Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Abanico veterinario
On-line version ISSN 2448-6132Print version ISSN 2007-428X
Abanico vet vol.9 Tepic 2019 Epub July 30, 2021
https://doi.org/10.21929/abavet2019.927
Original articles
Evaluation of the modified Giemsa staining technique in the acrosomal evaluation of mammalian sperm
1Departamento de Producción Agrícola y Animal. Universidad Autónoma Metropolitana Unidad Xochimilco, México, CDMX. proyo_manuel@hotmail.com, jaggnutricion@yahoo.com.mx acordova@correo.xoc.uam.mx mvz.osvaldo.ld@gmail.com
2Facultad de Agronomía. Universidad Autónoma de Sinaloa, México. juan_eulogio_guerra_liera@hotmail.com
3Facultad de Veterinaria. Benemérita Universidad Autónoma de Puebla, México. rubenhuertac@live.com.mx
4Departamento Reproducción Animal Instituto Nacional de Investigación Tecnología Agraria y Alimentaria, Madrid, España. raulss@inia.es
It is important to design efficient, practical and easy to perform techniques to assess the acrosomal integrity of the sperm. The objective was to modify the Giemsa staining technique for acrosomal NAR assessment in mammalian sperm. 140 lamellae of spermatozoa from 4 different mammals (bovine, ovine, porcine and human) were evaluated, divided into two groups (70 lamellae per group). The first group was evaluated acrosomal NAR with the classic technique of Giemsa staining and the second, with the modified Giemsa technique. The means of acrosomal NAR and the times of each of the techniques were compared. There was no difference between the means of acrosomal NAR in both techniques (p<0.05); however, while the conventional technique takes 115 minutes, the time of realization of the modified technique was 35 minutes, reducing the time 80 minutes, with better image clarity. In conclusion, the modified staining technique decreases the NAR titration time and shows a better sharpness of the image.
Keywords: mammal sperm; Giemsa; acrosomal integrity
Es importante diseñar técnicas eficientes, prácticas y fáciles de realizar para valorar la integridad acrosomal de los espermatozoides. El objetivo fue evaluar la técnica modificada de tinción Giemsa para valoración del borde apical normal (NAR) en espermatozoides de mamíferos. Se evaluaron 140 laminillas de espermatozoides de 4 diferentes mamíferos (bovinos, ovinos, porcinos y humanos), se dividieron en dos grupos (70 laminillas por grupo). El primer grupo, se evaluó NAR con la técnica clásica de tinción Giemsa y el segundo, con la técnica modificada de Giemsa. Se compararon los porcentajes de acrosomas NAR y los tiempos de cada una de las técnicas. No hubo diferencia entre las medias de acrosomas NAR en ambas técnicas (p<0.05); sin embargo, mientras la técnica convencional tarda 115 min, el tiempo de realización de la técnica modificada fue de 35 minutos, reduciendo el tiempo 80 minutos, con una mejor claridad de la imagen. En conclusión, la técnica de tinción modificada disminuye el tiempo de valoración de NAR y muestra una mejor nitidez de la imagen.
Palabras clave: espermatozoides de mamíferos; Giemsa; integridad acrosomal
INTRODUCTION
Semen freezing can cause damage to the sperm structure, or changes in the distribution of enzymes in the membranes; decreasing its fertilizing capacity (Hernández et al., 2017). In order to know this, various tests have been created in the laboratory that are based exclusively on the evaluation of the cellular structure, allowing to adequately assess a seminal sample and thus be able to predict male fertility (Díaz et al., 2009; Puente et al., 2016). The ideal tests are those that simply and efficiently allow diagnostic tests to evaluate the fertilizing capacity of an ejaculate (Osorio et al., 2007).
The acrosome is a structure located in the apical part of the sperm head. It plays a fundamental role in fertilization, since the damage in it generates the release of enzymes from its interior, losing the ability to fertilize the sperm (Osorio et al., 2007; Atuesta et al., 2012; Ugarelli et al., 2017); It is therefore important to assess the acrosomal integrity of sperm. The acrosome is a secretory vesicle of the sperm that contains different enzymes, especially acrosin, responsible for the digestion of cumulus oophorus and the pellucida zone.
The normal morphology of the acrosome has a normal apical ridge (NAR). The determination of NAR is one of the most important sperm parameters, due to its role in the acrosomal reaction for fertilization of the oocyte. In it, there is a relationship between the NAR and the fertilization rate, so it is convenient to perform specific tests for the assessment of NAR, as a form of fertility prediction (Bonet et al., 2006; Nieto, 2010; Puente et al., 2016).
There are several techniques that allow NAR titration, among which fluorescent methods stand out; where various high-cost and complicated substances are combined with specialized equipment, making these techniques uncommon to perform (Montesinos et al., 2014; Restrepo et al., 2016). Because of this, other tests commonly used in laboratories have been designed as routine practice and are more accessible, where only a phase contrast microscope is required, for example: Eosin-Nigrosine, Eosin-Fast Green, Triple Stain, Spermac Stain and Giemsa Stain; being the latter the most widely used staining technique (Bonet et al., 2006; Bernardi et al., 2011; Restrepo et al., 2013). Due to the above, it is important to design efficient, practical and easy to perform techniques to assess NAR of sperm.
The objective of the present work was to evaluate the modified technique of Giemsa staining, for the assessment of NAR in mammalian sperm.
MATERIAL AND METHODS
Location. This work was developed in the Laboratory of Clinical Analysis and Veterinary Histopathology Laboratory of the Autonomous Metropolitan University Unit Xochimilco, during the months of August to September 2018.
Population and sample. NAR of 140 sperm lamellae from four different species of mammals was evaluated: bovines (20), sheep (40), pigs (50) and human beings (30). In cattle and sheep, the samples were obtained from thawed semen, for pig samples it was semen diluted in commercial MRA® diluent, it was used two days after its extraction; and for human semen, these were fresh semen.
Staining methodologies. The lamellae were into two groups divided, with 70 lamellae each. The first group was with the classic Giemsa staining methodology stained, to assess NAR, proposed by Whatson y Martín (1972).
The second group was stained with the modified Giemsa staining technique; where the procedure was as follows: once the sperm smear dried, the fixation process was carried out by the addition of 96° ethyl alcohol, which was added with a tilt in the lamella of 35°, at a slow stream and it continued for five seconds (figure 1). It was allowed to dry on a plate at 36 °C, until the alcohol dehydrated completely. Subsequently, the staining of Giemsa (Sigma-Aldrich), previously prepared with 0.6 g of Giemsa, in 20 ml of distilled water was added and kept for 25 minutes. Then, it was with distilled water washed, with a slow jet and continued with a tilt of the lamella of 35°, ensuring that the jet did not touch the portion where the sperm smear was located (figure 1), allowing it to dry on a platen for two minutes at 36 °C. The NAR evaluation was performed under an optical microscope on the 1000X objective with immersion oil. The criterion of acrosome evaluation was to classify as NAR, those sperm in which the cap was gently attached to the nucleus and had an apical edge that formed a soft crescent.
Evaluation and statistical analysis. For both groups, 200 sperm were counted per lamella, and the percentage of NAR acrosomes was obtained, observing if there were differences in the sharpness (better image quality in the acrosomal membrane and apical edge of the acrosome) of acrosomal structures and time of performing the technique in both groups. A descriptive analysis of each of the species under study was performed; in addition to a t-student test for the contrast of means (p <0.05). The statistical package SPSS version 20.0 (IBM, 2011) was used.
RESULTS
Figure 1 shows the smear fixation, and Figure 2 shows the photographs of NAR acrosomes with sperm from bovines, pigs, humans and sheep, observed with a 1000X optical microscope. In the images, it is observed that in the modified Giemsa staining technique, the image is clearer, with acrosomal membranes and better stained acrosomal apical edges; also the head, body and sperm tail are observed with a better definition.
Table 1 NAR acrosome means with traditional and modified Giemsa staining of bovine, porcine, human and sheep sperm.
| Treatments | N | Mean | Standard Deviation | Mean of standard error | |
| Bovine | Original | 10 | 83.200 | 7.4803 | 2.3655 |
| Modified | 10 | 84.600 | 7.4416 | 2.3532 | |
| Porcine | Original | 25 | 89.320 | 2.6255 | .5251 |
| Modified | 25 | 90.800 | 2.2361 | .4472 | |
| Human | Original | 15 | 90.333 | 3.2440 | .8376 |
| Modified | 15 | 90.133 | 3.4614 | .8937 | |
| Sheep | Original | 20 | 94.550 | 2.5021 | .5595 |
| Modified | 20 | 94.850 | 2.4554 | .5490 |
No difference was found in the percentages of the means (p˃0.05).
There was no difference between the NAR acrosome means in both staining techniques; however, while the conventional technique takes 115 minutes, the time of realization of the modified technique was 35 minutes; reducing the time 80 minutes, with a better clarity of the image.
DISCUSSION
As for NAR acrosomes, there was no difference in the percentage of the means between both groups, which means that the modified Giemsa staining technique can be used, obtaining good evaluation of NAR acrosomes. On the other hand, it can also be observed that the modified Giemsa staining technique reduces the staining time of NAR acrosomes by 80 minutes, since it is not placed for 90 minutes in Giemsa staining and 15 minutes in 5% formaldehyde, as in original technique. It can be said that the modified Giemsa staining technique is efficient, fast and simple to perform; complying with the characteristics indicated by Osorio et al. (2007) of laboratory techniques for semen evaluation.
The modification of the Giemsa staining technique is not by the freezing diluents affected, which can be observed in Figure 2, in 1-B and 3-B; in which semen of bovine and defrosted sheep was used, obtaining better sharpness of the acrosome image with the modified Giemsa staining technique; as indicated by Muiño et al. (2005). This author mentions that the majority of stains used for optical microscopy are not suitable for semen evaluation since they require the use of fixatives, such as formaldehyde or glutaraldehyde, which usually interfere with the diluents used for freezing, making analysis difficult.
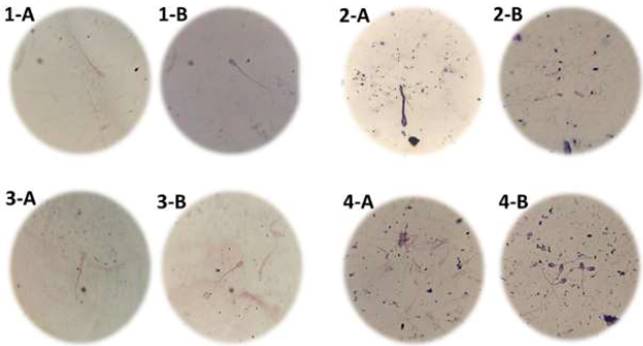
1-A bovine sperm stained with classical Giemsa staining technique and 1-B bovine sperm stained with modified Giemsa staining. 2-A human sperm stained with classical Giemsa staining technique and 2-B human sperm stained with modified Giemsa staining. 3-A sperm of sheep stained with classical Giemsa staining technique and 3-B sperm of sheep stained with modified Giemsa staining. 4-A porcine sperm stained with classical Giemsa staining technique and 4-B porcine sperm stained with modified Giemsa staining.
Figure 2 Photograph of NAR with sperm from bovine, pigs, humans and sheep, observed with a 1000X optical microscope.
In Figure 2, in 1-B, 2-B, 3-B and 4-B can be seen that when using the modified Giemsa staining technique, there is a better sharpness of the NAR acrosome image, in comparison to sperm stained with the original technique as seen in Figure 2 in 1-A, 2-A, 3-A and 4-A. Therefore, the 96° alcohol used plays an extremely important role in allowing sperm fixation in the smear for staining. This may be due to the use of 96° alcohol as a smear fixer, as mentioned by González et al., (2015) and Gorodner, (2013), which showed that 96° alcohol is a good fixative that preserves without, alter cellular components, but with poor penetration, so it is used to fix extended cytology.
The 96° alcohol is used as a spray for a few seconds and the smear is allowed to dry in the air, allowing fixing the sample obtained and then placing the stain (López y Casasbuenas, 2015); coinciding with Juárez et al., (2008), mention the use of 70° alcohol in conjunction with 96° alcohol and its combination with other reagents (xylol, formaldehyde, etc.) for the evaluation of normal sperm morphology. While Fernández et al. (2001) and Sánchez et al., (2014)used 96 ° alcohol to fix human sperm samples and to observe the degree of nuclear maturity and sperm morphology, obtaining good results.
ACKNOWLEDGMENT
To the Clinical Analysis Laboratory and the Veterinary Histopathology Laboratory of the Autonomous Metropolitan University Unit Xochimilco for the facilities provided for carrying out laboratory work. To CONACyT for the support of the Master of Agricultural Sciences scholarship with registration number 624422, provided to the first author.
REFERENCES
ATUESTA BJE, Grajales LHA, López BM. 2012. Evaluación de la integridad de la membrana acrosomal a la inducción física y química de la reacción acrosómica en espermatozoides de conejos línea Caldes. Revista Spei Domus. 8 (16): 16-28. ISSN 1794-7928. https://revistas.ucc.edu.co/index.php/sp/article/view/843 [ Links ]
BERNARDI SF, Allende R, Mazzeo MJ, Marini PR. 2011. Evaluación de los cambios ocasionados en espermatozoides bovinos por variaciones en el manejo de las dosis durante su manipulación en inseminación artificial. Investigación Veterinaria. 13: 25-38. ISSN 1668-3498. http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1668-34982011000200003&lng=es&nrm=iso [ Links ]
BONET S, Martínez E, Rodríguez EJ, Barrera X. 2006. Manual de Técnicas de Reproducción Asistida en Porcino. Universidad de Girona. Red Temática Nacional de Reproducción Porcina. Pp. 306. ISBN: 978-84-8458-241-084-8458-241-8. [ Links ]
DÍAZ FO, Mesa H, Valencia MJG, Gómez LGH, Uribe FJ. 2009. Evaluación de la integridad acrosomal y la funcionalidad bioquímica de la membrana espermática en cerdos productores con gotas citoplasmáticas persistentes. Revista Científica FCV-LUZ. 19 (5): 500-505. ISSN 0798-2259. http://www.redalyc.org/articulo.oa?id=95911615010 [ Links ]
FERNÁNDEZ VR, Hortas NML, Castilla JA, López TCM, Valladares MJC, Alaminos MM, Ruiz MA, Castejón CFJ, Sánchez LT. 2001. Alteraciones de la madurez nuclear en espermatozoides de varones con antecedentes de criptorquidia. Cirugía Pediatrica. 14: 95-97. ISSN: 0214-1221. https://www.secipe.org/coldata/upload/revista/2001;14.95-97.pdf [ Links ]
GONZÁLEZ VA, Mac DM, Grosman G. 2015. Normas para el procedimiento de registro, rotulación, traslado y recepción de biopsias y citología al servicio de anatomía patología del Hospital Provincia Neuquen. Metodología en elaboración de Guías de Prácticas Clínicas. Comité de Docencia e Investigación. Pp. 1-17. http://www.saludneuquen.gob.ar/wp-content/uploads/2016/10/35-NORMAS-PROCEDIMIENTO-REGISTRO-HPN-2015.pdf [ Links ]
GORODNER OZ. 2013. Histología: métodos e instrumentos de estudio de la histología parte 1: Técnicas histológica. Universidad Nacional del Noreste. Facultad de Medicina. 2013: 1-13. https://med.unne.edu.ar/sitio/multimedia/imagenes/ckfinder/files/files/histologia_med_cat2/GUIA%201%20%202013.pdf [ Links ]
IBM Corp. 2011. IBM SPSS Statistics para Windows, Versión 20.0. Armonk, Nueva York: IBM Corp [ Links ]
HERNÁNDEZ CL, Quintero MA, Camargo RO, Rojas LM. 2017. Evaluación de la integridad funcional y estructura de espermatozoides caprinos criopreservados mediante diluyentes comerciales. Revista Científica, FCV-LUZ. 27 (1): 35-43. ISSN 0798-2259. http://www.redalyc.org/articulo.oa?id=95950495006 [ Links ]
JUÁREZ DPF, De-Dios VMD, Sagrera RJD, Gutiérrez HPR. 2008. Reversión de vasectomía con criopreservación sistemática de espermatozoides testiculares. Revista Internacional de Andrología. 7: 215-221. ISSN: 1698-031X. http://dx.doi.org/10.1016/S1698-031X(09)73389-8 [ Links ]
LÓPEZ CP, Casasbuenas AJ. 2015. La biopsia y la citología, pilares del diagnóstico médico (II parte). Revista Médica Sanitas. 18 (2): 82-89. ISSN 0123-4250. http://www.unisanitas.edu.co/Revista/55/LA_BIOPSIA_Y_LA_CITOLOGIA.pdf [ Links ]
MONTESINOS IS, Carvalho JO, Malaquias JV, Arnhold E, Freneau GE, Dode MAN, Fioravanti MCS, Sereno JRB. 2014. Evaluación del semen criopreservado de toros Curraleiro Pé Duro, pertenecientes al banco brasilero de Germoplasma Animal. Animal Genetic Resources. 54: 135-140. 2078-6344. http://dx.doi.org/10.1017/S2078633614000101 [ Links ]
MUIÑO R, Fernández M, Areán H, Viana JL, López M, Fernández A, Peña AI. 2005. Nuevas tecnologías aplicadas al procesado y evaluación del semen bovino en centros de inseminación artificial. ITEA. 3: 175-191. ISSN 1699-6887. https://dialnet.unirioja.es/servlet/articulo?codigo=1271105 [ Links ]
NIETO DKD. 2010. Técnicas de reproducción asistida. Tinciones para la evaluación de la morfología espermática. Informe de pasantía. Universidad Simón Bolívar. https://studylib.es/doc/5936981/t%C3%A9cnicas-de-reproducci%C3%B3n-asistida-tinciones-para-la-evalu... [ Links ]
OSORIO SRE, Giraldo JF, Mesa H, Gómez LG, Henao UFJ. 2007. Evaluación de la integridad acrosómica en el semen de verraco. Vet.Zootec. 1 (1): 41-47. ISSN: 2310-2799. http://vetzootec.ucaldas.edu.co/downloads/v1n1a07.pdf [ Links ]
PUENTE MA, Rodríguez D, Tataglione M. 2016. Viabilidad y estado acrosomal en espermatozoides de conejo. Revista Veterinaria Argentina. 33 (339): 1-7. ISSN: 1852-317X. https://www.veterinariargentina.com/revista/2016/07/viabilidad-y-estado-acrosomal-en-espermatozoides-de-conejo/ [ Links ]
RESTREPO BG, Úsuga SA, Rojano BA. 2013. Técnicas para el análisis de la fertilidad potencial del semen equino. Revista CES Medicina Veterinaria y Zootecnia. 8: 115-127. ISSNe: 1900-9607. http://revistas.ces.edu.co/index.php/mvz/article/view/2838 [ Links ]
RESTREPO BG, Varela GE, Usuga SA. 2016. Evaluación de la calidad espermática epididimal en hipopótamos hippopotamus amphibius (artiodactyla: hippopotamidae) ubicados en el magdalena medio, Colombia. Acta Zoológica Mexicana. 32: 158-167. ISSN 2448-8445. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0065-17372016000200158&lng=es&nrm=iso [ Links ]
SÁNCHEZ SEM, Oláez HJR, Ávila CA, López ML, Sánchez RSH. 2014. Alteraciones en el semen de pacientes con problemas de infertilidad. Archivos de Medicina. 10 (1): 1-17. ISSN-e 1698-9465. https://dialnet.unirioja.es/servlet/articulo?codigo=5052080 [ Links ]
UGARELLI A, Evangelista VS, Santiani A. 2017. Evaluación de la integridad acrosomal en espermatozoides epididimarios de Alpaca mediante Citometría de Flujo. Revista de Investigación Perú. 28: 130-140. ISSN 1609-9117. http://dx.doi.org/10.15381/rivep.v28i1.12947 [ Links ]
WHATSON PF, Matin ICA.1972. A comparison of changes in the acrosome of Deep- frozen ram and bull spermatozoa. Journal of Reproduction and Fertility. 28: 99-101. ISSN 0449-3087. https://doi.org/10.1530/jrf.0.0280099. [ Links ]
Received: April 15, 2019; Accepted: December 09, 2019; Published: December 16, 2019











 text in
text in 




