Introduction
Subarachnoid hemorrhage (SAH) is a clinical condition in which severe life-threatening neurological destruction occurs1. Acidosis is the most dangerous complication of SAH. The carotid body network (CBs) is essential for pH regulation through its binuclear neurons (BNNs), and BNN degeneration after SAH exacerbates acidosis. CBs are mostly chemosensitive and highly vascularized structures2. Chemosensitive glomus cells are synaptically connected to glossopharyngeal nerve terminals in CBs3 and are often sensitive to blood pH changes4. A difference of < 2% O2 concentration in arteriovenous blood stimulates CBs5. Dysfunctions of CBs can result in cerebral circulatory disorders, cardiorespiratory disturbances, and acidosis4. CBs degeneration was explained to be responsible for the decrease in blood pH after SAH6. Interestingly, it was noticed that binuclear neuron degeneration was more prominent in animals with low blood pH after SAH7. Although obstructive hydrocephalus is a serious complication of SAH8, examination of the central canal has not been adequately investigated. Intraventricular blood causes dangerous ischemic degeneration of the choroid plexus9 and major periventricular tissue damage and ischemic damage to ependymal and arachnoid cells10, destruction of ciliated ependymal cells and accumulation of small amounts of blood along the ventricular system cause dilatation of the ventricles11. Spinal SAH causes normal pressure hydrocephalus12. Sometimes hydrocephalus can be associated with hydromyelia13. Although central canal dilatation is an important part of hydrocephalus, central canal dilatation has been overlooked in the progression of hydrocephalus. Although basic explanatory theories have been proposed, no mechanism based on the desquamation of ependymal cells and rupture of subependymal membrane related to central canal dilatation has been published in the literature. This study will aim to investigate the effect of blood metabolism changes that occur after SAH on pathological changes in the medulla spinalis.
Materials and methods
Animal husbandry and the study design followed the guidelines of the National Institutes of Health. The study design was approved by the Committee on Animal Research of Ataturk University.
Twenty-eight hybrid rabbits were used. Five rabbits (n = 5) were used to analyze the CBs network; six were used for the SHAM group (n = 6); 1 cc saline was injected into the cisterna magna and the remaining (n = 17) were used to form the SAH group; 1 cc autologous arterial blood was injected into the cisterna magna, and decapitated after 3 weeks of follow-up. Blood pH values of all animals were recorded three times a week before and after the experimental procedures. Blood samples (1 cc) were collected in a heparinized syringe and pH values were measured with a pH meter (Mettler Toledo MP 220 pH Meter, Schwarzenbach, Switzerland).
They were followed for 3 weeks and then decapitated under general anesthesia. Brain tissues were removed and stored in a 10% formalin solution for 7 days. The specimens were embedded in paraffin blocks and twenty consecutive 5 μm longitudinal/horizontal sections were taken from all preparations for stereological examinations. Spinal cord preparations were stained with hematoxylin and eosin (H&E) and glial fibrillary acidic protein (GFAP) immunostaining. All preparations were examined under a light microscope and the following method was used to determine the ependymal cell density of central canal ependymal cells.
The central canal is a cylindrical surface. Ependymal cells are arranged on this surface like single-layer round cakes in a tray. Therefore, ependymal cell density is considered to be the number of cells per square millimeter. Here, we calculated the surface area of an average central canal section of 1000 μm at the C5 level and determined the number of ependymal cells in this area. We then considered the number of cells per 1000 square micrometers as the ependymal cell density.
They were horizontally embedded in paraffin blocks to cut all ventricles and central canals during the histopathologic examination. Each spinal cord was sectioned in 20-50 consecutive 5-μm sections. The Cavalieri method was used to assess the volumes of the central canal. The advantages of this method were to avoid excessive preservation and cutting. Many consecutive sections were obtained from tissue samples from one side of the aqueducts to the other and stacked on top of each other and imaginary central canals were formed like cylinders and surfaces were calculated by cylinder methods using the following formula:
V = 2πrh
Histopathologically, cytoplasmic condensation, nuclear shrinkage, cellular angulations, and pericytoplasmic halo formation due to cytoplasmic regression were accepted as neuronal degeneration criteria. Central canal volume values and ependymal cell numbers of the central canal surfaces were compared statistically. The relationship between pH values of cerebrospinal fluid (CSF)/blood and degenerated epithelial cell densities was statistically analyzed by the Mann–Whitney U-test. Data analysis consisted of Kruskal–Wallis and Mann–Whitney U-test. Differences were considered significant at p < 0.05.
Results
Three of the 28 rabbits died within the 2nd week, probably due to cardiorespiratory irregularities, and new animals were re-studied. All animals showed signs of meningeal irritation, decreased consciousness scores, cardiac rhythm, and respiratory disturbances during the 1st week and returned to normal. Various electrocardiographic changes such as ST depression, ventricular extrasystoles, bigeminal pulses, QRS separation, and fibrillations were observed in animals with SAH. Further analysis of respiratory parameters showed a decrease in respiratory frequency (bradypnea) and an increase in respiratory amplitude (34%) during the first hours of SAH. Finally, increased respiratory frequency, decreased respiratory amplitude, prolonged expiration and shortened inspiration, apnea-tachypnea episode, diaphragmatic breathing, and respiratory arrest were observed in animals that died due to SAH. The heart rates of all normal animals were 252 ± 23/min; 224 ± 14/min in SHAM, and 182 ± 15/min in SAH. In severe SAH, heart rhythm disturbances were more frequent at 143 ± 12/min. Respiratory rhythms became acidotic and arrhythmic. Lower pH values were associated with more acidotic respiratory patterns. Brain edema, rigidity, leptomeningeal thickness, and increased brain weight were observed in all animals that developed decapt SAH. Brain laceration was detected in two animals. Although early decapitated animals showed no degenerative changes in the choroid plexus, late decapitated animals showed degenerative changes in the villus layer including scaling, choroidal cell shrinkage, angulation, cytoplasmic condensation, and cellular loss.
Histopathological results
GENERAL HISTOLOGICAL FINDINGS OF CENTRAL CANAL
The central ducts contained epithelial cells with numerous cuboidal epithelium with brush edges. In control rabbits, ependymal cells showed round centrally located nuclei. The SAH group had clear signs of nuclear condensation and cell body shrinkage, suggesting the presence of epithelial cell deformations. Deformed cells were detected by positive staining of the GFAP assay (Figs. 1 and 2). Ependymal cells showed significant cell damage, and severe shedding of villi and epithelial cell loss was observed in acidotic CSF. General examinations in the study group of acidotic CSF showed spinal cord swelling, edema, pia-arachnoid adhesions, ventral canal dilatation, spinal cord swelling, edema, arachnoiditis, central canal hemorrhage, occlusions, and dilatation (Fig. 3). Edema, swelling, desquamation, ependymal cell central canal occlusions, and basement membrane ruptures were detected in ependymal cells. There were clear signs of nuclear condensation and cell body shrinkage, suggesting the presence of apoptosis of choroidal epithelial cells. Severe villus shedding and cell loss were observed in the study group (Fig. 4).

Figure 1 In a normal rabbit, at the C5 level, in the cross-section perpendicular to the long axis of the spinal cord, the central canal (CC) is observed from the inside to the outside, A: the butterfly-shaped dark gray matter surrounding it and the white matter around it. B: magnified form of the central canal, C: long axis parallel section of the central canal, D: ependymal cells (E) and normal ciliary extensions (NC) are observed.

Figure 2 A: in a rabbit belonging to the control group, normal central canal (CC/A), surrounding normal ependymal cells (E) and ciliary extensions (CE/B) in the perpendicular section of the long axis of the spinal cord at C5 level. B: central canal with partially dilated turbid material and edematous deformed wall and deformed ependymal cells (DE) that have partially lost their ciliary extensions (DC-E) in a subject belonging to the SHAM group. C: a subject belonging to the study group has highly edematous-deformed ependymal cells (DC-E), whose cytoplasm is darkened and condensed and desquamated and has lost their ciliary extensions significantly, ruptured and desquamated basement membrane (Ds-E), and blood elements in the central canal LM, GFAP, × 1000/ABC).
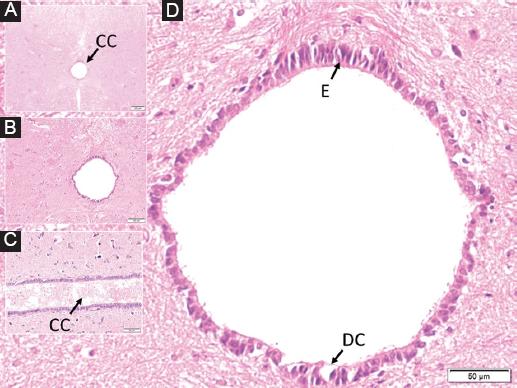
Figure 3 In a rabbit belonging to the study group, dilated central canal containing fuzzy material from the inside out, in the perpendicular section to the long axis of the spinal cord at C5 level, A: a butterfly-shaped dark gray matter surrounding it and white matter around it are observed. B: magnified form of the central duct with partially dilated turbid material, C: long axis parallel section of the central duct with partially turbid material and edematous ependymal cells, D: highly edematous-deformed, cytoplasm darkened and condensed and desquamated and ciliary extensions. Significantly lost ependymal cells (E), ruptured basement membrane, and blood elements are observed in the central canal.

Figure 4 A: normal ependymal cells (E) and ciliary extensions (CE) in a rabbit belong to the control anima, B: moderately deformed ependymal cells (DE) and desquamated ciliary extensions (DCE) in a SHAM animal, and separated basal membrane, C: highly edematous-deformed and desquamated ependymal cells (Ds-E) whose cytoplasm is darkened and condensed and lost their ciliary extensions significantly (DC-E) in a study animal (LM, GFAP, × 50/A, B, C).
Numerical results
The mean blood pH values and degenerated ependymal cells density (n/mm2) were 7.351 ± 0.033/23 ± 7 in control, 7.322 ± 0.059/78 ± 13 in SHAM, and 7.261 ± 0.048/254 ± 62 in slight study animals (Fig. 5). Mean cerebrospinal pH values were 7.322 ± 0.034 in the control group and 7.324 ± 0.056 in the SHAM group and 7.275 ± 0.049 in the SAH group. The mean cervical central canal volume was 1.056 ± 0.053 mm3 in control animals; 1.321 ± 0.12 mm3 in SHAM animals and 1.743 ± 0.245 mm3 in animals with low pH (n = 6). Low pH values cause greater ependymal cell desquamation (p < 0.01) with a direct linear link to degenerating carotid body neuron density (p < 0.01).

Figure 5 The central canal is basically a cylindrical surface. The ependymal cells are arranged on this surface like single-layer circular cakes in a tray. Therefore, ependymal cell density is considered as the number of cells per square millimeter. Here we calculated the surface area of an average 1000 μm central canal section at the C5 level. And we determined the number of ependymal cells in this area. We then considered the amount of cells per 1000 μm squared to be the ependymal cell density.
Discussion
Cells in the central canal of the spinal cord sense and regulate pH. The spinal cord transmits pH signals from the organs to the brain and controls the cerebrospinal fluid that flows around the brain and spinal cord and is lined with ciliated cells. These pH sensors in the central canal regulate fluctuations in pH, an elegant mechanism is vital so that it can sense pH levels. This cell membrane detects pH levels that react to acidic pH and alkaline pH levels. The normal body pH is 7.4. These ciliary neurons are highly sensitive to acid-base balance. Ciliated neurons have a very low level of activity at pH = 7.4. However, if the pH deviates in an acidic or alkaline direction, the activity increases markedly. Changes in pH activate all the highly regulating microcircuits of the nervous system and disrupt their normal function. If the nervous system secretes high levels of lactic acid during intense exercise or epilepsy, it causes an acidosis pH in the nervous system. Both more acidic and alkaline pH have an inhibitory effect on motor activity in the spinal cord14. The ependymal cells are responsible for the production of the CSF15. Although ependymal cell desquamation and subependymal basal membrane destruction related to choroidal artery vasospasm may lead to aqueductal stenosis and hydrocephalus after SAH8, acidosis-related ependymal desquamation, and subependymal basal membrane rupture as mechanisms of central channel stenosis which has not been suggested in the literature.
Central blood ependymal cell degeneration secondary to acidosis is an overlooked complication of hydrocephalus and can lead to flaking of ependymal cells, destruction of the subependymal basement membrane, accumulation of blood cells in the subependymal basement membrane, increased cerebrospinal fluid pressure, and consequently hydrospinal. Although central canal dilatation as a distant complication of SAH following SAH is an important part of hydrocephalus, central canal dilatation has been ignored in the progression of hydrocephalus. Although basic explanatory theories have not been proposed, no mechanism for central canal dilatation based on the desquamation of ependymal cells and subependymal membrane rupture has been published in the literature. We hypothesized that hydrospinal is an important and dangerous complication of hydrocephalus.
In our previous study6, we obtained findings as follows: due to increased intracranial pressure and vasospasm after SAH, the glossopharyngeal nerve is affected, and this effect is reflected to the carotid body through the neuronal network. The degeneration of the CBs leads to a disturbance of blood pH and the development of acidosis-related complications. Our results from the current study have shown that one of the most important complications of acidosis after CBs degeneration is that increased CSF acidosis leads to neuronal degeneration in the spinal canal. Morphologic examination of the spinal cord shows that this degeneration is significant and also hydromyelia has been observed16. This damage to the spinal cord, which is the connection between the brain and the end organs, allows a better understanding of the mortality and morbidity after SAH.
Although pH changes, especially acidosis, are the most common biochemical pathology in intensive care patients in medical practice17, the organ damage caused by it has almost never been examined. Especially in acidosis, while blood pH is taken into consideration, CSF pH has always been overlooked. Blood and CSF pH changes cause acid burns in the functional units of all organs in which these two fluids circulate18. Jalalvand et al. reported their study as follows: spinal pH is optimal at 7.4, at this level neuronal conduction functions normally. However, in both acidosis and alkalosis, neuronal conduction is significantly impaired and functional conduction is blocked. This impaired conduction in the neuronal network causes dysfunction in both motor and visceral organs18. As a matter of fact, in our previous studies, it has been reported that acidic CSF destroys the choroid plexuses, spinal cord, and intestines8. Histopathologic morphologic and mechanical changes that occur in the central canal together with all ventricular surfaces in acidosis may be the cause of many neurologic diseases of uncertain etiology. Gerstman et al. reported that circulating CSF causes stimulation in neurons around the central canal and this stimulation regulates locomotor functions19. Desquamation occurring after SAH proves that it disrupts this regulation system in two ways: first, the central canal surface where stimulation will be performed will be destructed, and second, the flow of CSF contaminated by desquamation will decrease. As a result of these physiopathologic processes, it will cause functional impairment in the end organs (extremities and visceral organs).
Blockage of endocrine, immune, and mechanical functions of ependymal cells due to acidosis disrupts signal flows in the spinal cord, which is the transit tunnel of afferent and efferent signals, and eliminates central and peripheral interactions. The conductivity of CSF in acid pH also increases. This triggers information anarchy by mixing efferent and afferent signals. Kuyama et al. showed that the electrical conductivity of CSF increased after SAH, which supports our theory and results20.
Importance of presented study
In the direction of mentioned literature, we theorized that CBs neuron ischemia-induced acidosis based on central canal pathologies should be remembered as a dangerous complication of SAH.
Limitations
This study does not include rich biochemical parameters. In addition, to elucidate the electrokinetic and thermodynamic properties of CSF, electrophysiologic parameters (the relationship between CSF conductivity and pH), evaluation of clinical presentation (evaluation of spasticity in the extremities), electromyographic studies, and measurement of CSF ion concentration will allow the elucidation of the dark spots on the subject.
Conclusions
An ignored pathological change that occurs after SAH is a deterioration in blood pH in favor of acidosis, which is reflected in the pH of CSF. Acidotic CSF causes neuronal degeneration and hydromyelia in the medulla spinalis. These disturbances are very valuable for a better understanding of end-organ damage after SAH and we think that clarifying this mechanism in the literature will pave the way for potential treatment methods.











 text new page (beta)
text new page (beta)


