HG, Becerril PCM, P. DR.2018. Factors influencing milk production of local goats in the Comarca Lagunera, Mexico. Livestock Research for Rural Development. 30(7):2-7. ISSN: 0121-3784. https://www.cabdirect.org/cabdirect/abstract/20183317336 [ Links ]
Manterola BH. 2011. Estrategias nutricionales y alimenticias para modificar los sólidos totales de la leche. En Seminario sobre productividad en sistemas pastoriles lecheros. Departamento de Producción Animal, (Ed.). Págs. 1-20. Circular de Extensión. Facultad de Ciencias Agronómicas, Universidad de Chile. https://www.paislobo.cl/2011/10/seminario-sobre-productividad-en.html [ Links ]
Martínez GM, Suárez VH. 2018. The mammary gland: morphology and development. Synthesis of milk components. In: Martínez GM , Suárez VH, Goat dairying: production, management, health, quality of milk and products. Buenos Aires, Argentina: Ediciones INTA. Pp. 37-41. ISBN: 978-987-521-972-4. https://repositorio.inta.gob.ar/xmlui/handle/20.500.12123/5408?locale-attribute=en [ Links ]
Mas OJ. 2018. Metabolismo de los lípidos. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 660-713. ISBN: 978-607-448708-4. https://libros.facmed.unam.mx/index.php/2021/07/22/bioquimica-de-laguna-ypina/ [ Links ]
McDonald P, Edwards RA, Greenhalgh JFD, Morgan CA, Sinclair LA, Wilkinson RG. 2011. Lipids. In: McDonald P, Animal Nutrition. New York, United States: Prentice Hall. Pp. 32-52. ISBN: 978-1408204238.https://www.pearson.com/en-gb/search.html?aq=Mc%20Donald-Animal-Nutrition-7th-Edition [ Links ]
McKerchar HJ, Lento C, Bennie RZ, Crowther JM, Dolamore F, Dyer JM, Clerens S, Mercadante D, Wilson DJ, Dobson RCJ. 2023. The protein dynamics of bovine and caprine β-lactoglobulin differ as a function of pH. Food Chemistry. 408(1):135229. ISSN: 1873-7072. https://doi.org/10.1016/j.foodchem.2022.135229 [ Links ]
Menzies P. 2021. Udder health for dairy goats. Veterinary Clinics of North America: Food Animal Practice. 37(1):149-174. ISSN: 1558-4240. https://doi.org/10.1016/j.cvfa.2020.12.002 [ Links ]
Monks J, Ladinsky MS, McManaman JL. 2020. Organellar contacts of milk lipid droplets. Thousand Oaks Journal. 3(1):2-12. ISSN: 2515-2564. https://doi.org/10.1177/2515256419897226 [ Links ]
National Chamber of Industrial Milk. 2021. CANILEC. Markets and statistics. http://www.canilec.org.mx/index.html [ Links ]
Nayik GA, Jagdale YD, Gaikwad SA, Devkatte AN, Dar AH, Ansari MJ. 2022. Nutritional profile, processing and potential products: A comparative review of goat milk. Dairy. 3(3):622-647. ISSN: 2624-862X. https://doi.org/10.3390/dairy3030044 [ Links ]
Nelson LD, Cox MM. 2017a. The citric acid cycle. In: Nelson LD , Cox MM, Lehninger. Principles of Biochemistry. New York, United States: Freeman, W. H. Pp. 1668-1743. ISBN: 9781464126116. https://link.springer.com/book/9781319381493 [ Links ]
Nelson LD, Cox MM. 2017b. Lipids. In: Nelson LD , Cox MM, Lehninger. Principles of Biochemistry. New York, United States: Freeman, W. H. Pp. 989-1056. ISBN: 9781464126116. https://link.springer.com/book/9781319381493 [ Links ]
Nelson LD, Cox MM. 2017c. Protein metabolism. In: Nelson LD , Cox MM, Lehninger. Principles of Biochemistry. New York, United States: Freeman, W. H. Pp. 2820-2941. ISBN: 9781464126116. https://link.springer.com/book/9781319381493 [ Links ]
Neville MC, McFadden TB, Forsyth I. 2002. Hormonal regulation of mammary differentiation and milk secretion. Journal of Mammary Gland Biology and Neoplasia. 7(1):49-66. ISSN: 1083-3021. https://doi.org/10.1023/A:1015770423167 [ Links ]
Nunes NA, Araujo WL, Obata T, Fernie AR. 2013. Regulation of the mitochondrial tricarboxylic acid cycle. Current Opinion in Plant Biology. 16(3):335-343. ISSN: 18790356. https://doi.org/10.1016/j.pbi.2013.01.004 [ Links ]
Olarte MJ, Kim S, Sharp ME, Swanson JMJ, Farese RV Jr ., Walther TC. 2020. Determinants of endoplasmic reticulum-to-lipid droplet protein targeting. Developmental Cell. 54(4):471-487 e477. ISSN: 1878-1551. https://doi.org/10.1016/j.devcel.2020.07.001 [ Links ]
Pacheco GV, Caballero ZA, Martínez GS, Prado ROF, García CAC. 2021. Biochemistry and metabolic pathways of polysaccharides, lipids, and proteins. Abanico Veterinario. 11(1):1-26. ISSN: 2448-6132. https://doi.org/10.21929/abavet2021.47 [ Links ]
Philipps WP. 2018. Proteases-general aspects. In: Simões NC , Kumar V, Enzymes in Human and Animal Nutrition: Principles and Perspectives. New York, United States: Academic Press. Pp. 257-264. ISBN: 9780128094266. https://shop.elsevier.com/books/enzymes-in-human-and-animal-nutrition/nunes/978-012-805419-2 [ Links ]
Piña GE, Flores HO. 2018. Metabolismo de los compuestos nitrogenados. En: Hernández MMT, Bioquímica de Laguna y Piña. Ciudad de México, México: El Manual Moderno. Pp. 714-763. ISBN: 978-607-448-708-4. https://libros.facmed.unam.mx/index.php/2021/07/22/bioquimica-de-laguna-y-pina/ [ Links ]
Pol A, Gross SP, Parton RG. 2014. Review: biogenesis of the multifunctional lipid droplet: lipids, proteins and sites. Journal of Cell Biology. 204(5):635-646. ISSN: 15408140. https://doi.org/10.1083/jcb.201311051 [ Links ]
Reece OW, Rowe WE. 2017a. Endocrine system. In: Reece OW, Rowe WE, Functional Anatomy and Physiology of Domestic Animals. New York, United States: John Wiley & Sons. Pp. 242-262. ISBN: 9781119270843. https://www.wiley.com/enus/Functional+Anatomy+and+Physiology+of+Domestic+Animals%2C+5th+Edition-p9781119270867 [ Links ]
Reece OW, Rowe WE. 2017b. Lactation / functional anatomy of female mammary glands. Mammogenesis, lactogenesis and lactation. In: Reece OW, Rowe WE, Functional Anatomy and Physiology of Domestic Animals. New York, EE. UU.: John Wiley & Sons. Pp. 660-678. ISBN: 9781119270843.https://www.wiley.com/en-us/Functional+Anatomy+and+Physiology+of+Domestic+Animals%2C+5th+Edition-p9781119270867 [ Links ]
Reese OW, Budras KD, Mülling C, Bragulla H, Hagen J, Witter K, König HE. 2020. Mammary gland (mamma, uber, mastos). In: König HE , Liebich GG, Veterinary Anatomy of Domestic Animals. Stuttgart, Germany: Georg Thieme Verlag KG. Pp. 642-648. ISBN: 978-3-13-242933-8 https://vetbooks.ir/veterinary-anatomy-of-domestic-animals-textbookand-colour-atlas-7th-edition/ [ Links ]
Rodwell WV. 2018. Catabolism of proteins and amino acid nitrogen. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 661-686. ISBN: 978-1-25-983793-7 https://accessmedicine.mhmedical.com/book.aspx?bookID=2386 [ Links ]
Rowland AA, Voeltz GK. 2012. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nature Reviews Molecular Cell. 13(10):607-625. ISSN: 1471-0080. https://doi.org/10.1038/nrm3440 [ Links ]
Saheki Y, De Camilli P. 2017. Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annual Review of Biochemistry. 86:659-684. ISSN: 1545-4509. https://doi.org/10.1146/annurev-biochem-061516-044932 [ Links ]
Saikia D, Hassani MI, Walia A. 2022. Goat milk and its nutraceutical properties. International Journal of Applied Research. 8(4):119-122. ISSN: 2394-5869. https://doi.org/10.22271/allresearch.2022.v8.i4b.9639 [ Links ]
Saipin N, Thuwanut P, Thammacharoen S, Rungsiwiwut R. 2020. Effect of incubation temperature on lactogenic function of goat milk-derived mammary epithelial cells. In Vitro Cellular & Developmental Biology. 56(10):842-846. ISSN: 1543-706X. https://doi.org/10.1007/s11626-020-00529-3 [ Links ]
Sanhueza J, Valenzuela R, Valenzuela A. 2012. Cholesterol metabolism: increasingly complex. Grasas y Aceites. 63(4):373-382. ISSN: 1988-4214. https://doi.org/10.3989/gya.035512 [ Links ]
Sansi MS, Iram D, Zanab S, Vij S, Puniya AK, Singh A, Ashutosh Meena S. 2022. Antimicrobial bioactive peptides from goat Milk proteins: In silico prediction and analysis. Journal of Food Biochemistry. 45(10):e14311. ISSN: 1745-4514. https://doi.org/10.1111/jfbc.14311 [ Links ]
Seoane A, Brea RJ, Fuertes A, Podolsky KA, Devaraj NK. 2018. Biomimetic generation and remodeling of phospholipid membranes by dynamic imine chemistry. Journal of the American Chemical Society. 140(27):8388-8391. ISSN: 1520-5126. https://doi.org/10.1021/jacs.8b04557 [ Links ]
Singh A , Rajeev S. 2020. DNA: an important component of life. In: Torrens F, Mahapatra KD , Haghi AK, Biochemistry, Biophysics, and Molecular Chemistry: Applied Research and Interactions. Florida, United States: Apple Academic Press, Inc. Pp. 195-208. ISBN: 9781771888165. https://www.routledge.com/Biochemistry-Biophysics-and-MolecularChemistry-Applied-Research-and/Torrens-Mahapatra-Haghi/p/book/9781774635100 [ Links ]
Song Z, Xiaoli AM, Yang F. 2018. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 10(10):1-10. ISSN: 2072-6643. https://doi.org/10.3390/nu10101383 [ Links ]
Sturley SL, Hussain MM. 2012. Lipid droplet formation on opposing sides of the endoplasmic reticulum. Journal of Lipid Research. 53(9):1800-1810. ISSN: 1539-7262. https://doi.org/10.1194/jlr.R028290 [ Links ]
Suburu J, Shi L, Wu J, Wang S, Samuel M, Thomas MJ, Kock ND, Yang G, Kridel S, Chen YQ. 2014. Fatty acid synthase is required for mammary gland development and milk production during lactation. American Journal of Physiology-Endocrinology and Metabolism. 306(10):E1132-E1143. ISSN: 1522-1555. https://doi.org/10.1152/ajpendo.00514.2013 [ Links ]
Svennersten SK, Olsson K. 2005. Endocrinology of milk production. Domestic Animal Endocrinology. 29(2):241-258. ISSN: 18790054. https://doi.org/10.1016/j.domaniend.2005.03.006 [ Links ]
Swaisgood HE. 2003. Protein composition of milk: identification, structure, and chemical composition. In: Fox PF , McSweeney PLH, Advanced dairy chemistry: proteins part A. Cork, Ireland: Springer Science. Pp. 140-225. ISBN: 9780306472718. https://link.springer.com/book/10.1007/978-1-4419-8602-3 [ Links ]
Thul TA, Corwin EJ, Carlson NS, Brennan PA, Young LJ. 2020. Oxytocin and postpartum depression: A systematic review. Psychoneuroendocrinology. 120(1):104-109. ISSN: 1873-3360. https://doi.org/10.1016/j.psyneuen.2020.104793 [ Links ]
Tortora JG, Funke RB, Case LC. 2019. Organic compounds. Structure and chemistry: carbohydrates, lipids, proteins, nucleic acids. In: Beauparlant S, Microbiology: An Introduction. New York, United States: Pearson. Pp. 33-47. ISBN: 978-0-13-460518-0. https://www.pearson.com/en-us/subject-catalog/p/microbiology-anintroduction/P200000006850/9780135789377 [ Links ]
Verschueren KHG, Blanchet C, Felix J, Dansercoer A, De Vos D, Bloch Y, Van Beeumen J, Svergun D, Gutsche I, Savvides SN, Verstraete K. 2019. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature. 568(7753):571575. ISSN: 1476-4687. https://doi.org/10.1038/s41586-019-1095-5 [ Links ]
Vidal ME. 2013. Lácteos: oferta y demanda en el contexto regional y mundial. En 1º Foro de Agricultura de América del Sur. Oficina de Programación y Política Agropecuaria, (Ed.). Consejo Agropecuario del Sur. Foz de Iguazú, Brasil. https://www.odepa.gob.cl/publicaciones/noticias/noticias-institucionales/primer-foro-dela-agricultura-en-america-del-sur [ Links ]
Weil AP. 2018a. Protein synthesis and the genetic code. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 955-991. ISBN: 978-1-25-983793-7. https://accessmedicine.mhmedical.com/book.aspx?bookID=2386 [ Links ]
Weil AP. 2018b. RNA synthesis, processing and modification. In: Rodwell WV, Bender AD, Botham MK, Kennelly JP , Weil AP, Harper’s Illustrated Biochemistry. United States: McGraw-Hill Education / Medical. Pp. 911-954. ISBN: 978-1-25-983793-7. https://accessmedicine.mhmedical.com/book.aspx?bookID=2386 [ Links ]
Wilson C, Venditti R, Rega LR, Colanzi A, D'Angelo G, De Matteis MA. 2011. The Golgi apparatus: an organelle with multiple complex functions. Biochemical Journal. 433(1):19. ISSN: 1470-8728. https://doi.org/10.1042/BJ20101058 [ Links ]
Zhang C, Liu P. 2019. The new face of the lipid droplet: lipid droplet proteins. Proteomics. 19(10):e1700223. ISSN: 1615-9861. https://doi.org/10.1002/pmic.201700223 [ Links ]
Received: January 02, 2023; Accepted: May 02, 2023











 text in
text in