Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.13 Tepic Jan./Dec. 2023 Epub Oct 27, 2023
https://doi.org/10.21929/abavet2023.16
Literature Review
Synchronization of estrus and ovulation in bovine females. Endocrine bases and treatments used
1Universidad Autónoma Metropolitana, Unidad Xochimilco, Departamento Producción Agrícola y Animal, Calzada del Hueso 1100, Coyoacán, CDMX 04960 México.
2Universidad Autónoma Metropolitana, Unidad Xochimilco. Maestría en Ciencias Agropecuarias. Calzada del Hueso 1100, Coyoacán, CDMX 04960 México.
In Mexico, reproductive efficiency of cows in calf production systems is low and the use of estrus and ovulation synchronization schemes can contribute to improve it. The objective of this review was to describe the endocrine regulation of the estrous cycle and follicular dynamics in cattle, highlighting how, using exogenous hormones, and these processes can be manipulated to implement different estrous and ovulation synchronization protocols. In addition, results of pregnancy rate at synchronized estrus (PRSE) with the different estrous and ovulation synchronization protocols reported in the literature are presented. Reviews and original articles on topics of estrous cycle and follicular development in cattle were collected and PRSE data were obtained from scientific articles published between 2000 and 2023. The reported PRSE ranges from 23 to 76 %, however, although the range is wide, in estradiol and GnRH based protocols about 50 % of the PRSE data collected are between 45 and 55 %. In progesterone-based protocols, 50 % of the data report PRSE between 55 and 65 %. Currently, there are three main estrous and ovulation synchronization protocols with which, according to the literature reviewed, PRSE between 45 and 65 % can be obtained in most occasions.
Keywords: oestrus cycle; waves of follicular growth; synchronization of estrus and ovulation; beef cows
En México, la eficiencia reproductiva de las vacas en los sistemas de producción de becerro es baja y el uso de esquemas de sincronización del estro y la ovulación puede contribuir a mejorarla. El objetivo de esta revisión fue describir la regulación endocrina del ciclo estral y la dinámica folicular en los bovinos, resaltando cómo, a través del uso de hormonas exógenas se pueden manipular estos procesos para implementar los diferentes protocolos de sincronización del estro y la ovulación. Además, se presentan resultados de tasa de preñez a estro sincronizado (TPES) con los diferentes protocolos de sincronización de estro y ovulación que se reportan en la bibliografía. Se recopilaron revisiones y artículos originales sobre temas de ciclo estral y desarrollo folicular en bovinos y se obtuvieron datos de TPES de artículos científicos publicados entre 2000 y 2023. La TPES reportada oscila entre 23 hasta 76%, sin embargo, aunque el rango es amplio, en protocolos basados en estradiol y GnRH alrededor del 50% de los datos de TPES colectados están entre 45 y 55%. En protocolos basados en progesterona el 50% de los datos reportan TPES de entre 55 y 65%. En la actualidad, existen tres principales protocolos de sincronización de estro y ovulación con los que, según la bibliografía revisada, se puede obtener TPES de entre 45 y 65% en la mayoría de las ocasiones.
Palabras clave: ciclo estral; olas de crecimiento folicular; sincronización de estro y ovulación; vacas productoras de carne
INTRODUCTION
Rearing calves for beef production depends largely on the reproductive efficiency of dams (Alvarez et al., 2018). In Mexico, in general and specifically in the tropical regions of the country, reproductive efficiency in calf production systems is low (Lassala et al., 2020). It has been reported that only 32.6 % of cows in the national herd are pregnant, cows in the Northeast and Central zone have the highest gestation percentage (41 %), while in the Northern region the gestation percentage is only 25 % (Gutiérrez, 2018). Although there are factors such as breed, postpartum anestrus, lactation and nutritional status affecting reproductive efficiency in these production systems, there are also some reproductive management aspects that can exacerbate this problem. In Mexico, about 90 % of the country's calf producers use continuous mating, only between 2.4 and 9.4 % use artificial insemination (AI) and less than 10 % perform estrus synchronization (Lassala et al., 2020).
Knowledge of the endocrine control of the estrous cycle and the follicular dynamics physiology in cows has allowed the development of biotechnologies such as estrus and ovulation synchronization that in turn facilitate and make AI more efficient in beef cattle (Marizancén & Artunduaga, 2017). These biotechnologies applied correctly improve the reproductive efficiency of cattle (Colazo et al., 2018; Barusseli et al., 2018). In postpartum Nelore cows, the use of an estrus synchronization protocol prior to the onset of mating increased 1.5 times the probability of cows becoming pregnant during this period, as well as the pregnancy rate of the mating (52.2 vs. 27.6 %; Ferreira et al., 2018). In beef cows, the use of estrus and ovulation synchronization protocols at the beginning of the mating, allows inducing and uniformizing the manifestation of estrus in a pre-established period of time of short duration, which facilitates performing AI, scheduling calving times and inducing ovarian activity in heifers and cows in anestrus (Colazo & Mapletoft, 2014; Baruselli et al., 2018).
Estrus and ovulation synchronization protocols are based on the use of hormones to manipulate follicular dynamics and the duration of the luteal phase of the estrous cycle (Lamb et al., 2010; Colazo & Mapletoft, 2014) so it is important to understand these processes in detail. Hormones used to manipulate follicular dynamics are gonadotropinreleasing hormone (GnRH), estrogens and progesterone. To manipulate the duration of the luteal phase, prostaglandin F2α (PGF2-α) and progesterone can be used (Lamb et al., 2010; Bó & Baruselli, 2014; Colazo & Mapletoft, 2014; Bó et al., 2016).
It is important when applying hormonal treatments to manipulate the reproductive response of cattle to know the mechanism by which physiological and endocrine changes occur at each stage of the estrous cycle to be modified, as well as effects that each hormone has in a given protocol. This paper aims to describe the endocrine regulation of the estrous cycle and follicular dynamics in cattle, highlighting how, using exogenous hormones, and these processes can be manipulated to implement different estrus and ovulation synchronization protocols. In addition, results of pregnancy rate at synchronized estrus (PRSE) with different estrus and ovulation synchronization protocols reported in the literature are presented.
MATERIAL AND METHODS
We searched Pubmed, ScienceDirect, Google Scholar and SciELO databases using the keywords "Bovine estrus cycle" and "Bovine follicular waves" and selected the most important reviews and original articles on these topics, including those published by our group. To describe the synchronized estrus pregnancy rate (PRSE) results obtained with the main protocols currently in use, a search only in Pubmed was performed using the keywords "estrus synchronization beef cows estradiol" to obtain data reported using estradiol-based protocols and with the keywords "estrus synchronization beef cows GnRH", articles with the use of GnRH-based protocols were retrieved. In addition to the articles we retrieved in the latter search, we selected those that used progestogen-based protocols. Publications from 2000 to 2023 were selected in both searches. The main selection criteria for PRSE data were those where treatment assignment was completely randomized. Additionally, data were taken from experimental groups that evaluated the effect of equine chorionic gonadotropin (eCG) use, estrus presentation effect, cyclicity and temporary weaning. When in an article they evaluated variables other than those mentioned above, only the data from the control group were taken. With the PRSE data from each experimental group, frequency histograms or scatter plots were made to describe the variation of the data.
Endocrine Control of the Estrous Cycle in Cattle
Female domestic mammals from puberty and throughout their reproductive life will periodically present estrous cycles, which are defined as the period from the onset of one estrus to the onset of the next (Lamb & Mercadante, 2016). The estrous cycle is characterized by a series of anatomical, endocrine and behavioral changes aimed at ovulation and preparing the uterine environment for possible gestation (Bó et al., 2003). In cattle, each estrous cycle has an average duration of 20 days in heifers and 21 days in cows (Sartori & Barros, 2011). The estrous cycle is composed of four stages: estrus, metaestrus, diestrous, proestrus and two phases: the follicular, estrogenic or proliferative phase, which includes proestrus and estrus, and the luteal, progestational or secretory phase, which includes metaestrus and diestrous (Figure 1). In the follicular phase the ovulatory follicle develops, so the dominant hormone is estradiol. This steroid promotes, at the uterine level, the proliferation of endometrial cells. In the luteal phase, the development and functionality of the CL occurs, so the dominant hormone is progesterone that stimulates the endometrium to secrete uterine milk or histone (Rathbone et al., 2001; Sartori & Barros, 2011).

Figure 1 Schematic representation of the stages and phases of the estrous cycle in cattle and of the follicular development waves
The estrous cycle establishment and the changes that occur in it are regulated by the synthesis and secretion of hormones from the hypothalamus. GnRH, from the pituitary gland produces luteinizing hormone and follicle stimulating hormone (LH and FSH), from the follicle that secretes estrogen and inhibin, from the LC that produces progesterone and oxytocin, and from the endometrium that releases PGF2α (Sartori & Barros, 2011).
During proestrus, FSH production remains low and constant because it is not regulated by GnRH, while LH begins to increase its frequency of secretion and decrease the amplitude of its pulses. This promotes the final maturation of the follicle by increasing the synthesis of estradiol and inhibin (Rathbone et al., 2001). The increase of these two hormones exerts a negative feedback on FSH synthesis at the pituitary level (Aerts & Bols, 2010). On the other hand, towards the end of proestrus and beginning of estrus, estradiol exerts a positive feedback effect on GnRH by acting on α and β receptors located on glutamatergic and kispeptinergic neurons (Kenealy & Terasawa, 2012). This estradiol effect triggers the preovulatory GnRH-LH peak, and with it ovulation (Garverick & Smith, 1993; Forde et al., 2011; De Graaff & Grimard, 2018). To trigger ovulation, which occurs between 10 to 12 hours after the end of estrus, LH promotes the synthesis of prostaglandins and enzymes to facilitate the rupture of follicular walls. In addition, LH stimulates the restart of meiosis in the oocyte (Delgado et al., 2011). After ovulation, the CL develops from the ovulated follicle by the effect of LH and progesterone concentrations gradually increase to reach their maximum concentration at the end of metaestrus and beginning of diestrous (Forde et al., 2011). Peak progesterone concentrations are kept constant throughout the entire estrus to prevent ovulation of dominant follicles developed during the luteal phase of the cycle (Baruselli et al., 2007).
If the ovulated oocyte is not fertilized, around days 16 and 17 of the estrous cycle, the endometrium will secrete PGF2α to induce CL regression (Rosales-Torres & Guzmán, 2011; Sartori & Barros, 2011). For this, PGF2α by binding to its receptor on CL blood vessels causes an elevation of intracellular Ca++ to induce vasoconstriction. Vasoconstriction reduces the supply of nutrients, oxygen and cholesterol to the LC, causing a reduction in progesterone synthesis and apoptosis of the luteal cells (RosalesTorres & Guzmán, 2008; Shirasuna et al., 2012). Luteolysis causes a reduction in the serum concentration of progesterone so that the negative feedback exerted by progesterone on GnRH disappears (Rathbone et al., 2001).
Waves of Follicular Growth
Follicular development during the estrous cycle in cattle occurs in waves and it is a highly selective process where usually only one follicle can ovulate and the fate of the rest of the follicles in the same cohort is atresia (Rosales-Torres et al., 2012). In each wave of follicular growth, a dominant follicle develops, although only the dominant follicle of the last wave will ovulate (Aerts & Bols, 2010; Bó et al., 2016; Ginther, 2016). Follicular growth waves are composed of three phases: cyclic recruitment, selection and dominance (Aerts & Bols, 2010; Fortune et al., 1991; Rosales-Torres et al., 2012).
During cyclic recruitment, a cohort of small antral follicles initiate growth in response to a transient FSH spike (Mihm et al., 2000; Driancourt, 2001; Aerts & Bols, 2010). This phase lasts about 2 days during which FSH stimulates follicle growth (Webb et al., 2004; Rodgers & Rodgers, 2010). Selection occurs at the end of the common growth period, when one of the recruited follicles, is selected as dominant to continue its growth (Sirard, 2016; Rosales-Torres et al., 2012). Finally, dominance refers to the mechanism by which the follicle selected as dominant has a rapid development and suppresses the growth of subordinate follicles Ginther et al., 1989; Webb et al., 2004). To this end, estradiol and the inhibin produced in it prevent the synthesis and secretion of FSH (Beg & Ginther, 2006). This reduction of FSH causes atresia of the subordinate follicles, while the dominant follicle continues its growth at a rate of up to 1.6 mm per day (Sirois & Fortune 1988; Rosales-Torres et al., 2012). If the dominant follicle develops during the luteal phase of the estrous cycle, circulating concentrations of progesterone prevent the preovulatory LH peak so it will be unable to ovulate and will become atretic (Figure 1). In the absence of a functional CL, the dominant follicle by its high estradiol production induces the preovulatory LH peak and thus ovulation (Driancourt, 2001; Moore & Thatcher, 2006).
It is important to note that as long as the dominant follicle is estrogenically active, a new wave of follicular growth cannot emerge (Webb et al., 2004; Rosales-Torres et al., 2012). However, if the dominant follicle ovulates or becomes atretic, the blockade on FSH will disappear as estradiol and inhibin are no longer produced and a new transient elevation of this hormone will occur, which will stimulate the emergence of a new wave of follicular growth (Sartori et al., 2004; Beg & Ginther, 2006).
There are some species and breed differences in follicular dynamics (Baruselli et al., 2007). In Bos taurus cattle, the selection of the dominant follicle occurs 2 days after the initiation of the follicular wave and generally the follicle that reaches 8.5 mm in diameter will be the one selected as dominant (Fortune et al., 1991; Fortune et al., 2001). In the case of Bos indicus cows, the selection of the dominant follicle will be 2.6 days after the start of the wave and the follicle with a diameter of approximately 5.9 ± 0.4 mm will be selected (Sartori & Barros, 2011). Baruselli et al. (2018) report that Brahman, Nelore and Gyr cows can present between two and four waves of follicular development per estrous cycle. While in Bos taurus cows two to three waves predominate (Sartori et al., 2004). In addition to the difference in the number of follicular waves, Bos indicus females recruit a greater number of follicles per follicular growth wave than Bos taurus females (33.4±3.2 versus 25.4±2.5 respectively). Finally, in Bos taurus females with two follicular growth waves the largest diameter of the dominant follicle is 17.1 mm for the first wave and 16.5 mm for the second wave (Ginther, 2016). In Bos indicus the largest diameters of the dominant follicle are 11.3 mm, 12.1 mm and 10.4 mm for the first, second and third growth waves respectively (Figueiredo et al., 1997).
Oestrus and Ovulation Synchronization
Oestrus and ovulation synchronization protocols have important advantages for the reproductive management of beef cattle and have evolved over time to be more effective. The implementation of these protocols facilitates the use of fixed-time artificial insemination (FTAI) or estrus detected, homogenizes calf birth, allows calving scheduling, newborn calf care, and increases pregnancy rates (Abel et al., 2017). Currently, hormonal manipulation of the estrous cycle in cattle to synchronize estrus and ovulation is based on four major principles;
Simulate the luteal phase of the estrous cycle. This is achieved by exogenous administration of progesterone using intravaginal devices that release progesterone (DIP) constantly (Colazo & Mapletoft, 2014) or by using oral progestogens such as melengestrol acetate (MGA; Lamb et al., 2010). DIPs are currently the most commonly used and are recommended for use for periods of 5 to 7 days (Abel et al., 2017; Williams & Stanko, 2020). The physiological rationale for the use of DIPs is that, upon withdrawal of treatment, blood progesterone concentrations are rapidly reduced. This causes animals that do not have a corpus luteum to go into proestrus and maturation of a dominant follicle occurs that produces sufficient estradiol to increase the frequency of pituitary LH pulses and the likelihood of ovulation (Rathbone et al., 2001; Lamb et al., 2010; Sartori & Barros, 2011).
Accelerate CL regression by administration of PGF2α or its synthetic analogues. The application of PGF2α induces lysis of the CL to cause reduction in endogenous progesterone concentrations and induce a new follicular phase in animals that have a functional CL (Abreu et al., 2018). PGF2α induces lysis of the CL when applied at least 6 to 7 days after the onset of estrus. Since prior to this time, the CL is in development and there are many survival factors in the surrounding environment such as LH, VEGF and IGF-I that prevent the luteolytic effect of PGF2α (Wenzinger & Bleul, 2012; Abel et al., 2017; Scarpa et al., 2019). It is important to note that in the absence of a CL PGF2α has no effect, however for management issues and the need to have experience to palpate the presence of a CL, PGF2α is usually applied even when there is no in CL in ovary.
Synchronize the onset of a new wave of follicular growth. This is achieved by the application of GnRH or estradiol. When GnRH or its analogues are applied, an LH peak is induced and if the female has a dominant follicle, it will ovulate. Ovulation of the dominant follicle causes a reduction of estradiol and inhibin, which allows an elevation of FSH to initiate a new wave of follicular growth (Adams et al., 1992; Aerts & Bols, 2010). As for the use of estradiol or its analogues, they are applied in high doses (2 mg), to induce a negative feedback on FSH at the pituitary level and thereby cause atresia of the follicles of the growth wave in turn present in the ovary. When the follicles become atretic, they stop secreting estradiol, inhibin, the negative feedback they exerted on FSH is eliminated, and the concentrations of this gonadotropin are elevated to initiate a new wave of follicular growth (Bó et al., 1994; Scarpa et al., 2019).
Induce ovulation of the dominant follicle of the synchronized growth wave. In beef cattle, ovulation can be induced by application of GnRH at the time of artificial insemination or with low-dose (1 ng) application of estradiol in proestrus animals to induce estrous behavior and the preovulatory LH peak (Bó et al., 2016).
Main Protocols Currently Used
To date, the most commonly used treatments for estrus and ovulation synchronization are based on DIP's. The protocols differ in the hormone used to synchronize follicular development, which can be GnRH, synthetic estrogens or progestogens. Thus, the estrous and ovulation synchronization protocols currently used are based on estradiol, GnRH or progesterone (Figure 2), although most protocols use a combination of these hormones (Bó & Baruselli, 2014; Lamb & Mercadante, 2016; Bó et al., 2016).
Estradiol-based protocols
Estradiol-based treatments (Figure 2A to 2C) consist of the insertion of a DIP plus an injection of 2 mg estradiol benzoate (BE) intramuscularly on day 0. Estradiol allows for the induction of the onset of the follicular growth wave and thus ensures the presence of an estrogenically active follicle containing a viable oocyte at the time of AI (Bó et al., 1994; Uslenghi et al., 2014; Bó et al., 2016). The DIP is removed 7, 8 or 9 days after insertion and PGF2α is applied to lyse a possible CL. This ensures the reduction of progesterone in blood and the onset of the follicular phase. After this, ovulation of the dominant follicle of the synchronized wave should be induced, for which one can proceed in several ways in relation to DIP withdrawal: 1) apply 1 mg of BE 24 hours later, 2) apply GnRH 54 hours later or 3) apply estradiol cypionate (ECP; 0.5 or 1 mg) upon DIP withdrawal. With the use of these protocols, it is recommended to perform IATF between 54 and 64 hours after DIP withdrawal (Colazo et al., 2003; Sales et al., 2012).

Figure 2 Main estrous and ovulation synchronization protocols currently used in beef cattle. A-C) Estradiol-based protocols using BE (A), GnRH (B) or ECP (C) as ovulation inducers. D-F) GnRH-based protocols without (D) or with the use of a progesterone-releasing intravaginal device (DIP) for seven (E) or five (F) days. G and H) Progesterone-based protocols without the use of GnRH prior to PGF2α application (G) or with the use of GnRH seven days prior to PGF2α application (H)
PRSE in heifers treated with estradiol-based protocols
Using estradiol-based protocols in heifers, the lowest PRSE reported in the articles consulted was 39 % (Reineri et al., 2023) while the highest was 59 % (Silva et al., 2018a). Regarding differences by ovulation inducer, PRSE did not differ statistically in heifers with the use of GnRH (59 %; Silva et al., 2018b) or DBS (53 %; Silva et al., 2018b) or between the use of BE (48 %) and DBS (47 %; (Pfeifer et al., 2014).
The use of eCG at the time of DIP withdrawal in heifers has not been as extensively evaluated as in cows (see below), however, the use of 200, 300 or 400 IU of eCG does not seem to modify PRSE (Pinto et al., 2020). From the data collected in heifers, in no case are differences in PRSE between animals in estrus or pubertal status reported. However, the presence of CL at the beginning of treatment indicates that heifers have started to cycle. In this regard, Silva et al. (2018b) report that PRSE is similar in heifers without the presence of CL at the start of treatment when using GnRH (50.5 %) or ECP (50.5 %) as ovulation inducers. However, PRSE was higher in animals with CL when GnRH (68 %) was used to induce ovulation than when DBS (55 %) was used. This evidence suggests that, in heifers treated with estradiol-based protocols, GnRH, BE or DBS can be used interchangeably as ovulation inducers. However, if cycling heifers can be detected at the start of treatment, it is better to use GnRH to induce ovulation in these heifers.
PRSE in cows treated with estradiol-based protocols
In lactating cows, the lowest reported PRSE was 23 % (Malik et al., 2012) and the highest was 72 % (Rodrigues et al., 2018). In this group of animals treated with estradiol-based protocols most of the PRSE data (73%) reported in the articles consulted was between 41 and 60 % (Figure 3A). For non-lactating cow data, the lowest PRSE was 44 % and the highest was 62 % (Uslenghi et al., 2014).
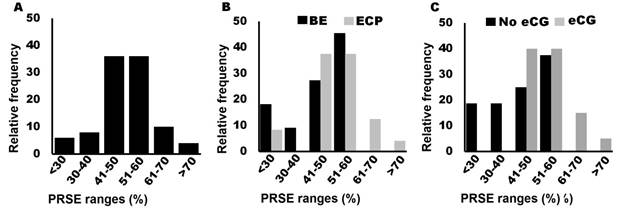
Figure 3 Pregnancy rate to synchronized estrus (PRSE) with estradiol-based protocols in lactating cows. A) Distribution of total PRSE data. B) Distribution of PRSE data with the use of estradiol benzoate (BE) and estradiol cypionate (ECP) as ovulation inducers. C) Distribution of PRSE data with or without the use of 300 IU of equine chorionic gonadotropin (eCG) at the time of DIP withdrawal. Information compiled from: Ross et al., 2004; Sa Filho et al., 2010; Malik et al., 2012; Campos et al., 2013; Pfeifer et al., 2015; Uslenghi et al., 2016; Pessoa et al., 2016; Cooke et al., 2016; Rodrigues et al., 2018; Santos et al., 2018; Silva et al., 2018a, Crepaldi et al., 2019; Oliveira-Filho et al., 2020; Noronha et al., 2020; Diniz et al.,2021; Alves et al., 2021; Pfeifer et al., 2022; Barbosa et al., 2022; Rodriguez et al., 2023; Aragunde-Vieytes et al., 2023
As an ovulation inducer, in estradiol-based protocols, range PRSE reported in lactating cows with the use of from 23 to 58 %, whereas when DBS was used as an ovulation inducer, 88 % of the reported PRSE data ranged from 41 to 70 % (Figure 3B). These data suggest that PRSE may be increased if DBS is used compared to BE as ovulation inducers. However, in articles where different ovulation inducers were compared in lactating cows treated with estradiol-based protocols, no differences were observed between the use of BE or ECP (Sales et al., 2012; Uslenghi et al., 2014; Uslenghi et al., 2016).
The use of eCG (300 IU) at the time of DIP withdrawal is a strategy to increase PRSE in lactating cows treated with estradiol-based protocols. As we can see in Figure 3C, PRSE reported when eCG was not used, in 100 % of the data retrieved from the literature, was less than 61 %, whereas when eCG was used 100 % of the PRSE data were between 45 and 72 %. These reports suggest that PRSE in lactating cows treated with estradiol-based protocols can be improved with the use of eCG. This hypothesis was confirmed by Pessoa et al. (2016) who report that the use of eCG at the time of DIP withdrawal increases PRSE (45%) compared to cows not given eCG (30 %). ECG acts primarily on FSH receptors in the follicle to promote estradiol synthesis and follicular growth (Murphy & Martinuk, 1991). It has been reported that increased follicular development can improve oocyte quality and thus fertility (Simões et al., 2018) which explains why, the use of this hormone improves PRSE in estradiol-based protocols.
In lactating beef cows, the presence of calf reduces GnRH/LH secretion (Crowe, 2008), so temporary weaning is recommended from DIP removal until FTAI (Crowe, 2008). However, Cooke et al. (2016)) report that PRSE is similar in cows. Temporary weaning was performed (45.6 %) than in cows where it was not performed (46.6 %). Pfeifer et al., (2014) report a PRSE of 58.1 % with temporary weaning, which is within the range of PRSE reported in the rest of the articles reviewed where temporary weaning is not reported to have been performed (Malik et al., 2012; Rodrigues et al., 2018). This evidence suggests that temporary weaning is not necessary and that estradiol-based protocols can counteract the negative effect of calf presence on GnRH/LH secretion.
Another factor that may affect PRSE is the presentation of estrus after DIP withdrawal. PRSE was higher (P<0.05) in lactating and non-lactating cows that presented estrus, than in those that did not present estrus (Cedeño et al., 2021; Pessoa et al., 2016; Rodrigues et al., 2018). The lack of estrous behavior may be indicative of the absence of a mature preovulatory follicle capable of producing sufficient estradiol to stimulate estrus and ovulation, which would explain why animals that do not present estrus have a lower PRSE.
Recently, a variation on estradiol-based protocols has been reported where DIP is used for only 5 or 6 days. This protocol has been termed J-synch and in beef heifers treated with this protocol there is a higher PRSE (61.9 %) compared to heifers treated with the conventional protocol (51.4 %; Bó et al., 2016). Similar to what was observed in heifers treated with the conventional estradiol-based protocol, heifers treated with J-synch cycling have a higher PRSE than heifers in anestrus (Núñez-Olivera et al., 2022; Zwiefelhofer et al., 2021). Likewise, in lactating cows, the use of J-synch tends to increase PRSE (74.1 %) when compared to the GnRH-based protocol (66.5 %; Macmillan et al., 2020).
Additionally, in this type of protocols, the application of eCG at the time of DIP withdrawal increases PRSE (Núñez-Olivera et al., 2020). The reduction from 8 to 5 days in the use of DIP in estradiol-based protocols avoids the presence of persistent follicles since the release of progesterone by DIP is sufficient to exert negative feedback for GnRH, which favors follicular turnover (Day, 2015).
GnRH-based protocols
GnRH-based treatments consist of the administration of this hormone on day zero to induce ovulation of the dominant follicle and thereby promote the initiation of a new wave of follicular growth. Subsequently, on day seven, PGF2α is applied to induce regression of the CL and 48 to 72 hours later a second dose of GnRH is applied (Figure 2D) to induce ovulation and TFAI is performed (Martínez et al., 1999; Martínez et al., 2000). The effectiveness of this treatment depends largely on whether the animals ovulate after the first application of GnRH. If animals ovulate, a CL will form, so by applying PGF2α on day 7, serum progesterone concentrations will be reduced, animals will enter a follicular phase and there will be a pre-ovulatory follicle that will ovulate with the second application of GnRH (Bó et al., 2016; Ginther, 2016). Because the percentage of ovulation after the first application of GnRH is highly variable (Bó et al., 2016), this protocol has been supplemented with the use of a DIP along with the first application of GnRH, which must be removed at the time of PGF2α application (Figure 2E). Thus, regardless of whether animals ovulate after the first application of GnRH, when the DIP is removed, all will be in proestrus. Additionally, this protocol has been shortened, using DIP for only 5 days and applying two injections of PGF2α, one at the time of DIP withdrawal and the second 6 to 8 hours later (Figure 2F).
PRSE in heifers treated with GnRH-based protocols
In heifers treated with GnRH-based protocols using DIP for 5 days, most PRSE data (91%) were between 51-70 %. In contrast, when DIP was used for 7 days, most PRSE data were between 41-60 % (Figure 4A). These data suggest that PRSE may be increased if DIP is used for 5 days compared to 7 days.

Figure 4 Pregnancy rate to synchronized estrus (PRSE) in female cattle treated with GnRH-based protocols. A) PRSE data distribution in heifers in which a progesterone-releasing intravaginal device (DIP) was used for 5 or 7 days after the first GnRH application. B) PRSE data distribution in lactating cows without the use of a DIP or with its use for 5 and 7 days after the first GnRH application. C) PRSE data distribution at different times between PGF2-α application and AI in lactating cows treated with GnRH-based protocols and the use of a DIP for 7 days. D) PRSE data distribution at different times between PGF2-α application and AI in lactating cows treated with GnRH-based protocols without use of a DIP. Data collected from: Abel et al., 2017; Martínez et al., 2000; Bishop et al., 2017; Bridges et al., 2014; Macmillan et al., 2020; Burns et al., 2008; Busch et al., 2007; Busch et al., 2008; Cruppe et al., 2014; Dahlen et al., 2010; Echternkamp & Thallman, 2011; Esterman et al., 2016; Geary et al., 2001; Giles et al., 2013; Hall et al., 2017; Helguera et al., 2018; Hill et al., 2014; Hill et al., 2016a; Hill et al., 2016b; Kasimanickam et al., 2006; Kasimanickam et al., 2010; Kasimanickam et al., 2014; Kasimanickam et al., 2009; Kasimanickam et al., 2012; Knickmeyer et al., 2019; Lamb et al., 2001; Lamb et al., 2006; Larson et al., 2006; Marquezini et al., 2011; Marquezini et al., 2013a; Marquezini et al., 2013b; Martínez et al., 2002; Mercadante et al., 2015; Mialot et al., 2003; Nash et al., 2012; Oosthuizen et al., 2018a; Oosthuizen et al., 2018b; Oosthuizen et al., 2018c; RosalesTorres et al., 2017; Small et al., 2009; Stevenson et al., 2003; Thomas et al., 2014; White et al., 2016; Whittier et al., 2010; Whittier et al., 2013; Williams & Stanko, 2020; Wilson et al., 2010; Rodriguez et al., 2023; Bonacker et al., 2020; Rojas-Canadas et al., 2023
When DIP is used for 5 days in GnRH-based protocols, 2 injections of PGF2α are recommended at the time of DIP withdrawal because ovulating animals, with the first application of GnRH, have a young CL that are under the influence of trophic stimuli that promote their development (Niswender et al., 2000). According to the information consulted, PRSE is reduced in heifers treated with protocols based on GnRH+DIP for 5 days, if a single injection of PGF2α is used compared to the use of two injections of this hormone (Kasimanickam et al., 2012; Kasimanickam et al., 2014; White et al., 2016; Helguera et al., 2018). For this reason, it is most advisable to give two injections of PGF2α when deciding to use DIP for 5 days.
Estrous presentation prior to FTAI has shown contrasting results on PRSE in heifers treated with GnRH-based protocols. Heifers synchronized with the GnRH protocol and DIP for 7 days that present estrus have similar PRSE to heifers that did not present estrus (Martínez et al., 2002; Knickmeyer et al., 2019). On the other hand, Oosthuizen et al. (2018a) and Speckhart et al. (2022), using the same protocol, report that PRSE is higher in heifers that present estrus prior to insemination than those that do not. Thus, it appears that PRSE in heifers treated with GnRH-based protocols may be partially dependent on estrus presentation. However, inseminating only heifers in estrus, in addition to presenting a management challenge to detect estrus, may reduce the calving rate at the end of mating, so it is recommended to inseminate all animals regardless of whether they are in estrus or not.
Regarding pubertal status at the start of treatment, it appears that PRSE does not differ between pubertal and peri-pubertal animals subjected to GnRH-based protocols with use of DIP for 7 or 5 days (Busch et al., 2007; Helguera et al., 2018; Knickmeyer et al., 2019). The use of GnRH and DIP in these protocols, besides synchronizing estrus and ovulation, can induce ovarian activity in peri-pubertal heifers with good body development Colazo & Mapletoft, 2014; Baruselli et al., 2018), explaining why, there are no differences in PRSE between pubertal and peri-pubertal heifers.
The time between DIP withdrawal and AI has shown contradictory effects on PRSE. Helguera et al., (2018) report no difference in PRSE between heifers treated with GnRH and DIP for 5 days and inseminated at 66 hours after DIP withdrawal with heifers inseminated at 72 hours. In contrast, in heifers treated with the same protocol, PRSE is higher when insemination was performed at 54 hours after DIP withdrawal than when insemination was performed at 72 hours (Kasimanickam et al., 2012). Although the differences in these results may be due to many factors, it is important to note that the longer the time between DIP withdrawal and AI, the risk that the dominant follicle will ovulate, compromising oocyte viability and fertility.
PRSE in lactating cows treated with GnRH-based protocols
The distribution of PRSE data in lactating cows treated with GnRH-based protocols without the use of DIP or with its use for 5 or 7 days is shown in Figure 4B. The range of PRSE in lactating cows treated with GnRH-based protocols without the use of DIP was from 31 % (Stevenson et al., 2003) to 64 % (Small et al., 2009). While in females subjected to GnRH-based protocols and DIP for 5 days, the range was 33% (Williams & Stanko, 2020) to 69 % (Kasimanickam et al., 2009) and animals where the GnRH-based protocol was used, but with DIP for 7 days, the range was 38% (Rosales-Torres et al., 2017) to 71 % (Lamb et al., 2001).
PRSE is higher in animals in which DIP was used for 7 days after the first application of GnRH compared to those without (Lamb et al., 2001 and Larson et al., 2006). Regarding differences between using DIP for 7 or 5 days, the PRSE data collected suggest no difference (Figure 4B), however, it has been reported that PRSE is higher when using DIP for 5 days compared to 7 days (Whittier et al., 2013). Based on these data, the use of DIP for either 5 or 7 days is recommended in cows treated with GnRH-based protocols to ensure that animals that do not ovulate with the first application of GnRH enter the follicular phase at the time of DIP withdrawal (Bó et al., 2016; Ginther, 2016). This increases the number of animals responding to treatment and with it the PRSE.
As mentioned above, the time between DIP removal and AI can vary between 48 and 72 hours. From the PRSE data we collected from lactating cows treated with GnRH+DIPbased protocols for 5 days, in the majority (85 %) FTAI was performed within 72 hours of DIP withdrawal, the lowest PRSE was 33 % (Williams & Stanko, 2020) and the highest was 69 % (Kasimanickam et al., 2009). In lactating cows treated with the GnRH-based protocol with and without the use of DIP for 7 days, the time between PGF2-α application and AI as well as the reported PRSE results were highly variable (Figure 4C and 4D). In lactating cows in which DIP was used for 7 days, the highest PRSE (71 %) was reported when FTAI was performed at 48 (Lamb et al., 2001) and 72 hours (Nash et al., 2012), while the lowest (38 %) was when AI was performed at 72 hours (Rosales-Torres et al., 2017). Busch et al. (2008) , show that PRSE is higher when AI is done at 66 h than when it is done at 54 h after DIP withdrawal. However, due to the variation in PRSE shown in Figure 4C the effect of time between DIP withdrawal and AI in 7-day GnRH and DIP-based protocols may not be significant. Finally, in lactating cows treated with GnRH-based protocols without the use of DIP, PRSE ranged from 31% when FTAI was performed at 48 hours after PGF2α application (Stevenson et al., 2003) to 64 % reported when FTAI was performed at 64 hours after PGF2α application (Small et al., 2009).
Pre-AI estrus presentation, ovarian status at baseline, eCG use, and temporary weaning can affect PRSE in lactating cows treated with GnRH-based protocols. Several reports show the effect of presenting estrus prior to AI on PRSE (Nash et al., 2012; Thomas et al., 2014; Hill et al., 2016a; Hill et al., 2016b; Abel et al., 2017) and conclude that females presenting estrus have higher PRSE than those not presenting estrus. In works that used the protocol based on GnRH+DIP for 7 days, it is reported that the highest PRSE was 48 % (Abel et al., 2017) and the lowest was 42 % (Nash et al., 2012; Hill et al., 2016a) in cows that do not present estrus prior to AI. In animals that present estrus, the highest PRSE was 71 % (Nash et al., 2012) and the lowest 64 % (Ferreira et al., 2018). As in estradiol-based protocols, in those based on GnRH, the presentation of estrus can improve PRSE, since in animals with estrous behavior they are more likely to have mature preovulatory follicle capable of inducing their own ovulation via estradiol. It is recommended, as in heifers, for management reasons and to increase the calving rate at mating end, that FTAI be performed in all animals.
Regarding the effect of ovarian status on PRSE, in females cycling at the beginning of treatment with GnRH+DIP for 7 days, the range of PRSE reported is from 49 % (Marquezini et al., 2013a) to 69 % (Bridges et al., 2014), while, in anestrus animals the range is from 47 % (Nash et al., 2012) to 63 % (Busch et al., 2008). For their part, Giles et al. (2013), using the protocol based on GnRH and DIP for 5 days report that PRSE is 50 % in cycling cows and 52 % in anestrus cows. As mentioned, in the case of heifers treated with GnRH-based protocols, these can induce cyclicity in anestrus animals (Colazo & Mapletoft, 2014; Baruselli et al., 2018), explaining why there is no difference in PRSE between cycling and anestrus cows.
Even though the presence of the calf reduces GnRH secretion (Martínez et al., 2000), few studies include temporary weaning as a factor to improve PRSE in the GnRH-based protocols reviewed in this paper. Marquezini et al. (2013a and 2013b) report, in animals treated with GnRH+DIP-based protocols for 7 days, that PRSE is similar when temporarily weaned for 72 hours than when not temporarily weaned. In contrast, Geary et al. (2001) report that temporary weaning for 48 hours tends (P=0.09) to increase PRSE in cows treated with GnRH-based protocols with or without DIP. Although the response to GnRHbased synchronization protocols may be compromised by the presence of the calf and its negative effects on GnRH secretion, as a practical matter and it is based on this evidence, the suggestion is not to temporarily wean.
The use of eCG at the end of GnRH-based protocols is not as common as in estradiolbased protocols. Some reports show, in lactating cows treated with GnRH-based protocols with and without use of DIP, that PRSE is higher in animals where eCG (400 IU) is used at the time of PGF2α application than in cows where eCG is not used (Small et al., 2009; Randi et al., 2021). In contrast, in animals treated with GnRH+DIP-based protocols for 7 days plus temporary weaning, PRSE is not different in animals treated or not with 400 IU eCG at the time of DIP withdrawal (Marquezini et al., 2013b). These results suggest that further studies should be conducted to validate whether the use of eCG can improve PRSE in lactating cows treated with GnRH-based protocols.
Progesterone-based protocols
Progesterone-based protocols consist of the use of DIP or progesterone analogs such as MGA for prolonged periods to synchronize estrus in females, followed by PGF2α and GnRH or GnRH, PGF2α and GnRH. The DIP is inserted at day 1 and removed 7, 9 or 14 days later. The purpose of this is that the animals go into estrus and ovulate synchronously. Subsequently, when the animals are in the luteal phase, PGF2α is applied to induce the regression of the CL so that the animals enter proestrus homogeneously (Figure 2G). The most commonly used time, between DIP withdrawal and PGF2α treatment is 16 days, although this hormone can be applied at 11 days (Eborn & Grieger, 2013). After PGF2α application, FTAI can be performed at 66 or 72 hours. A variation to this protocol involves the application of GnRH 7 days prior to PGF2α application (Figure 2H). It is important to note that GnRH can be applied at 2, 4, 9 and 12 days after DIP withdrawal (Mallory et al., 2011; Eborn & Grieger, 2013).
PRSE in heifers treated with progesterone-based protocols
In heifers treated with the P-PGF2α-GnRH protocol the lowest reported PRSE was 31 % (Thomas et al., 2017) and the highest was 63 % (Mallory et al., 2011). For heifers treated with the variation of this protocol (P-GnRH-PGF2α-GnRH), the range of PRSE was 44 (Mallory et al., 2011) to 62 % (Busch et al., 2007). From the PRSE data collected from the selected articles 45% report a PRSE between 61 and 70 % in heifers treated with the PGnRH-PGF2α-GnRH protocol, while only 25 % of the data are within this range in heifers treated with the P-PGF2α-GnRH protocol (Figure 5A). This suggests, that the P-GnRHPGF2α-GnRH protocol increases PRSE compared to the P-PGF2α-GnRH protocol. In this regard, Eborn & Grieger (2013), using MGA for 7 days, show that PRSE is higher when GnRH is used after MGA withdrawal (55%) than when it is not used (38 %). Similarly, PRSE is higher in heifers when GnRH is used after DIP withdrawal than in heifers in which it is not used (Kasimanickam et al., 2016). This evidence suggests that use of the PGnRH-PGF2α-GnRH protocol may improve PRSE because a new wave of follicular growth is synchronized with the use of the first GnRH.
Other factors that may modify PRSE in heifers treated with progesterone-based protocols are the type of progestogen, estrous presentation, and pubertal status. In females treated with the P-GnRH-PGF2α-GnRH protocol, no differences in PRSE were observed between the use of progesterone or MGA (Eborn & Grieger, 2013). Whereas, in heifers that present estrus before PRSE and are treated with the P-PGF2α-GnRH protocol, they have a higher PRSE than when they did not present estrus (Thomas et al., 2014; Thomas et al., 2017). Regarding the pubertal status of heifers, no differences in PRSE were observed between peri-pubertal heifers and pubertal heifers subjected to a progesterone-based protocol regardless of whether or not GnRH was used after progesterone withdrawal (Busch et al., 2007; Mallory et al., 2011). The use of progestogens in these protocols may sensitize the hypothalamus of peri-pubertal animals to induce cyclicity (Perry, 2016). This explains why there is no difference in PRSE between pubescent and peri-pubertal animals when treated with these protocols.
PRSE in lactating cows treated with progesterone-based protocols
From the data collected from the selected articles, the lowest PRSE reported in lactating cows treated with the P-GnRH-PGF2α-GnRH protocol was 50 % (Schafer et al., 2007) and the highest was 70 % (Bader et al., 2005). In contrast, cows treated with the P-PGF2αGnRH protocol the lowest reported PRSE was 46 % (Abel et al., 2017) and the highest was 76 % (Abel et al., 2017). According to the PRSE data shown in Figure 5B, the use of the P-GnRH-PGF2α-GnRH protocol in lactating cows appears to increase PRSE compared to cows treated with the P-PGF2α-GnRH protocol. This is because the use of GnRH prior to PGF2α application synchronizes a new wave of follicular growth.
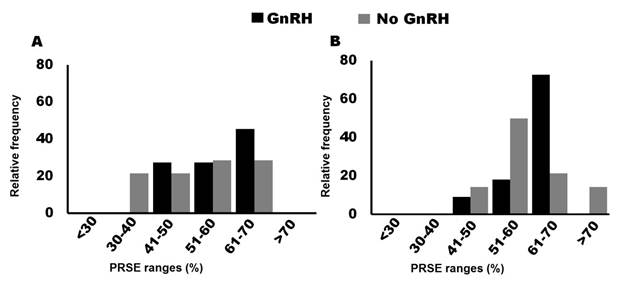
Figure 5 Pregnancy rate to synchronized estrus (PRSE) in female cattle treated with progesteronebased protocols. A) Distribution of PRSE data in heifers in which GnRH was or was not applied after progesterone withdrawal. B) Distribution of PRSE data in lactating cows in which GnRH was or was not applied after progesterone withdrawal. Data compiled from Bader et al., 2005; Busch et al., 2007; Eborn & Grieger, 2013; Schafer et al., 2007; Mallory et al., 2011; Martin et al., 2014; Kasimanickam et al., 2016; Stegner et al., 2004; Thomas et al., 2014; Thomas et al., 2017; Ketchum et al., 2021
In lactating cows submitted to these protocols, PRSE is higher when animals present estrus before FTAI than when they do not present estrus (Thomas et al., 2014). In lactating cows treated with the P-PGF2α-GnRH protocol PRSE was 71 and 76 % when they presented estrus before insemination while when they did not present estrus PRSE was 43 and 53 % (Abel et al., 2017). Regarding postpartum ovarian status, Schafer et al. (2007) using the P-GnRH-PGF2α-GnRH protocol reported that PRSE is similar between cycling cows (59 %) and cows in postpartum anestrus (64 %). Similarly, in cows treated with the same protocol, regardless of whether MGA is used for 7 or 14 days, PRSE is similar between cycling and postpartum anestrus cows (Bader et al., 2005). As in heifers, the presentation of estrus in lactating cows ensures the presence of a mature preovulatory follicle capable of ovulation when GnRH is applied in conjunction with AI. Whereas, progesterone-based protocols as well as GnRH-based protocols can induce ovarian activity in postpartum anestrus cows.
Conclusions
Currently used estrous and ovulation synchronization protocols manipulate follicular development with the use of estrogens, GnRH or progestogens to subsequently induce ovulation of a preovulatory follicle for FTAI. In addition, it must be ensured that the animals enter a follicular phase synchronously using PGF2α and DIPs. Based on the information reviewed in this work, the reported PRSE data are highly variable, however, independent of the protocol type used, it is possible to obtain PRSE of between 40 and 60 %. Depending on the protocol type used and the physiological status of the animal, some factors can be used to improve PRSE. In lactating cows the use of eCG increases PRSE when included in estradiol-based protocols, while in heifers and lactating cows treated with GnRH-based protocols, PRSE can be increased by using DIP for 5 or 7 days. Finally, in females treated with progesterone-based protocols, the use of GnRH prior to PGF2α application improves PRSE.
REFERENCES
Abel, JM, Bishop BE, Thomas JM, Ellersieck MR, Poock SE, Smith MP, Patterson DJ. 2017. Comparing strategies to synchronize estrus before fixed-time artificial insemination in primiparous 2-year-old beef cows. Theriogenology. 87:306-315. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2016.09.010 [ Links ]
Abreu FM, Da Silva MAC, Cruppe LH, Mussard ML, Bridges GA, Harstine BR. 2018. Role of progesterone concentrations during early follicular development in beef cattle: I. Characteristics of LH secretion and oocyte quality. Animal Reproduction Science. 196:59-68. ISSN:0378-4320. https://doi.org/10.1016/j.anireprosci.2018.06.020 [ Links ]
Adams GP, Matteri RL, Ginther OJ. 1992. Effect of progesterone on ovarian follicles, emergence of follicular waves and circulating follicle-stimulating hormone in heifers. Journal of Reproduction and Fertility. 96(2):627-40. ISSN: 2228-5482. https://pubmed.ncbi.nlm.nih.gov/1339842/ [ Links ]
Aerts JMJ, Bols PEJ. 2010. Ovarian follicular dynamics. A review with emphasis of the bovine species. Part II: antral development, exogenous influence and future prospects. Reproduction in Domestic Animals45:180-7. ISSN: 1439-0531. https://doi.org/10.1111/j.1439-0531.2008.01298.x [ Links ]
Alves RLOR, Silva MA, Consentini CEC, E Silva LO, Folchini NP, Oliva AL, Prata AB, Gonçalves JRS, Wiltbank MC, Sartori R. 2021. Hormonal combinations aiming to improve reproductive outcomes of Bos indicus cows submitted to estradiol/progesterone-based timed AI protocols. Theriogenology. 15;169:89-99. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2021.04.007 [ Links ]
Alvarez RH, Pugliesi G, Nogueira-Natal FL, Rocha CC, Ataide-Júnior GA, FerreiraMelo AJ. 2018. Reproductive performance of Bos indicus beef cows treated with different doses of equine chorionic gonadotropin at the end of a progesterone-estrogen based protocol for fixed-time artificial insemination. Theriogenology. 118:150-156. ISSN: 0093691X. https://doi.org/10.1016/j.theriogenology.2018.06.003 [ Links ]
Aragunde Vieytes R, Viñoles Gil C, Gastal GDA, Cavestany D. 2023. Equine chorionic gonadotropin administered on day 5 of a 7-days fixed-time artificial insemination program improves ovulation synchrony and corpus luteum function in anestrous beef cows. Theriogenology. 195:62-68. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2022.10.016 [ Links ]
Bader JF, Kojima FN, Schafer DJ, Stegner JE, Ellersieck MR , Smith MF, Patterson DJ. 2005. A comparison of progestin-based protocols to synchronize ovulation and facilitate fixed-time artificial insemination in postpartum beef cows. Journal of Animal Science. 83:136-143. ISSN: 1525-3163. https://doi.org/10.2527/2005.831136x [ Links ]
Barbosa IP, Cestaro JP, Silva SA, Noleto GS, Gonçalves RL, Silva GM, Paes FH, Gasperin BG, Rovani MT, Pfeifer LFM. 2022. GnRH34: An alternative for increasing pregnancy in timed AI beef cows. Theriogenology. 179:1-6. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2021.11.014 [ Links ]
Baruselli P, Gimenes L, Sales JN, 2007. Fisiologia reprodutiva de fêmeas taurinas e zebuínas. Revista Brasileira de Reprodução Animal. Belo Horizonte. 31(2):205-211. ISSN: 1809-3000. Disponible en https://www.academia.edu/18955421/ [ Links ]
Baruselli PS, Ferreira RM, Sa MF, Bo GA. 2018. Review: Using artificial insemination v. natural service in beef herds. Animal. 12:S45-S52. ISSN: 1751-732X. https://doi.org/10.1017/S175173111800054X [ Links ]
Beg MA, Ginther OJ, 2006. Follicle selection in cattle and horses: role of intrafollicular factors. Reproduction. 132:365-377. ISSN: 1741-7899. https://doi.org/10.1530/rep.1.01233 [ Links ]
Bishop BE , Thomas JM , Abel JMPoock SE , Ellersieck MR , Smith MF . 2017. Split-time artificial insemination in beef cattle: III. Cmparing fixed-time artificial insemination to Splittime artificial insemination with delayed administration of GnRH in postpartum cows. Theriogenology. 99:48-52. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2017.04.046 [ Links ]
Bó GA, Adams GP, Pierson RA, Tribulo HE, Caccia M, Mapletoft RJ. 1994. Follicular wave dynamics after estradiol-17β treatment of heifers with or without a progestogen implant. Theriogenology. 41:1555-1569. ISSN: 0093-691X. https://doi.org/10.1016/0093-691X(94)90821-Y [ Links ]
Bó GA, Baruselli PS, Martinez MF. 2003. Pattern and manipulation of follicular development in Bos indicus cattle. Animal Reproduction Science. 78:307326. ISSN:0378-4320. https://doi.org/10.1016/s0378-4320(03)00097-6 [ Links ]
Bó GA, Baruselli, PS. 2014. Synchronization of ovulation and fixed-time artificial insemination in beef cattle. Animal. 8:144-150. ISSN: 1751-732X. https://doi.org/10.1017/S1751731114000822 [ Links ]
Bó GA, De La Mata JJ, Baruselli PS, Menchaca A. 2016. Alternative programs for synchronizing and resynchronizing ovulation in beef cattle. Theriogenology. 86:388-396. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2016.04.053 [ Links ]
Bonacker RC, Stoecklein KS, Locke JWC, Ketchum JN, Knickmeyer ER, Spinka CM, Poock SE , Thomas JM . 2020. Treatment with prostaglandin F2α and an intravaginal progesterone insert promotes follicular maturity in advance of gonadotropin-releasing hormone among postpartum beef cows. Theriogenology. 157:350-359. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2020.08.018 [ Links ]
Bridges GA, Lake SL, Kruse SG, Bird SL, Funnell BJ, Arias R. 2014. Comparison of three DIP-based fixed-time AI protocols in beef heifers. Journal of Animal Science. 93:3127-313. ISSN: 1525-3163. https://doi.org/10.2527/jas.2013-7404 [ Links ]
Burns MG, Buttrey BS, Dobbins C.A, Martel CA, Olson KC, Lamb GC. 2008. Evaluation of human chorionic gonadotropin as a replacement for gonadotropin-releasing hormone in ovulation-synchronization protocols before fixed timed artificial insemination in beef cattle. Journal of Animal Science. 86:2539-2548. ISSN: 1525-3163. https://doi.org/10.2527/jas.2008-1122 [ Links ]
Busch DC, Schafer DJ , Wilson DJ, Mallory DA, Leitman NR, Haden JK. 2008. Timing of artificial insemination in postpartum beef cows following administration of the CO.synch+cotrolled internal drug-release protocol. Journal of Animal Science. 86:15191525. ISSN: 1525-3163. https://doi.org/10.2527/jas.2008-0925 [ Links ]
Busch DC , Wilson DJ , Schafer DJ , Leitman NR , Haden JK, Ellersieck. 2007. Comparison of progestin-based estrus sinchronization protocols before fixed-time artificial insemination on pregnancy rate in beef heifers. Journal of Animal Science. 85:1933-1939. ISSN: 1525-3163. https://doi.org/10.2527/jas.2006-845 [ Links ]
Campos JT, Marinho LS, Lunardelli PA, Morotti F, Seneda MM. 2013. Resynchronization of estrous cycle with eCG and temporary calf removal in lactating Bos indicus cows. Theriogenology. 80:619-23. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2013.05.029 [ Links ]
Cedeño AV, Cuervo R, Tríbulo A, Tríbulo R, Andrada S, Mapletoft R, Menchaca A, Bó GA. 2021. Effect of expression of estrus and treatment with GnRH on pregnancies per AI in beef cattle synchronized with an estradiol/progesterone-based protocol. Theriogenology. 161:294-300. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2020.12.014 [ Links ]
Colazo MG, Mapletoft RJ. 2014. A review of current timed-AI (TAI) programs for beef and dairy cattle. Canadian Veterinary Journal. 55:772-780. ISSN: 85286. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4095965/ [ Links ]
Colazo MG, Kastelic JP, Mapletoft RJ. 2003. Effects of estradiol Cypionate (ECP) on ovarian follicular dynamics, synchrony of ovulation, and fertility in DIP-based, fixed-time AI programs in beef heifers. Theriogenology. 60:855-865. ISSN: 0093-691X. https://doi.org/10.1016/s0093-691x(03)00091-8 [ Links ]
Colazo MG, Whittaker P, Macmillan K, Bignell D, Boender G, De Carvalho-Guimaraes R. 2018. Evaluation of a modified GnRH-based timed-AI protocol associated with estrus detection in beef heifers inseminated with sex-selected or conventional semen. Theriogenology. 118:90-95. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2018.05.037 [ Links ]
Cooke RF, Peres RFG, Cipriano RS, Guarnieri-Filho TA, Marques RS, Rodrigues MC. 2016. Impacts of meloxicam prior to temporary calf weaning on physiological and reproductive responses of Bos indicus beef cows. Journal of Animal Science. 94:406-411. ISSN: 1525-3163. https://doi.org/10.2527/jas2015-9222 [ Links ]
Crepaldi GA, Sales JNS, Girotto RW, Carvalho JGS, Baruselli PS. 2019. Effect of induction of ovulation with estradiol benzoate at P4 device removal on ovulation rate and fertility in Bos indicus cows submitted to a TAI protocol. Animal Reproduction Science. 209:106141. ISSN: 1873-2232. https://doi.org/10.1016/j.anireprosci.2019.106141 [ Links ]
Crowe MA. 2008. Resumption of ovarian cyclicity in post-partum beef and dairy cows. Reproduction in Domestic Animals Suppl. 5:20-8. ISSN: 0936-6768. https://doi.org/10.1017/S1751731114000251 [ Links ]
Cruppe LH, Day ML, Abreu FM, Kruse S, Lake SL, Biehl MV. 2014. The requirement of GnRH at the beginning of the five-day Co-Synch+controlled internal drug release protocol in beef heifers. Journal of Animal Science. 92:4198-4203. ISSN: 1525-3163. https://doi.org/10.2527/jas.2014-7772 [ Links ]
Dahlen CR, Bird SL, Martel CA, Olson KC, Stevenson JS, Lamb GC. 2010. Administration of human chorionic gonadotropin 7 days after fixed-time artificial insemination of suckled beef cows. Journal of Animal Science. 88:2337-2345. ISSN: 1525-3163. https://doi.org/10.2527/jas.2009-2596 [ Links ]
Day ML. 2015. State of the art of GnRH - based timed AI in beef cattle. Animal Reproduction. 12:473-478. ISSN 0378-4320. https://www.animal-reproduction.org/journal/animreprod/article/5b5a6032f7783717068b4611 [ Links ]
De Graaff W, Grimard B. 2018. Progesterone-releasing devices for cattle estrus induction and synchronization: Device optimization to anticipate shorter treatment durations and new device developments. Theriogenology. 112:34-43. ISSN: 0093691X. https://doi.org/10.1016/j.theriogenology.2017.09.025 [ Links ]
Delgado PAM, Cuéllar NR, Sánchez CMG, Rojas ECC. 2011. Dinámica folicular en la vida reproductiva de la hembra bovina. Veterinária e Zootecnia. 5:88-99. ISSN: 21783764. http://vetzootec.ucaldas.edu.co/downloads/v5n2a08.pdf [ Links ]
Diniz JHW, Peres RFG, Teixeira ACB, Riveros JAN, Noronha IM, Martins CFG, Oliveira CS, Pohler KG, Pugliesi G, Oliveira LZ. 2021. Administration of PGF2α at the moment of timed-AI using sex-sorted or conventional semen in suckled nelore cows with different intensity of estrus behavior. Theriogenology. 15;174:169-175. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2021.08.023 [ Links ]
Driancourt MA. 2001. Regulation of ovarian follicular dynamics in farm animals. Implications for manipulation of reproduction. Theriogenology. 55:1211-1239. ISSN: 0093-691X. https://doi.org/10.1016/s0093-691x(01)00479-4 [ Links ]
Eborn DR, Grieger, DM. 2013. Timed insemination of beef heifers using the 7-11 Synch protocol. Journal of Animal Science. 91:666-672. ISSN: 1525-3163. https://doi.org/10.2527/jas.2011-4951 [ Links ]
Echternkamp SE, Thallman RM. 2011. Factors affecting pregnancy rate to estrus synchronization and fixed-time artificial insemination in beef Cattle. Journal of Animal Science. 89:3060-3068. ISSN: 1525-3163. https://doi.org/10.2527/jas.2010-3549 [ Links ]
Esterman RD, Alava EN, Austin BR, Hersom MJ. 2016. Select Synch and Co-Synch protocols using a DIP yield similar pregnancy rate after a fixed-time insemination in suckled Bos indicus x Bos taurus cows. Theriogenology. 85:870-876. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2015.10.035 [ Links ]
Ferreira RM, Conti TL, Goncalves RL, Souto LA, Sales JNS, Sa MF. 2018. Synchronization treatments previous to natural breeding anticipate and improve the pregnancy rate of postpartum primiparous beef cows. Theriogenology. 114:206-211. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2017.11.022 [ Links ]
Figueiredo RA, Barros CM, Pinheiro OL, Soler JMP. 1997. Ovarian follicular dynamics in nelore breed (Bos indicus) cattle. Theriogenology. 47:1489-1505. ISSN: 0093-691X. https://doi.org/10.1016/S0093-691X(97)00156-8 [ Links ]
Forde N, Beltman ME, Lonergan P, Diskin M, Roche JF, Crowe MA. 2011. Oestrous cycles in Bos taurus cattle. Animal Reproduction Science. 124:163-169. ISSN:0378-4320. https://doi.org/10.1016/j.anireprosci.2010.08.025 [ Links ]
Fortune JE, Sirois J, Turzillo AM, Lavoir M. 1991. Follicle selection in domestic ruminants. Journal Reproduction and Fertility Supplement. 43:187-98. ISSN:04493087. https://www.biosciproceedings.org/bp/0002/pdf/bp0002rdr15.pdf [ Links ]
Fortune JE, Rivera GM, Evans ACO, Turzillo AM. 2001. Differentiation of dominant versus subordinate follicles in cattle. Biology of Reproduction. 65:648-654. ISSN: 00063363. https://doi.org/10.1095/biolreprod65.3.648 [ Links ]
Garverick HA, Smith MF . 1993. Female reproductive physiology and endocrinology of cattle. Veterinary Clinics of North America: Food Animal Practice. 9(2):223-247. ISSN: 07490720. https://doi.org/10.1016/s0749-0720(15)30643-5 [ Links ]
Geary TW, Whittier JC, Hallford DM, MacNeil MD. 2001. Calf removal improves conception rates to the Ovsynch and Co-Synch protocols. Journal of Animal Science. 79:1-4. ISSN: 1525-3163. https://doi.org/10.2527/2001.7911 [ Links ]
Giles RL, Ahola JK, Whittier JC, French JT, Repenning PE, Kruse SG. 2013. Administration of a GnRH analog on day 9 of a 14-day controlled internal drug release insert with timed artificial insemination in lactating beef cows. Journal of Animal Science. 91:1866-1873. ISSN: 1525-3163. https://doi.org/10.2527/jas.2012-5497 [ Links ]
Ginther OJ, Kastelic JP, Knopf L. 1989. Intraovarian relationships among dominant and subordinate follicles and the corpus luteum in heifers. Theriogenology. 32:787-795. ISSN: 0093-691X. https://doi.org/10.1016/0093-691x(89)90467-6 [ Links ]
Ginther OJ. 2016. The theory of follicle selection in cattle. Domestic Animal Endocrinology. 57: 85-99. ISSN: 18790054. https://doi.org/10.1016/j.domaniend.2016.06.002 [ Links ]
Gutiérrez GC. 2018. Simposio Vaca-Cría. Manejos Ganaderos en el sistema Vaca Cría En: 54 Reunión Nacional de Investigación Pecuaria. Año 4. Vol. 1 Núm.1. Nayarit México. [ Links ]
Hall JB, Kasimanickam RK, Glaze Jr JB, Roberts-lew MC. 2017. Impact of delayed insemination on pregnancy rates to gender selected semen in a fixed-timeAI system. Theriogenology. 102:154-161ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2017.07.014 [ Links ]
Helguera IL, Whittaker P , Behrouzi A, Mapletoft RJ, Colazo MG . 2018. Effect of initial GnRH and time of insemination on reproductive performance in cyclic and acyclic beef heifers subjected to a 5-d Co-synch plus progesterone protocol. Theriogenology. 106:39-45. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2017.10.001 [ Links ]
Hill SL, Grieger DM, Olson KC, Jaeger JR, Dahlen CR, Bridges GA. 2016a. Using estrus detection patches to optimally time insemination improved pregnancy risk in suckled beef cows enrolled in a fixed-time artificial insemination program. Journal of Animal Science. 94(9):3703-3710. ISSN: 1525-3163. https://doi.org/10.2527/jas.2016-0469 [ Links ]
Hill SL, Grieger DM, Olson KC, Jaeger JR, Dahlen CR, Crosswhite MR. 2016b. Gonadotropin-releasing hormone increased pregnancy risk in suckled beef cows not detected in estrus and subjected to a Split-time artificial insemination program. Journal of Animal Science. 94:3722-3728. ISSN: 1525-3163. https://doi.org/10.2527/jas.20160582 [ Links ]
Hill SL, Perry GA, Mercadante VRG, Lamb GC, Jaeger JR, Olson KC., 2014. Altered progesterone concentrations by hormonal manipulations before a fixed-time artificial insemination CO-Synch+DIP program in suckled beef cows. Theriogenology. 82:104-113. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2014.03.008 [ Links ]
Kasimanickam R, Asay M, Firth P, Whittier WD, Hall JB. 2012. Artificial insemination at 56 h after intravaginal progesterone device removal improved AI pregnancy rate in beef heifers synchronized with five-day CO-Synch+controlled internal drug release (DIP) protocol. Theriogenology. 77:1624-1631. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2011.12.007 [ Links ]
Kasimanickam R, Collins JC, Wuenschell J, Currin JC, Hall JB, Whittier DW. 2006. Effect of timing of prostaglandin administration, controlled internal drug release removal and gonadotropin releasing hormone administration on pregnancy rate in fixed-time AI protocols in crossbred Angus cows. Theriogenology. 66:166-172. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2005.10.019 [ Links ]
Kasimanickam R, Day ML, Rudolph JS, Hall JB, Whittier WD. 2009. Two doses of prostaglandin improve pregnancy rates to timed-AI in a 5-day progesterone-based synchronization protocol in beef cows. Theriogenology. 71:762-767. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2008.09.049 [ Links ]
Kasimanickam R, Hall JB, Currin JF, Inman B, Rudolph JS, Whittier WD. 2010. Pregnancy rates in angus cross beef cows bred at observed oestrus with or without second GnRH administration in fixed-timeprogesterone-supplemented ovsynch and cosynch protocols. Reproduction in Domestic Animals. 45:487-492. ISSN: 1439-0531. https://doi.org/10.1111/j.1439-0531.2008.01269.x [ Links ]
Kasimanickam RK, Firth P, Schuenemann GM, Whtlock BK, Gay JM, Moore DA. 2014. Effect of the first GnRH and two doses of PGF2α in a 5-day progesterone-based COSynch protocol on heifer pregnancy. Theriogenology. 81:797-804. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2013.12.023 [ Links ]
Kasimanickam RK, Hall JB, Whittier WD. 2016. Fertility of Angus cross beef heifers after GnRH treatment on day 23 and timing of insemination in 14-day DIP protocol. Journal of Animal Science. 52(1):122-129. ISSN: 1525-3163. https://doi.org/10.1111/rda.12866 [ Links ]
Keneal YBP, Terasawa E. 2012. Rapid direct action of estradiol in GnRH neurons: findings and implications. Front Endocrinol (Lausanne). 3(2):106. https://doi.org/10.3389/fendo.2011.00106 [ Links ]
Ketchum JN , Bonacker RC , Andersen CM, Smith EG, Stoecklein KS , Spinka CM , Thomas JM . 2021. Evaluation of later timepoints for split-time artificial insemination when using sex-sorted semen among beef heifers following the 14-d CIDR®-PG protocol. Animal Reproduction Science. 224:106649. ISSN: 1873-2232J. https://doi.org/10.1016/j.anireprosci.2020.106649 [ Links ]
Knickmeyer ER , Thomas JM , Locke JWC , Bonacker RC , Ellersieck MR , Poock SE . 2019. Evaluation of split-time artificial insemination following administration of a long or short-term progestin-based estrus synchronization protocol in beef heifers. Theriogenology. 133:179-186. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2019.04.011 [ Links ]
Lamb GC, Stevenson JS, Kesler DJ, Garverick HA , Brown DR, Salfen BE. 2001. Inclusion of an intravaginal progesterone Insert plus GnRH and prostaglandin F2α for ovulation control in postpartum suckled beef cows. Journal of Animal Science. 79:22532259. ISSN: 1525-3163. https:/doi.org/10.2527/2001.7992253x [ Links ]
Lamb GC , Larson JE, Geary TW, Stevenson JS , Johnson SK, Day ML. 2006. Synchronization of estrus and artificial insemination in replacement beef heifers using gonadotropin-releasing hormone, prostaglandin F2α and progesterone. Journal of Animal Science. 84:3000-3009. ISSN: 1525-3163. https://doi.org/10.2527/jas.2006-220 [ Links ]
Lamb GC , Mercadante VRG. 2016. Synchronization and Artificial Insemination Strategies in Beef Cattle. Veterinary Clinics of North America: Food Animal Practice. 32:335-347. ISSN: 07490720. https://doi.org/10.1016/j.cvfa.2016.01.006 [ Links ]
Lamb GC , Dahlen CR, Larson JE , Marquezini G, Stevenson JS . 2010. Control of the estrous cycle to improve fertility for fixed-time artificial insemination in beef cattle: a review. Journal of Animal Science. 88:E181-192. https://doi.org/10.2527/jas.2009-2349 [ Links ]
Larson JE , Lamb GC , Stevenson JS , Johnson SK , Day ML, Geary TW . 2006. Synchronization of estrus in suckled beef cows for detected estrus and artificial insemination and timed artificial insemination using gonadotropin-releasing hormone, prostaglandin F2α, and progesterone. Journal of Animal Science. 84:332-342. ISSN: 1525-3163. https://doi.org/10.2527/2006.842332x [ Links ]
Lassala A, Hernández-Cerón J, Pedernera M, González-Padilla E, Gutierrez CG. 2020. Cow-calf management practices in Mexico: Reproduction and breeding. Veterinaria Mexico OA. 7(1). ISSN: 2448-6760. https://doi.org/10.22201/fmvz.24486760e.2020.1.839 [ Links ]
Macmillan K , Gobikrushanth M, Sanz A, Bignell D , Boender G , Macrae L. 2020. Comparison of the effects of two shortened timed-AI protocols on pregnancy per AI in beef cattle. Theriogenology. 15:142:85-91. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2019.09.038 [ Links ]
Malik A, Wahid H, Rosnina Y, Kasim A, Sabri M. 2012. Effects of timed artificial insemination following estrus synchronization in postpartum beef cattle. Open Veterinary Journal. 2(1):1-5. ISSN:2218-6050. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4655776/ [ Links ]
Mallory DA , Nash JM, Ellersieck MR , Smith MF , Patterson DJ. 2011. Comparison of long-term progestine-based protocols to synchronize estrus before fixed-time artificial insemination in beef heifers. Journal of Animal Science. 89:1358-1365. ISSN: 15253163. https://doi.org/10.2527/jas.2010-3694 [ Links ]
Marizancén SMA, Artunduaga PL. 2017. Mejoramiento genético en bovinos a través de la inseminación artificial y la inseminación artificial a tiempo fijo. Revista de Investigación Agraria y Ambiental. 8(2): 247-259. ISSN-e:1989-6352. https://doi.org/10.22490/21456453.2050 [ Links ]
Marquezini GHL , Dahlen CR , Bird SL, Lamb GC . 2011. Administration of human chorionic gonadotropin to suckled beef cows before ovulation synchronization and fixedtime insemination: Replacement of gonadotropin-releasing hormone with human chorionic gonadotropin. Journal of Animal Science. 89:3030-3039. ISSN: 15253163. https://doi.org/10.2527/jas.2010-3455 [ Links ]
Marquezini G HL , Mercadante VRG, Bischoff KM, Black TE, DiLorenzo N, Bird SL . 2013a. Effects of temporary calf removal before fixed-time artificial insemination on pregnancy rates and subsequent calf performance in suckled beef cows. Journal of Animal Science. 91:2414-2425. ISSN: 1525-3163. https://doi.org.10.2527/jas.20125743 [ Links ]
Marquezini G HL , Mercadante VRG , Olson KC, Jaeger JR, Perry GA, Stevenson JS . 2013b. Effects of equine chorionic gonadotropin on follicle development and pregnancy rates in suckled beef cows with or without calf removal. Journal of Animal Science. 91:1216-1224. ISSN: 1525-3163. https://doi.org/10.2527/jas.2012-5382 [ Links ]
Martin NT, Thomas JM , Nash JM , Mallory DA , Ellersieck MR , Poock SE . 2014. Comparison of a 16- versus a 19-day o interval between controlled internal drug release removal and prostaglandin F2α following a 14-day controlled internal drug release treatment and fixed-time artificial insemination in postpartum beef cows. Journal of Animal Science. 92:1759-1767. ISSN: 1525-3163. https://doi.org/10.2527/jas.2013-7045 [ Links ]
Martínez MF, Adams GP, Bergfelt DR, Kastelic JP, Mapletoft RJ . 1999. Effect of LH or GnRH on the dominant follicle of the first follicular wave in beef heifers. Animal Reproduction Science. 57:23-33. ISSN:0378-4320. https://doi.org/10.1016/s03784320(99)00057-3 [ Links ]
Martínez MF , Kastelic JP , Adams GP , Janzen E, McCartney DH, Mapletoft J. 2000. Estrus synchronization and pregnancy rates in beef Cattle given DIP-B, prostaglandin and estradiol, or GnRH. Canadian Veterinary Journal. 41:786-790. ISSN: 0008-5286. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1476379/ [ Links ]
Martínez MF , Kastelic JP , Adams GP , Mapletoft RJ . 2002. The use of a progesterone -releasing device (DIP-B) or melengestrol acetate with GnRH, LH, or estradiol benzoate for fixed-time AI in beef heifers. Journal of Animal Science. 80:1746-1751. ISSN: 1525-3163. https:/doi.org/10.2527/2002.8071746x [ Links ]
Mercadante VRG, Kozicki LE, Ciriaco FM, Henry DD, Dahlen CR , Crosswhite MR. 2015. Effects of administration of protaglandin F2α at initiation of the seven-day COSynch+controlled internal drug release ovulation synchronization protocol for suckled beef cows and replacement beef heifers. Journal of Animal Science. 93:5204-5213. ISSN: 1525-3163. https://doi.org/10.2527/jas.2015-8967 [ Links ]
Mialot JP, Constant F, Dezaux P, Grimard B, Deletang F, Ponter AA. 2003. Estrus synchronization in beef cows: comparison between GnRH+PGF2α+GnRH and PRID+PGF2α+eCG. Theriogenology 60:319-330. ISSN: 0093-691X. https://doi.org/10.1016/S0093-691X(02)01371-7 [ Links ]
Mihm M, Austin EJ, Good TEM, Ireland JLH, Knight PG, Roche JF. 2000. Identification of potential intrafollicular factors involved in selection of dominant follicles in heifers. Biology of Reproduction. 63:811-819. ISSN: 00063363. https://doi.org/10.1016/S0093691X(02)01371-7 [ Links ]
Moore K, Thatcher W. 2006. Major advances associated with reproduction in dairy cattle. Journal of Dairy Science. 89:1254-1266. ISSN: 1525-3198. https://doi.org/10.3168/jds.S0022-0302(06)72194-4 [ Links ]
Murphy BD, Martinuk SD. 1991. Equine chorionic gonadotropin. Endocrine Reviews.12:27-44. ISSN: 1945-7189. https://doi.org/10.1210/edrv-12-1-27 [ Links ]
Nash JM , Mallory DA , Ellersieck MR , Poock SE , Smith MF , Patterson DJ. 2012. Comparison of long-versus short-term DIP-based protocols to synchronize estrus prior to fixed-time AI in postpartum beef cows. Animal Reproduction Science. 132:11-16. ISSN:0378-4320. https://doi.org/10.1016/j.anireprosci.2012.03.013 [ Links ]
Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. 2000. Mechanisms controlling the function and life span of the corpus luteum. Physiological Reviews. 80:1-29. ISSN: 1522-1210. https://doi.org/10.1152/physrev.2000.80.1.1 [ Links ]
Noronha IM, Cooke RF, Martins CFG, Oliveira Filho RV, Pohler KG, Vasconcelos JLM. 2020. Administering an additional prostaglandin F2α injection to Bos indicus beef cows during a treatment regimen for fixed-time artificial insemination. Animal Reproduction Science. 219:106535. ISSN: 1873-2232. https://doi.org/10.1016/j.anireprosci.2020.106535 [ Links ]
Núñez-Olivera R, Cuadro F, Bosolasco D, de Brun V, de la Mata J, Brochado C, Meikle A, Bó GA, Menchaca A. 2020. Effect of equine chorionic gonadotropin (eCG) administration and proestrus length on ovarian response, uterine functionality and pregnancy rate in beef heifers inseminated at a fixed-time. Theriogenology. 15;151:16-27. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2020.03.031 [ Links ]
Núñez-Olivera R, Bó GA, Menchaca A. 2022. Association between length of proestrus, follicular size, estrus behavior, and pregnancy rate in beef heifers subjected to fixed-time artificial insemination. Theriogenology. 15;181:1-7. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2021.12.028 [ Links ]
Oliveira Filho RV, Cooke RF, de Mello GA, Pereira VM, Vasconcelos JLM, Pohler KG. 2020. The effect of clitoral stimulation post artificial insemination on pregnancy rates of multiparous Bos indicus beef cows submitted to estradiol/progesterone-based estrus synchronization protocol. Journal of Animal Science. 7:1-5. ISSN 1525-3163. https://doi.org/10.1093/jas/skaa195 [ Links ]
Oosthuizen N, Canal LB, Fontes PLP, Sanford CD, Dilorenzo N, Dahlen CR . 2018a. Prostaglandin F-2 alpha, 7 d prior to initiation of the 7-d CO-synch plus DIP protocol failed to enhance estrus response and pregnancy rates in beef heifers. Journal of Animal Science. 96:1466-1473. ISSN: 1525-3163. https://doi.org/10.1093/jas/sky058 [ Links ]
Oosthuizen N , Fontes PLP, Sanford CD, Ciriaco FM , Henry DD , Canal LB . 2018b. Estrus synchronization and fixed-time artificial insemination alter calving distribution in Bos indicus influenced beef heifers. Theriogenology. 106:210-213. ISSN: 0093691X. https://doi.org/10.1016/j.theriogenology.2017.10.028 [ Links ]
Oosthuizen N , Lansford AC, Canal LB , Fontes PLP , Sanford CD , Dahlen CR . 2018c. Comparison of two alternate PGF (2 alpha) products in two estrus synchronization protocols in beef heifers. Journal of Animal Science. 96:1388-1395. ISSN: 15253163. https://doi.org/10.1093/jas/sky059 [ Links ]
Perry GA . 2016. Factors affecting puberty in replacement beef heifers. Theriogenology. 86:373-378. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2016.04.051 [ Links ]
Pessoa GA, Martini AP, Carloto GW, Rodrigues MC, Claro-Júnior I, Baruselli PS. 2016. Different doses of equine chorionic gonadotropin on ovarian follicular growth and pregnancy rate of suckled Bos Taurus beef cows subjected to timed artificial insemination protocol. Theriogenology. 15:792-799. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2015.09.057 [ Links ]
Pfeifer LF, Castro NA, Melo VT, Neves PM, Cestaro JP, Schneider A. 2015. Timed artificial insemination in blocks: A new alternative to improve fertility in lactating beef cows. Animal Reproduction Science. 163:89-96. ISSN:0378-4320. https://doi.org/10.1016/j.anireprosci.2015.10.002 [ Links ]
Pfeifer LF, Leonardi CE, Castro NA, Viana JH, Siqueira LG, Castilho EM. 2014.The use of PGF2α as ovulatory stimulus for timed artificial insemination in cattle. Theriogenology. 15:81:689-695. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2013.11.016 [ Links ]
Pfeifer LFM, Gasperin BG, Cestaro JP, Schneider A. 2022. Postponing TAI in beef cows with small preovulatory follicles. Animal Reproduction Science. 242:107006. ISSN: 1873-2232. https://doi.org/10.1016/j.anireprosci.2022.107006 [ Links ]
Pinto HF, Missio D, Dos Santos Brum D, Carloto GW, Martini AP, Pessoa GA, Neto NA, Claro I Jr , Sá Filho MF, Leivas FG. Decreasing the dose of equine chorionic gonadotropin does not affect ovarian or pregnancy responses of purebred taurine and crossbred beef heifers. Animal Reproduction Science. 218:106474. ISSN: 1873-2232. https://doi.org/10.1016/j.anireprosci.2020.106474 [ Links ]
Randi F, Kelly AK, Parr MH, Diskin MG, Lively F, Lonergan P, Kenny DA.2021. Effect of ovulation synchronization program and season on pregnancy to timed artificial insemination in suckled beef cows. Theriogenology. 15;172:223-229. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2021.06.021 [ Links ]
Rathbone MJ, Kinder JE, Fike K, Kojima F, Clopton D, Ogle CR. 2001. Recent advances in bovine reproductive endocrinology and physiology and their impact on drug delivery system design for the control of the estrous cycle in cattle. Advanced. Drug Delivery Reviews. 50:277-320. ISSN: 1872-8294. https://doi.org/10.1016/s0169409x(01)00156-9 [ Links ]
Reineri PS, Bernhard SDR, Principi SA, Gerlero GD, Aller JF. 2023. Effects of two protocols of ovulation synchronization on corpus luteum size and blood flow, progesterone concentration, and pregnancy rate in beef heifers. Animal Reproduction Science. 251:107223. ISSN: 1873-2232. https://doi.org/10.1016/j.anireprosci.2023.107223 [ Links ]
Rodgers RJ, Rodgers HFI. 2010. Morphological classification of bovine ovarian follicles. Reproduction Review. 139:309-318. ISSN: 1741-7899. https://doi.org/10.1530/REP-09-0177 [ Links ]
Rodrigues AD, Cooke RF, Cipriano RS, Silva LGT, Cerri RLA, Cruppe LH. 2018. Impacts of estrus expression and intensity during a timed-AI protocol on variables associated with fertility and pregnancy success in Bos indicus-influenced beef cows. Journal of Animal Science. 96(1):236-249. ISSN: 1525-3163. https://doi.org/10.1093/jas/skx043 [ Links ]
Rodriguez AM, Maresca S, López-Valiente S, Bilbao MG, Moran KD, Bartolome JA, Pratt SL, Long NM. 2023. Comparison of the 7-day CO-Synch and 8-day estradiol-based protocols for estrus synchronization and timed artificial insemination in suckled Bos taurus cows. Theriogenology. 1;200:70-76. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2023.02.003 [ Links ]
Rojas-Canadas E, Battista SE, Kieffer JD, Wellert SR, Mussard ML, Garcia-Guerra A. 2023. GnRH dose at initiation of a 5-day CO-Synch + P4 for fixed time artificial insemination in suckled beef cows. Animal Reproduction Science. 250:107210. ISSN: 1873-2232J. https://doi.org/10.1016/j.anireprosci.2023.107210 [ Links ]
Rosales-Torres AM, Guzmán A. 2008. Apoptosis in follicular atresia and luteal regression. Review. Técnica Pecuaria en México. 46:159-182. ISSN: 0040-1889. https://www.redalyc.org/pdf/613/61346205.pdf [ Links ]
Rosales-Torres AM, Guzmán A, Gutiérrez AC. 2012. Follicular development in domestic ruminants. Tropical and Subtropical Agroecosystems. 1:147-160. https://www.revista.ccba.uady.mx/ojs/index.php/TSA/article/view/1299/709 [ Links ]
Rosales-Torres AM, Guzmán-Sánchez A. 2011. Role of Vascular Endothelial Growth Factor and its receptors during the ovarian cycle. Review. Revista Mexicana de Ciencias Pecuarias. 3(1):89-111. ISSN: 2448-6698. https://www.redalyc.org/pdf/2656/265622705008.pdf [ Links ]
Rosales-Torres AM, López-Cedillo Z.B, Hernández-Coronado CG. RoseteFernández JV, Mendoza GD, Guzmán A. 2017. Short-term dietary concéntrate supplementation during estrus synchronization treatment in beef cows increased IGF-I serum concentration but did not affect the reproductive respons. Tropical Animal Health and Production. 49: 221-226. ISSN: 15737438. https://doi.org/10.1007/s11250016-1166-7 [ Links ]
Ross PJ, Aller JF, Callejas SS, Butler H, Alberio RH. 2004. Estradiol benzoate given 0 or 24 h after the end of a progestagen treatment in postpartum suckled beef cows. Theriogenology . 62:265-273. ISSN: 0093-691X. https:doi.org/10.1016/j.theriogenology.2003.10.013 [ Links ]
Sa Filho MF, Crespilho AM, Santos JE, Perry GA , Baruselli PS. 2010. Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows. Animal Reproduction Science. 120(1-4):23-30. ISSN:03784320. https://doi.org/10.1016/j.anireprosci.2010.03.007 [ Links ]
Sales JNS, Carvalho JBP, Crepaldi GA, Cipriano RS, Jacomini JO, Maio JRG. 2012. Effects of two estradiol esters (benzoate and cypionate) on the induction of synchronized ovulations in Bos indicus cows submitted to a timed artificial insemination protocol. Theriogenology . 78:510-516. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2012.02.031 [ Links ]
Santos MH, Ferraz Junior MVC, Polizel DM, Barroso JPR, Miszura AA, Martins AS, and Bertoloni AV. 2018. Decreasing from 9 to 7 days the permanence of progesterone inserts makes possible their use up to 5 folds in suckled Nellore cows. Theriogenology . 111:56-61. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2018.01.017 [ Links ]
Sartori R, Barros CM. 2011. Reproductive cycles in Bos indicus cattle. Animal Reproduction Science. 124 :244-250. ISSN:0378-4320. https://doi.org/10.1016/j.anireprosci.2011.02.006 [ Links ]
Sartori R, Haughian JM, Shaver RD, Rosa GJM, Wiltbank MC. 2004. Comparison of Ovarian Function and Circulating Steroids in Estrous Cycles of Holstein Heifers and Lactating. Journal of Dairy Science. 87:905-920. ISSN: 1525-3198. https://doi.org/10.3168/jds.S0022-0302(04)73235-X [ Links ]
Scarpa JO, O’neil MM, Cardoso RC, Stanko RL, Williams GL. 2019. Ovarian follicular and luteal characteristics in Bos indicus-influenced beef cows using prostaglandin F2α with or without GnRH at the onset of the 5-day CO-Synch + controlled internal drug release (DIP) protocol. Animal Reproduction Science. 204:1-9. ISSN:0378-4320. https://doi.org/10.1016/j.anireprosci.2019.02.013 [ Links ]
Schafer DJ , Bader JF , Meyer JP, Haden JK , Ellersieck MR , Lucy MC. 2007. Comparison of preogestin-based protocols to synchronize estrus and ovulation before fixed-time artificial insemination in postpartum beef cows. Journal of Animal Science. 85:1940-1945. ISSN: 1525-3163. https://doi.org/10.2527/jas.2006-836 [ Links ]
Shirasuna K, Nitta A, Sineenard J, Shimizu T, Bollwein H, Miyamoto A. 2012. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow. Domestic Animal Endocrinology. 43(2):198-211. ISSN: 18790054. https://doi.org/10.1016/j.domaniend.2012.03.007 [ Links ]
Silva MAV, Santos CS, Franca IG, Pereira HG, Sa Filho MF , Freitas BG. 2018a. Hormonal strategy to reduce suckled beef cow handling for timed artificial insemination with sex-sorted semen. Theriogenology . 114:159-164. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2018.03.020 [ Links ]
Silva EP, Wiltbank MC, Machado AB, Gambin LS, Dias MM, Chaiben MFC. 2018b. Optimizing timed AI protocols for Angus beef heifers: Comparison of induction of synchronized ovulation with estradiol cypionate or GnRH. Theriogenology . 121:7-12. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2018.07.019 [ Links ]
Simões LMS, Orlandi RE, Massoneto JPM, Scandiuzzi LA Jr , Freitas BG , Bastos MR, Souza JC, Sales JNS. 2018. Exposure to progesterone previous to the protocol of ovulation synchronization increases the follicular diameter and the fertility of suckled Bos indicus cows. Theriogenology . 116:28-33. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2018.04.031 [ Links ]
Sirard MA. 2016. Somatic environment and germinal differentiation in antral follicle: The effect of FSH withdrawal and basal LH on oocyte competence acquisition in cattle. Theriogenology . 86(1):54-61. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2016.04.018 [ Links ]
Sirois J, Fortune JE. 1988. Ovarian Follicular Dynamics during the Estrous Cycle in Heifers Monitored by Real-Time UItrasonography. Biology of Reproduction. 39:308317. ISSN:00063363. https:/doi.org/10.1095/biolreprod39.2.308 [ Links ]
Small JA, Colazo MG , Kastelic JP , Mapletoft RJ . 2009. Effect of progesterone presynchronization and eCG on pregnancy rates to GnRH-based, timed-AI in beef cattle. Theriogenology . 71:698-706. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2008.09.045 [ Links ]
Speckhart SL, Oliveira Filho RV, Franco GA, Vasconcelos JLM, Schrick FN, Edwards JL, Pohler KG. 2022. Short Communication: Influence of estrus activity and reproductive tract size and position scores on fertility in Bos indicus and Bos taurus suckled beef cows. Journal of Animal Science. 100(6):skac141. ISSN 1525-3163. https://doi.org/10.1093/jas/skac141 [ Links ]
Stegner JE , Bader JF , Kojima, FN, Ellersieck MR , Smith MF , Petterson DJ. 2004. Fixed-time artificial insemination of postpartum beef cows at 72 or 80 h after treatment with the MGA® Select protocol. Theriogenology . 61:1299-1305. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2003.07.023 [ Links ]
Stevenson JS , Lamb GC , Jhonson MA, Medina-Britos MA, Grieger DM, Harmoney, KR. 2003. Supplemental norgestomet, progesterone, or melengestrol acetate increases pregnancy rates in suckled beef cows after timed inseminations. Journal of Animal Science. 81:571-586. ISSN: 1525-3163. https://doi.org/10.2527/2003.813571x [ Links ]
Thomas JM, Locke JWC , Bishop BE , Abel JM , Ellersieck MR , Yelich JV. 2017. Evaluation of the 14- DIP-PG and 9-d DIP-PG protocols for synchronization of estrus in íuBos indicus-influenced and Bos taurus beef heifers. Theriogenology . 92:190-196. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2017.01.020 [ Links ]
Thomas JM , Poock SE , Ellersieck MR , Smith MF , Patterson DJ. 2014. Delayed insemination for non-estrous heifers and cows when using conventional semen in timed artificial insemination. Journal of Animal Science. 92:4189-4197. ISSN: 1525-3163. https://doi.org/10.2527/jas.2014-7827 [ Links ]
Uslenghi G, Chaves SG. Cabodevila J, Callejas S. 2014. Effect of estradiol cypionate and amount of progesterone in the intravaginal device on synchronization of estrus, ovulation and on pregnancy rate in beef cows treated with FTAI based protocols. Animal Reproduction Science. 145:1-7. ISSN:0378-4320. https://doi.org/10.1016/j.anireprosci.2013.12.009 [ Links ]
Uslenghi G, Vater A, Rodríguez-Aguilar S, Cabodevila J , Callejas S. 2016. Effect of estradiol cypionate and GnRH treatment on plasma estradiol-17β concentrations, synchronization of ovulation and on pregnancy rates in suckled beef cows treated with FTAI-based protocols. Reproduction in Domestic Animals. 51(5):693-699. ISSN: 14390531. https://doi.org/10.1111/rda.12732 [ Links ]
Webb R, Garnsworthy PC, Gong JG, Armstrong DG. 2004. Control of follicular growth: Local interactions and nutritional influences1,2. Journal of Animal Science. 82:E63-E74. ISSN: 1525-3163. https:doi.org/10.2527/2004.8213_supplE63x [ Links ]
Wenzinger B, Bleul U, 2012. Effect of a prostaglandin F2α analogue on the cyclic corpus luteum during its refractory period in cows. BMC Veterinary Research. 14:8:220. ISSN: 1746-6148. https://doi.org/10.1186/1746-6148-8-220 [ Links ]
White SS, Kasimanickam RK, Kasimanickam VR. 2016. Fertility after two doses of PGF2α concurrently or at 6-hour interval on the day of DIP removal in 5-day CO-Synch progesterone-based synchronization protocols in beef heifers. Theriogenology . 86:785790. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2016.02.032 [ Links ]
Whittier WD, Currin JF, Schramm H, Holland S. 2013. Fertility in Angus cross beef cows following 5-day CO-Synch+DIP or 7-day CO-Synch estrus synchronization and timed artificial insemination. Theriogenology . 80:963-969. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2013.07.019 [ Links ]
Whittier WD, Kasimanickam RK, Currin JF, Schramm HH, Vlcek M., 2010. Effect of timing of second prostaglandin F2α administration in a 5-day, progesterone-based Co- Synch protocol on AI pregnancy rates in beef cows. Theriogenology . 74:1002-1009. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2010.04.029 [ Links ]
Williams GL, Stanko RL. 2020. Pregnancy rates to fixed-time AI in Bos indicusinfluenced beef cows using PGF2α with (Bee Synch I) or without (Bee Synch II) GnRH at the onset of the 5-day Co-Synch+DIP protocol. Theriogenology . 142:229-235. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2019.09.047 [ Links ]
Wilson DJ , Mallory, DA, Busch DC , Leitman NR , Haden JK , Schafer DJ . 2010. Comparison of short-term progestin-based protocols to synchronize estrus and ovulation in postpartum beef cows. Journal of Animal Science. 88:2045-2054. ISSN: 15253163. https://doi.org/10.2527/jas.2009-2627 [ Links ]
Zwiefelhofer EM, Macmillan K , Gobikrushanth M , Adams GP , Yang SX, Anzar M, Asai-Coakwell M, Colazo MG . 2021. Comparison of two intravaginal progesteronereleasing devices in shortened-timed artificial insemination protocols in beef cattle. Theriogenology . 1;168:75-82. ISSN: 0093-691X. https://doi.org/10.1016/j.theriogenology.2021.03.023 [ Links ]
Received: February 07, 2022; Accepted: June 08, 2023











 text in
text in 



