Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Sanus
On-line version ISSN 2448-6094
Sanus vol.6 Sonora Jan./Dec. 2021 Epub Oct 04, 2021
https://doi.org/10.36789/sanus.vi1.182
Review
Vaccine hesitancy: A review to address the phenomenon in Latin America
1Estudiante de Maestría en Ciencias de Enfermería. Universidad de Guanajuato. Campus Celaya-Salvatierra. Celaya, Guanajuato, México,
2Doctor en Ciencias de Enfermería. Universidad Autónoma de Coahuila. Escuela de Licenciatura en Enfermería Unidad Torreón. Torreón, Coahuila, México.
3Doctora en Ciencias de Enfermería. Universidad de Guanajuato. Departamento de Enfermería Clínica. Celaya, Guanajuato, México.
Introduction:
In recent years there have been movements of people, usually parents, who reject or are undecided about the use of vaccines due to the lack of awareness of its risks and the severity of diseases that can be prevented by vaccination, as well as the dissemination of false news about the safety and efficacy of vaccines in the social networks. Research in countries in Europe and North America have come up with a term to study this phenomenon, this term is Vaccine Hesitancy. However, this research is scarce in Latin America.
Objective:
The objective of this review is to identify potentially valid and reliable instrument that can be adapted and modified both to Spanish and to the Latin America context, related to the indecision to use vaccines within parents.
Methodology:
A search was conducted on three PubMed, EBSCOhost and BVS databases; with the descriptive terms of health sciences: "vaccine hesitancy", "parents", and "vaccination refusal". The results yielded 394 articles of which 19 were chosen for the assessment.
Results:
The main tools identified to measure vaccine hesitancy were the questionnaires named "Parental Attitudes about Childhood Vaccines" and "Vaccine Hesitancy Scale".
Conclusion:
“The Parent Attitudes on Childhood Vaccines” questionnaire is the most widely used and validated tool in different languages to measure vaccine hesitancy.
Key words: Vaccine; Vaccination refusal; Parents; Vaccination (DeCS)
Introducción:
En los últimos años se han desarrollado movimientos de personas, generalmente padres, que rechazan o están indecisas en cuanto al uso de las vacunas por la falta de conciencia de riesgos y la gravedad de las enfermedades prevenibles por vacunación, así como, por la difusión en las redes sociales de noticias falsas sobre la inocuidad y la eficacia de las vacunas. Investigaciones en países de Europa y América del Norte han descrito un término para estudiar este fenómeno el cual es Indecisión a las Vacunas, estas investigaciones son escasas en Latinoamérica.
Objetivo:
identificar instrumentos potencialmente válidos y confiables que puedan ser adaptados y modificados tanto al idioma español como al contexto de América Latina relacionados a la indecisión a las vacunas entre padres.
Metodología:
Se realizó una búsqueda en tres bases de datos PubMed, EBSCOhost y Biblioteca Virtual en Salud; con los términos descriptores de ciencias de la salud: “vaccine hesitancy”, “parents” y “vaccine refutation”.
Resultados:
Los resultados arrojaron 394 artículos de los cuales 19 fueron elegidos para el análisis. Los principales instrumentos identificados para medir la indecisión a las vacunas fueron los cuestionarios “Parental Attitudes About Childhood Vaccines” y “Vaccine Hesitancy Scale”.
Conclusiones:
El cuestionario “Parental Attitudes About Childhood Vaccines” es el instrumento más usado y validado en diferentes idiomas para medir la indecisión a las vacunas.
Palabras clave: Vacunas; Padres; Negativa a la vacunación; Vacunación (DeCS)
Introdução:
Nos últimos anos, houve movimentos de pessoas, geralmente pais, que rejeitam ou estão indecisos quanto ao uso de vacinas devido à falta de consciência dos riscos e à gravidade das doenças evitáveis pelas vacinas, além da disseminação de redes sociais de notícias sobre a segurança e eficácia das vacinas. Pesquisas em países da Europa e América do Norte descreveram um termo para estudar esse fenômeno, que é a vacinação vacinal, essas investigações são escassas na América Latina.
Objetivo:
O objetivo desta revisão é pesquisar na literatura científica estudos onde você estudou a hesitação da vacina entre os pais e identificar ferramentas potencialmente válidas e confiáveis que possam ser adaptadas e modificadas tanto no idioma espanhol quanto no contexto da América Latina.
Metodologia:
Uma pesquisa foi realizada em três bancos de dados PubMed, EBSCO Host e BVS; com os termos descritivos das ciências da saúde: "vaccine hesitancy", "parents" e "vaccination refusal".
Resultados:
Os resultados renderam 394 artigos, dos quais 19 foram escolhidos para a análise. A principal ferramenta identificada para medir a hesitação vacinal foram os questionários " Parental Attitudes About Childhood Vaccines” e “Vaccine Hesitancy Scale".
Conclusões:
O questionário “The Parent Attitudes on Childhood Vaccines” é a ferramenta mais amplamente utilizada e validada em diferentes idiomas para medir a hesitação da vacina.
Palavras-chave: Vacina; Recusa de vacinação; Pais; Vacinação (DeCS)
Introduction
In the last decades, vaccination reflects limited progress worldwide. In the report of the World Health Organization (WHO) global coverage remains at approximately 86% and it was estimated that more than 19 million infants were not vaccinated against tetanus, diphtheria, and pertussis (1). According to the 2019 Immunization in the Americas report (2), in the region, there are outbreaks of measles and diphtheria due to low coverage of vaccines against measles, rubella, and mumps (SRP) 82% and diphtheria, tetanus and pertussis (DPT) 88%; other vaccines also show low coverage such as hepatitis B in newborns, 80%, rotavirus 79% and oral polio vaccine 87%. In addition, communication with society has also become complicated by the lack of awareness of the risks and seriousness of Vaccine Preventable Diseases (VPD) as well as by the dissemination of false news on social networks about the safety and efficacy of vaccines (3-9).
Due to these events there is a risk of increased outbreaks of Vaccine Preventable Diseases (VPD); therefore, health personnel, especially nurses, being responsible for the implementation of vaccination programs in several countries, should design studies to generate knowledge and inform that vaccines are like any drug tested before marketing and use in the population, that vaccination is susceptible to cause minimal side effects or Vaccine or Immunization Attributable Events (VIAE); however, the individual and collective benefit far outweighs the alleged risks (9).
According to some studies (3-9), parents' refusal to have their children vaccinated is centered on values, lack of information, deep distrust of health institutions, parents' lack of knowledge about vaccines and their benefits, which makes it difficult to make decisions about vaccinating children and, consequently, it is believed that the authorities make vaccination mandatory due to private interests.
McDonald and his Strategic Advisory Group of Experts (SAGE) (10) have conducted and reviewed research studies to name and give meaning to a construct under which this situation can be studied, thus, the most associated words in English language is "Vaccine Hesitancy" (VH). According to the English-Spanish dictionary (11) "Vaccine" is translated as "vacuna" and "hesitancy" as "vacilación o indecisión", together the Spanish translation can be taken as "Indecisión a las Vacunas (IV)". The definition by the SAGE Working Group on VH is "delay in accepting or refusing vaccination despite the availability of vaccination services" (10).
Countries in Europe, Asia, and North America have begun to conduct research to address this situation, with positive results in identifying this group of people as they make up a much larger group than those who totally reject vaccines (10); they found that they are more susceptible to behavioral changes because they tend to seek information from those responsible for the vaccination of their children. However, in Latin America these studies are scarce but necessary, as parents with Vaccine Hesitancy (VH) are a group of interest because they represent an opportunity to work on how to detect and address doubts and insecurities to help devise effective interventions to increase vaccination coverage.
Therefore, the objective of this review was to search the scientific literature for studies that have addressed the Vaccine Hesitancy (VH) phenomenon, and to identify valid and reliable tools for recognizing and measuring VH that can be adapted to the Spanish language and the Latin American context.
Methodology
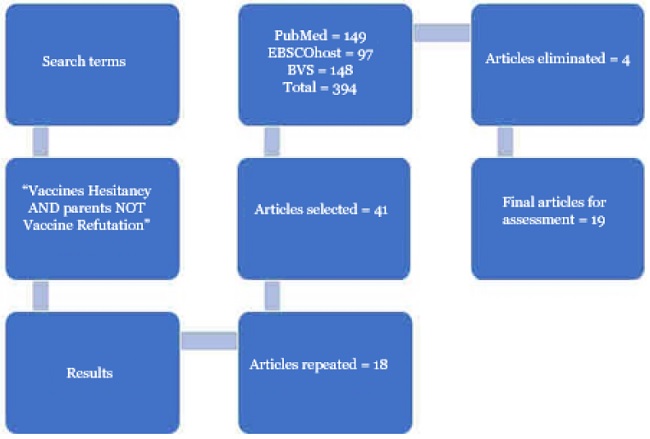
A systematic review was carried out starting with the question that guided the review, where the problem is Vaccine Hesitancy (VH), the intervention is the measurement of the phenomenon with valid and reliable instruments, and a comparison was made between them according to their reliability and results when translated into another language and measured in the population. The question was set forth as follows: Is there scientific evidence of valid and reliable tools to measure vaccine hesitancy that can be adapted to the Spanish language? The search was conducted to identify studies that investigated VH in parents considering the terms "vaccine hesitancy" and "parents" with the Boolean operator "AND". The results included numerous studies analyzing vaccine refusal, so "vaccine refutation" was added to the search with the Boolean operator "NOT". The search was limited to full-text articles published in the last five years in PubMed, Virtual Health Library (BVS), and EBSCO Host databases with the search protocol: "vaccine hesitancy AND parents NOT vaccine refutation" (Table 1).
Table 1 Database Search Protocol
Database |
Search Protocol |
PubMed |
((("vaccines"[MeSH Terms] OR "vaccines"[All Fields] OR "vaccine"[All Fields]) AND hesitancy[All Fields]) AND ("parents"[MeSH Terms] OR "parents"[All Fields])) NOT ("vaccination refusal"[MeSH Terms] OR ("vaccination"[All Fields] AND "refusal"[All Fields]) OR "vaccination refusal"[All Fields]) AND ("2014/10/11"[PDat] : "2019/10/09"[PDat]) |
EBSCOhost |
Vaccine hesitancy AND parents NOT vaccination refusal. Limiters - Date of publication: 20141001-20191031 Search Modes - Boolean/Phrase |
BVS |
(tw:(vaccine hesitancy)) AND (tw:(parents)) AND NOT (tw:(vaccination refusal)) |
Source: Our development
The selection of articles consisted of one of the variables in the studies being Vaccine Hesitancy (VH) measured in parents, following the review of the methodology of the articles; it was found that some made the measurement of the variable through a self-report item, which asked parents if they felt undecided about vaccines. This gave a new guideline to eliminate those studies in which VH was measured through parental self-report (limitations of these studies considered that there was a risk of social desirability bias and parents may not have answered honestly) and those that measured it through a developed instrument were selected. It was also reviewed the variables that can be measured and that are relevant to VH, whether they are sociodemographic variables or other study variables. The articles were analyzed and the necessary information was concentrated in a data matrix, where, in addition to the identification data of the article and authors, the methods of the study, the definition of Vaccine Hesitancy (VH), dimensions, identification of the measurement instrument, results, limitations, and conclusion were identified.
Results
The results of the search in the three databases yielded a total of 394 articles, 41 were selected and 18 were eliminated due to duplication, leaving 23 articles, all of which were published in English. Four articles were eliminated because the variable VH was measured by a self-report item. The total number of articles for analysis was n=19 (Figure 1).
Of the total number of articles selected for assessment, 100% were published in English, 9 studies were conducted in the United States, 2 in Canada, 3 in Europe, 3 in Asia, 1 in Latin America, and 1 was in several regions of different countries. Nine of the studies focused on developing a questionnaire or modifying an existing one to measure VH in parents, and 10 were descriptive, correlational, or clinical trials with intervention with or without randomization of the sample, having this construct among their study variables (Table 2).
Table 2 Articles assessed
Title |
Authors |
Objective |
Measurement instrument |
Percentage identified with levels of VHa % |
Evidence level |
Promoting vaccination in maternity wards motivational interview technique reduces hesitancy and enhances intention to vaccinate, results from a multicentre non-controlled pre- and post-intervention RCT-nested study, Quebec, March 2014 to February 2015 (12) |
Arnaud Gagneur, et al. |
To assess vaccination and VHa intention among parents who received an intervention based on individual motivational interview on infant immunization during postpartum stay in a maternity ward between March 2014 and February 2015. |
PACVb |
II-1f |
|
Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument (13) |
Jeroen Luyten, Luk Bruyneel, Albert Jan van Hoek |
Use a modified version of the VHSg to examine Vaccine Hesitancy (VH) among a representative UK sample. |
VHS (modified)) |
4 |
III |
Measuring vaccine hesitancy: The development of a survey tool (14) |
Heidi J. Larson, et al. |
Mapping the determinants of VH and develop tools to measure and address the nature and level of hesitancy. |
VHS version 1.0 VHS Likert Scale VHS open questions. |
NA |
III |
Overview of knowledge, attitudes, beliefs, vaccine hesitancy and vaccine acceptance among mothers of infants in Quebec, Canada (15) |
Dubé È, et al. |
Assess mothers' VH level and knowledge, attitudes and beliefs about vaccination. |
PACV |
28,6/15c |
III |
Development of a Spanish version of the parent attitudes about childhood vaccines survey (16) |
Rachel M. Cunningham, et al. |
Develop a culturally appropriate Spanish version of the PACV, and its accompanying demographic elements. |
PACV in Spanish |
NA |
III |
Reliability and validity of a survey to identify vaccine hesitancy among parents in Changxing county, Zhejiang province (17) |
Yu Hu, Yaping Chen, Hui Liang, Ying Wang |
To assess the validity and reliability of a survey to identify VH among parents. |
Survey of Vaccine Hesitancy |
24,9 |
III |
Physician Communication Training and Parental Vaccine Hesitancy: A Randomized Trial (19) |
Nora B. Henrikson, et al. |
To reduce VH in mothers of children seen by trained physicians and increase physician confidence in vaccine communication. |
PACV |
12.6 /9.8h |
I |
Longitudinal Trends in Vaccine Hesitancy in a Cohort of Mothers Surveyed in Washington State, 2013-2015 (20) |
Nora B. Henrikson, Melissa L. Anderson, Douglas J. Opel, John Dunn, Edgar K. Marcuse, David C. Grossman |
To assess the trend in parental VH during the first 2 years of their children's lives in a group of mothers in Washington State. |
PACV |
9.7 d |
II-3 |
Previsit Screening for Parental Vaccine Hesitancy: A Cluster Randomized Trial (21) |
Douglas J. Opel, et al. |
To evaluate the effect of identifying parents with VH, prior to their health surveillance visits on early childhood immunizations. |
PACV |
19.6 d |
II-3 |
Validation study of the Parent Attitudes About Childhood Vaccines (PACV) questionnaire: The Malay version (22) |
Haizlene Abd Halim, Suraya Abdul-Razak, Mazapuspavina Md Yasin, Mohamad Rodi Isa |
To adapt and translate the original PACV questionnaire from English language to Malay language and examine its psychometric properties. |
PACV Malay version |
NA |
III |
Comparative analysis of the Parent Attitudes about Childhood Vaccines (PACV) short scale and the five categories of vaccine acceptance identified by Gust et al (23) |
Omolade Oladejo, Kristen Allen, Avnika Amin, Paula M. Frew, Robert A. Bednarczyk, Saad B. Omer |
Evaluate how the vaccine acceptance categories of Gust et al. correspond to the PACV short scale. |
PACV short scale / HealtStyles survey adopted from the analysis of Gust et al. |
13/15.2c |
III |
Measuring vaccine hesitancy: Field testing the WHO SAGE Working Group on Vaccine Hesitancy survey tool in Guatemala (24)) |
Gretchen J. Domek, et al. |
To provide information on shared understanding of the VHS construct using the tool in diverse global settings. |
VHS |
1,1 |
III |
Vaccine hesitancy among parents in a multi-ethnic country, Malaysia (25) |
Fatin Shaheera Mohd Azizi, Yueting Kew, Foong Ming Moy |
To assess the reliability of the Malay-language PACV questionnaire to determine the prevalence of VH among parents and its association with parental sociodemographic characteristics. |
PACV Malay version |
11,6 |
III |
Implementing vaccine hesitancy screening for targeted education (26) |
John T. Connors, Eric A. Hodges, Jennifer DAuria, Laura Windham |
Determine whether using a screening tool (VHS) in conjunction with discussions of parental concerns affects parental intention to vaccinate. |
VHS |
18.9d |
II-3 |
Parental Vaccine Hesitancy and Declination of Influenza Vaccination Among Hospitalized Children (27) |
Annika M. Hofstetter, et al. |
To determine the proportion of parents of children hospitalized in a pediatric hospital that have VH and to examine the relationship between VH and parental declination of influenza vaccination for their children during hospitalization. |
Modified PACV for influenza vaccine |
24 |
III |
Vaccine hesitancy in the French population in 2016, and its association with vaccine uptake and perceived vaccine risk–benefit balance (28) |
Dominique Rey, et al. |
To estimate the prevalence and sociodemographic relationships of VH in French population subgroups, and to investigate the association of VH with vaccine administration and the perception of the risk-benefit balance for four vaccines. |
3 ítems of VHS |
46/48i |
III |
Investigating Italian parents' vaccine hesitancy: A cross-sectional survey (29) |
Francesco Napolitano, Alessia D'Alessandro, Italo Francesco Angelillo |
To assess the prevalence of VH and identify factors associated with VH among a sample of parents of children aged 2-6 years in Italy. |
PACV Italian version |
32,2 |
III |
Prevalence of Vaccine Hesitancy Among Expectant Mothers in Houston, Texas (30) |
Rachel M. Cunningham, Charles G. Minard, Danielle Guffey, Laurie S. Swaim, Douglas J. Opel, Julie A. Boom |
To assess the prevalence of VH among expectant parents who received obstetric care at clinics in Houston, Texas. |
Modified PACV for expectant parents. |
8 |
III |
a Vaccine Hesitancy (VH).
b Parental Attitudes about Childhood Vaccines (PACV).
c NI intermediate level/NAL high level of VH.
d In studies with pre-test and post-test, the pre-test result was taken.
e Not Applied, N/A
f Levels of Evidence by Type of Design (USPSTF)
g Vaccine Hesitancy Scale (VHS).
h Control Group/Intervention Group
i Parents of children/parents of teenage women
Source: Own development
The definition of Vaccine Hesitancy (VH) is constant in the studies, mostly following the definition of the SAGE Working Group, adding that it is a complex and context-specific phenomenon that changes with time, place, and type of vaccine. The questionnaires or scales used that underwent modification to be used were: Parental Attitudes About Childhood Vaccines (PACV) n=12 (12,15,16,18-22,25,27,29,30), Vaccine Hesitancy Scale (VHS) n= 5 (13,14,24,26,28), Survey of Vaccine Hesitancy n= 1 (17) and a comparative study between PACV and Healt Styles Survey from the analysis of Gust et al. (23), n= 1.
When the reliability of the instruments was checked through Cronbach's Alpha, it was found that the PACV in the original English version consists of fifteen items in three factors: "safety and efficacy", "general attitudes" and "behaviors" with Cronbach's Alpha of 0.74 0.84, and 0.74 respectively, which measure dimensions such as vaccination behaviors, beliefs about the safety and efficacy of vaccines, attitudes about mandatory and exemptions from vaccination, and confidence. The Malaysian version of the PACV had an overall Cronbach's Alpha of 0.77 and the Italian version had one of 0.91 (12,15,18-22,25,27,29,30).
The Parental Attitudes About Childhood Vaccines (PACV) instrument was also translated into Spanish, peer-reviewed, and tested in a Spanish-speaking population in the United States (16). However, the validity and reliability of the instrument was not evaluated, but the fact that it was peer-reviewed may provide some degree of validity. Similarly, the PACV was modified to be applied to parents of adolescents with a factor called "security and doubts" (18). To evaluate the PACV the score used was from 0 to 100 points, interpreted in two ways; the first: 0-29 low level of VH, 30-49 intermediate level of VH and >50 high level of VH, the second way: <50 points without presence of IV and >50 points with HV.
The VHS scale developed by SAGE Working Group on VH is a compendium of different versions which are VHS version 1.0, VHS 5-point Likert scale, VHS open-ended survey (14), although the scales have not been validated, in some studies in which it was used there were favorable results in the identification of parents with VH. Recommendations are made to work on it. The scale assesses dimensions such as complacency, convenience, and confidence (13,14,24,26,28). A modified UK version of the 9-item VHS consists of factors such as "lack of confidence and perceived risk" (13).
The instrument developed in China, Survey of Vaccine Hesitancy, has a total of fifteen items, grouped into three factors: "behaviors" with Cronbach's alpha of 0.71, "safety and efficacy" with Cronbach's alpha of 0.83, and "attitudes" with Cronbach's alpha of 0.72 (17).
In the studies in which the objective was not only to develop a measurement instrument, but to detect parents with VH through the instruments already developed, as well as to apply interventions to solve the problem, good results were obtained by identifying this population, since the results of the studies show that from 1% to 48% of the population could be identified with VH (Table 2).
The sociodemographic variables of interest for the studies were gender, age, education, income, number of children and age of children, employment and marital status. Other study variables were the child's vaccination status and whether they had ever delayed or refused the administration of at least one vaccine, the perception of the Vaccine Preventable Diseases (VPD) and Vaccine or Immunization Attributable Events (VIAE) were used in a correlational and predictive manner, that is, whether the result obtained from the instruments can have an effect on the child's vaccination status and the probability that they delay or refuse at some point the administration of any vaccine (15,17,18,23,25,27-30).
In general, the limitations of the studies were related to the lack of validation of the instrument, the sample size with a low percentage of participation or lack of representative samples of the study region, limiting the generalization of the results, the possible presence of social desirability bias in variables such as the vaccination status of the child and delay or refusal of the application of any vaccine (12,14,16,18-22,24-30).
Discussion
The Parental Attitudes About Childhood Vaccines (PACV) instrument was the most widely used instrument among the studies analyzed and has proven validity and reliability; it has been modified to be used in specific situations such as the type of vaccine, parents in different situations and in different countries of the world. In the specific context of a vaccine, a study was conducted to determine parental hesitancy towards the anti-influenza vaccine during the child's hospitalization (27), with minimal modifications in the PACV only to highlight and make clear the orientation towards this vaccine. The results showed the presence of Vaccine Hesitancy (VH) as 53% of parents declined influenza vaccination during hospitalization, 24% of respondents had a high indecision score >50, and a higher proportion of parents with high scores were found to decline vaccination compared to those with low scores. These results of Vaccine Hesitancy (VH) regarding influenza vaccine are consistent with the definition as it mentions that it can be vaccine-specific.
The PACV was also applied in expectant mothers (30) and 1 in 12 (8%) of pregnant women were found to have VH, and a cohort study (20) found a significant decrease between and proportion of mothers with VH of 9.7% at birth vs. 5.9% at 24 months of age of their child. The results suggest that Vaccine Hesitancy (HV) may decline over time for many parents as maternal experience with vaccination accumulates. In a study using a modified version of the PACV for parents of adolescents (18) it was found that only 39% expressed concern about preventable diseases, 41% expressed concern about serious side effects, and 46% disagreed that adolescents can receive all recommended vaccines at a single visit; only two items of the instrument were associated with low vaccination among adolescents. These results show that levels of VH are present in different time and context as in expectant mothers, parents of children or adolescents and again this is consistent with the stated definition of VH.
The Parental Attitudes About Childhood Vaccines (PACV) instrument translated into different languages and used in different countries, shows results where vaccine hesitancy is present, in Canada the presence of HV was found among mothers as the mean score was 27, with 28.6% with an intermediate score of hesitancy and 15% with a high score of hesitancy (15). A significant linear trend was also found between VH score and intention to vaccinate their child. A study with motivational interviewing-based intervention to reduce VH (12) reported that after the intervention, the population with lower VH increased from 55.9% to 78.8% (41% increase), whereas those with intermediate levels decreased from 44.1% to 21.1%; 15.6% of the population that showed a high level decreased to only 5.2% after the intervention. A similar proportion can be observed in the two studies in which the proportion of parents present high levels of indecision taking into account the pretest measurement of the intervention study. In Italy (29) the mean PACV score in Italian was 45.8 with 34.7% parents with high levels of VH, which were associated with variables such as being a mother, young, low level of education, belief that Vaccine Preventable Diseases (VPD) are not serious and concern about side effects. They stated that the most frequent reason for refusing or delaying vaccines was the lack of recommendation by pediatricians (35.1%). In Malaysia according to the PACV (25) 11.6% were categorized as parents with VH and were associated with the following characteristics: parents without employment, with minor children, non-Muslims and mothers expecting their first child, suggesting that the factors associated with VH may vary with respect to the characteristics of each country's population. In the study carried out in Washington where they evaluated a communication training intervention for physicians (19), VH was detected at baseline in the control (12.6%) and intervention (9.8%) groups. The performance of the PACV in different countries and languages and its ability to detect parents with VH is optimal and proves to be a valid and reliable instrument in different places and contexts beyond the Cronbach's alpha score, likewise, the place will differentiate the sociodemographic and study variables that can be associated and related to the PACV scores.
The Vaccine Hesitancy Scale (VHS) is an instrument under development, which aims to be a global instrument endorsed by the WHO to measure VH (10,14); however, in the United Kingdom (13), a study with a modified questionnaire found that 4% of the sample responded with indecision in all items and 19% in at least one item. In Guatemala (24), the Likert-type VHS scale showed that no parents had ever refused any vaccination, and only 1.1% had ever hesitated, none thought of any reason not to vaccinate, but thoughts of hesitancy were detected, of which 3 (0.4%) did not believe that vaccines protect against serious illness, 59% of parents think that other people's children do not have all the recommended vaccines, which suggests that the survey has limitations in identifying hesitant behaviors and may be difficult to understand but shows that thoughts and beliefs towards VH may be present. However, in France (28), 3 survey items were used and the presence of VH was identified among parents of children and adolescent women, with respect to vaccination of their children the VH was 46% to 48% and was significantly associated with demographic variables such as education, low income, and a poor perception of Vaccine Preventable Diseases (VPD). Parents with at least one child aged 10-15 years were more likely to be hesitant than parents with younger children and levels were significantly lower in persons with reported measles and hepatitis vaccination in their children. This suggests that the VHS has the elements and capabilities to be able to detect VH, although more studies, especially validation studies, are needed to fully meet the objective of being used globally as recommended by the WHO SAGE Working Group on VH.
In studies where any intervention focused on modifying VH levels was developed, through motivational interviewing-based techniques, communication training for physicians to deal with parental insecurities toward vaccination, early detection of parents with hesitancy prior to their child's health visit, and providing talks about parental concerns (12,19,21,26), the success or failure of the study was due to the design of the intervention and not the ability to detect VH levels. The limitations of the instruments found were that in some studies where the instrument was modified, validity and reliability were not evaluated, which could affect the validity of the construct. The authors mentioned that considering the results, despite not having measured the validity and reliability in some modified versions of the instruments, they are tools that allowed detecting parents who present VH (14,16,22,24,26,29,30).
Conclusions
The Opel PACV is a reliable and validated instrument that has been used and modified in different contexts and has effectively measured Vaccine Hesitancy (VH). The VHS of the SAGE Working Group on HV is a potential instrument for measuring HV; validation studies of this scale are recommended to expand the options for assessing this phenomenon. The variables that were mainly associated with VH were the vaccination status of the child, education, number of children, low perception of Vaccine Preventable Diseases (VPD). Studies on VH in Latin America are scarce, but necessary, as they can contribute to the development of strategies to improve vaccination coverage in the region
Referencias bibliográficas
1. OMS: Organización Mundial de la Salud [Internet] Suiza. 2019. [Citado 2019 Nov 06]. Organización Mundial de la Salud Cobertura Vacunal. [aprox. 1 pantalla]. Disponible en: Disponible en: https://www.who.int/es/news-room/fact-sheets/detail/immunization-coverage [ Links ]
2. Organización Panamericana de la Salud. Inmunización en las Américas, Resumen 2019. [Internet]. Washington: Inmunización Integral de la Familia, Familia, Promoción de la Salud y Curso de Vida; 2019 [Citado 2019 Nov 06]. Disponible en: Disponible en: https://www.paho.org/hq/index.php?option=com_docmanview=downloadcategory_slug=folleto-inmunizaciones-2646alias=50554-inmunizacion-en-las-americas-resumen-2019Itemid=270lang=es [ Links ]
3. Lopera Pareja EH. El movimiento antivacunas. Argumentos, causas y consecuencias. [Internet] Madrid: Catarata, 2016 [Citado 2019 Nov 06]. Disponible en: Disponible en: https://www.oei.es/historico/divulgacioncientifica/IMG/pdf/antivacunas_tripas.pdf [ Links ]
4. Cuesta Cambra U, Gaspar Herrero S. La “reputación online” de la información de vacunas en internet. Historia y Comunicación Social. 2014 [Citado 2019 Nov 06];19:15-29. Disponible en: http://dx.doi.org/10.5209/rev_HICS.2014.v19.45007 [ Links ]
5. Martínez Diz S, Martínez Romero M, Fernández Prada M., Cruz Piqueras M., Molina Ruano R, Fernández Sierra MA. Demandas y expectativas de padres y madres que rechazan la vacunación y perspectiva de los profesionales 43 sanitarios sobre la negativa a vacunar. An Pediatr. [Internet] 2014 [Citado 2019 Nov 06];80(6):370-378. Disponible en: Disponible en: http://www.sciencedirect.com/science/article/pii/S1695403313003779 [ Links ]
6. Véliz L, Campos C, Vega P. Conocimiento y actitudes de los padres en relación a la vacunación de sus hijos. Rev. chil. infectol. [Internet]. 2016 [Citado 2019 Nov 06];33(1):30-37. Disponible en: Disponible en: https://scielo.conicyt.cl/scielo.php?script=sci_arttextpid=S0716- [ Links ]
7. Cruz Piqueras M, García de Cortazar AR, Hortal Carmona J, Padilla Bernaldez. Reticencia vacunal: análisis del discurso de madres y padres con 44 rechazo total o parcial a las vacunas. Gac. Sanit. [Internet] 2019 [Citado 2019 Nov 06];33(1):53-59. Disponible en: Disponible en: https://www.sciencedirect.com/science/article/pii/S0213911117301838?via%20%3Dihub [ Links ]
8. Guadarrama Orozco JH, Vargas López G, Viesca Treviño C. Decisiones de los padres que no arriesgan la vida de sus hijos, pero que los exponen a daños serios: no a las vacunas. Bol. Med. Hosp. Infant. Mex. [Internet] 2015 [Citado 2019 Nov 06];72(5):353-357. Disponible en: http://dx.doi.org/10.1016/j.bmhimx.2015.09.007 [ Links ]
9. Domínguez A. Astray J, Castilla J, Godoy P, Tuells J, Barrabeig I. Falsas creencias sobre vacunas. Aten Primaria. [Internet] 2019 [Citado 2019 Nov 06];51(1):40-46. Disponible en: https://doi.org/10.1016/j.aprim.2018.05.004 [ Links ]
10. MacDonald NE SAGE Working Group on Vaccine Hesitancy. Vaccine hesitancy: Definition, scope and determinants. Vaccine. [Internet] 2015 [Citado 2019 Nov 06] 2015;33(34):4161-4. Disponible en: https://doi.org/10.1016/j.vaccine.2015.04.036 [ Links ]
11. Diccionario Inglés/Español. Barcelona: Editorial Océano. p 199. [ Links ]
12. Gagneur A, et al. Promoting vaccination in maternity wards motivational interview technique reduces hesitancy and enhances intention to vaccinate, results from a multicentre non-controlled pre- and post-intervention RCT-nested study, Quebec, March 2014 to February 2015. Euro Surveill. [Internet] 2019. [Citado 2019 Oct 11];24(36). Disponible en: https://doi.org/10.2807/1560-7917.ES.2019.24.36.1800641. [ Links ]
13. Luyten J, Bruyneel L, van Hoek AJ. Assessing vaccine hesitancy in the UK population using a generalized vaccine hesitancy survey instrument. Vaccine. [Internet] 2019. [Citado 2019 Oct 11];37(18):2494-2501. Disponible en: https://doi.org/10.1016/j.vaccine.2019.03.041. [ Links ]
14. Larson HJ, Jarrett C, Schulz WS, Chaudhuri M, Zhou Y, Dube E, Schuster M, MacDonald NE , Wilson R. Measuring vaccine hesitancy: The development of a survey tool. Vaccine. [Internet] 2015. [Citado 2019 Oct 14]:33(34):4165-4175. Disponible en: https://doi.org/10.1016/j.vaccine.2015.04.037. [ Links ]
15. Dubé È, et al. Overview of knowledge, attitudes, beliefs, vaccine hesitancy and vaccine acceptance among mothers of infants in Quebec, Canada. Hum Vaccin Immunother. [Internet] 2019. [Citado 2019 Oct 14];15(1):113-120. Disponible en: https://doi.org/10.1080/21645515.2018.1509647 [ Links ]
16. Cunningham RM, et al. Development of a Spanish version of the parent attitudes about childhood vaccines survey. Hum Vaccin Immunother. [Internet] 2019. [Citado 2019 Oct 15];15(5):1106-1010. Disponible en: https://doi.org/10.1080/21645515.2019.1578599 [ Links ]
17. Yu Hu, Yaping Chen, Hui Liang, Ying Wang. Reliability and validity of a survey to identify vaccine hesitancy among parents in Changxing county, Zhejiang province. Hum Vaccin Immunother. [Internet] 2019. [Citado 2019 Oct 15];15(5):1092-1099. Disponible en http://doi.org/10.1080/21645515.2019.1572409 [ Links ]
18. Roberts JR, et al. Vaccine hesitancy among parents of adolescents and its association with vaccine uptake. Vaccine. [Internet] 2015. [Citado 2019 Oct 16];33(14):1748-1755. Disponible en: https://doi.org/10.1016/j.vaccine.2015.01.068 [ Links ]
19. Henrickson NB, et al. Physician Communication Training and Parental Vaccine Hesitancy: A Randomized Trial. Pediatrics. [Internet] 2015. [Citado 2019 Oct 17];136(1):70-19. Disponible en: https://doi.org/10.1542/peds.2014-3199 [ Links ]
20. Henrikson NB, Anderson ML, Opel DJ, Dunn J, Marcuse EK, Grossman DC. Longitudinal Trends in Vaccine Hesitancy in a Cohort of Mothers Surveyed in Washington State, 2013-2015. Public Health Reports. [Internet] 2017. [Citado 2019 Oct 21];132(4):451-454. Disponible en: https://doi.org/10.1177/0033354917711175 [ Links ]
21. Opel DJ, Henrikson N, Lepere K, et al. Previsit Screening for Parental Vaccine Hesitancy: A Cluster Randomized Trial. Pediatrics. [Internet] 2019 [Citado 2019 Oct 21];144(5). Disponible en: https://doi.org/10.1542/peds.2019-0802 [ Links ]
22. Abd Halim H, Abdul-Razak S, Md Yasin M, Isa MR. Validation study of the Parent Attitudes About Childhood Vaccines (PACV) questionnaire: The Malay version. Hum Vaccin Immunother. [Internet] 2019 [Citado 2019 Oct 22]. Disponible en: https://doi.org/10.1080/21645515.2019.1674112 [ Links ]
23. Oladejo O, Allen K, Amin A, Frew PM, Bednarczyk RA, Omer SB. Comparative analysis of the Parent Attitudes about Childhood Vaccines (PACV) short scale and the five categories of vaccine acceptance identified by Gust et al. [Internet] Vaccine. 2016. [Citado 2019 Oct 24];34(41):4964-4968. Disponible en: https://doi.org/10.1016/j.vaccine.2016.08.046 [ Links ]
24. Domek GJ. Measuring vaccine hesitancy: Field testing the WHO SAGE Working Group on Vaccine Hesitancy survey tool in Guatemala. Vaccine. [Internet] 2018. [Citado 2019 Oct 25];36(35):5273-5281. Disponible en: https://doi.org/10.1016/j.vaccine.2018.07.046 [ Links ]
25. Mohd Azizi FS, Kew Y, Moy FM. Vaccine hesitancy among parents in a multi-ethnic country, Malaysia. Vaccine [Internet]. 2017 May 19 [Citado 2019 Oct 25];35(22):2955-2961. Disponible en: https://doi.org/10.1016/j.vaccine.2017.04.010. [ Links ]
26. Connors JT, Hodges EA, D’auria J, Windham L. Implementing vaccine hesitancy screening for targeted education. J Am Acad Nurse Pract. 2018. [Internet]. 2018 [Citado 2019 Oct 28];30(8):450-459. Disponible en: https://doi.org/10.1097/JXX.0000000000000056 [ Links ]
27. Hofstetter AM, et al. Parental Vaccine Hesitancy and Declination of Influenza Vaccination Among Hospitalized Children. HOSPITAL PEDIATRICS. [Internet] 2018. [Citado 2019 Oct 29];8(10):6628-6635. Disponible en: https://doi.org/10.1542/hpeds.2018-0025 [ Links ]
28. Rey D, Fressard L, Cortaredona S, Bocquier A, Gautier A, Peretti-Watel P, Verger P, on behalf of the Baromètre santé 2016 group. Vaccine hesitancy in the French population in 2016, and its association with vaccine uptake and perceived vaccine risk-benefit balance. Euro Surveill. [Internet] 2018 [Citado 2019 Oct 30];23(17):pii=17-00816. Disponible en: https://doi.org/10.2807/1560-7917.ES.23.17.17-00816 [ Links ]
29. Napolitano F, D'Alessandro A, Angelillo IF. Investigating Italian parents' vaccine hesitancy: A cross-sectional survey. Hum Vaccin Immunother. [Internet] 2018. [Citado 2019 Nov 04];14(7):1558-1565. Disponible en: https://doi.org/10.1080/21645515.2018.146394 [ Links ]
30. Cunningham, Rachel M. et al. Prevalence of Vaccine Hesitancy Among Expectant Mothers in Houston, Texas. Academic Pediatrics. [Internet] 2018 [Citado 2019 Nov 04];18(2):154-160. Disponible en: https://doi.org/10.1016/j.acap.2017.08.003 [ Links ]
Received: February 03, 2020; Accepted: January 18, 2021











 text in
text in