Introductıon
Coronary artery bypass grafting is performed worldwide for the relief of angina symptoms and long survival in the presence of coronary artery disease. Although there are attempts to develop technical and medical procedures to increase graft patency, new studies are still needed. Saphenous veins are frequently used as grafts due to the insufficient number of arterial grafts. Graft patency rates depend on many factors and are affected by the patient's risk factors, high arterial pressure, and surgical techniques. The overall angiographic patency rates for arteries were 95.7%, 90.1%, and 92.2% on early, 1-year, and 5-year angiograms, respectively, in a study. The angiographic patency rates of saphenous vein grafts (SVG) were 93.1%, 85.6%, and 91.3% on early, 1-year, and 5-year angiograms, respectively, in the same study1. Many factors such as endothelial damage, intimal hyperplasia and intraluminal thrombus are among the causes of early SVG failure2. Minimizing SVG damage will significantly reduce patients' mortality and morbidity rates.
The SVGs are prone to pulsatile arterial pressure related damage in the post-operative period. Permanent injury occurs especially in the endothelial layer and an inflammatory process begins. Uncontrolled proliferation and cell migration in endothelial cells, hyperplasia in vascular smooth muscle cells and formation of myofibroblasts result in neointimal hyperplasia3,4. Therefore, SVG occlusion starts and patency is shortened. To prevent damage due to intraluminal arterial pressure in vein grafts; there are studies in the literature regarding perivenous support, tissue adhesives, and various medical treatments3-6.
The aim of this study is to investigate the effect of perivascular application of fibrin glue (FG), which is used as a tissue adhesive, against endothelial damage and intimal thickening in SVG.
Material and methods
This ex vivo study was conducted following approval from the Local Ethics Committee (Bursa City Hospital Ethics Committee, Date: 16/06/2021, No: 2021-11/4). In this study, 20 excess SVG from patients who underwent coronary artery surgery at our institution were used. Average length of 4 cm excess SVG were divided into two segments. Perivascular FG was applied to one segment and was included in the treatment group. The other saphenous vein was not treated with FG and was included in the control group. A total of 40 SVGs were studied, from 20 subjects in each group.
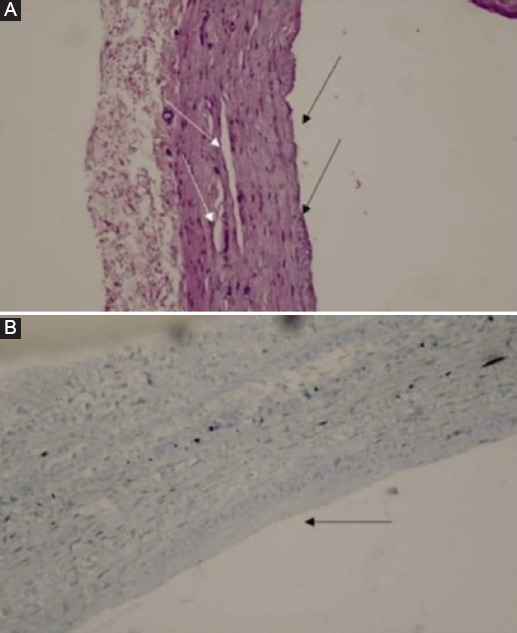
The intraluminal pressure was provided with the cardiopulmonary bypass machine which was used during coronary artery bypass grafting in each patient. The experiment began after the patient was transferred from the operating room after CABG surgery. Appropriate cannulae were connected to both ends of the SVG. An arterial line was connected to one end of the saphenous vein and a venous line was connected to the other end, and an arterial system model was created with a cardiopulmonary bypass machine (Fig. 1). A manometer was connected to the venous line to record the pressure within the SVG. FG was sprayed on the outer surface of one of the SVGs and left to dry for 3 min. The other SVG did not receive any treatment. This arterial system model was exposed to blood flow for 60 min with a mean pressure of 120 mmHg. After the experiment, the materials were collected for histopathological examination.

Figure 1 A: setting up the experimental setup and creating an arterial model with saphenous veins. B: perivascular fibrin glue applied to one of the saphenous vein grafts, prepared from the same patient, for the purpose of external support.
The patients who received emergency operation, whom underwent off-pump surgery, had insufficient excess saphenous vein graft, had hematologic and oncologic comorbidities, and were not volunteered were excluded from the study.
Histopathological examination
The extracted grafts were kept in 10% buffered formaldehyde solution for 24 h. After tissue sampling and routine tissue follow-up procedures, 5 mm-thick sections were taken from the samples fixed in the formalin of the material and embedded in paraffin, and stained with hematoxylin-eosin (H&E). Preparations stained with H&E were examined and the preparations that best represented vessel morphology were selected. In the immunohistochemical study, endothelial markers CD31 (clone EP78) and CD34 (clone QBend-10) dyes were used. Immunohistochemical staining was done with an automatic stainer (Ventana BenchMark Ultra) according to the protocols recommended by the instructions of use of the manufacturers.
The histomorphological classification of endothelial damage was made according to the classification made by Ip et al. as follows7.
No damage: All endothelial cells are interconnected. There is no change in cell sizes, no separation between layers (Fig. 2).
‒ Type 1 damage: Endothelial cell integrity is preserved and all the cells are in contact with each other, but a decrease in the diameter of the endothelial cells is observed. There are slight separations in the intima and/or media layers of the vessel (Fig. 3).
‒ Type 2 damage: The connections between endothelial cells are broken in places and there is loss of endothelial cells in places. There are more distinct separations in the intima and/or media layers of the vessel (Fig. 4).
‒ Type 3 damage: The endothelial cell layer is lost. There are significant separations in the intima and/or media layer of the vessel (Fig. 5).

Figure 2 A: all endothelial cells are in contact with each other, no separation between layers (H&E, ×4). B: endothelial layer shows all-round labeling with CD31 (CD31 immunohistochemistry, ×10).

Figure 3 A: slight loss of endothelial cells (black arrows) and slight separation in the tunica media (white arrows) (H&E, ×4). B: local endothelial cell losses are observed between CD31-marked endothelial cells (black arrows) (CD31 immunohistochemistry, ×20).
Statistical analysis
Data were categorical and calculated using IBM SPSS Statistics v.25. The Chi-square method was used to analyze two independent categorical data. Pearson Chi-square method was used for analysis if the expected count below 20% of the cells was <5. Fisher's Exact Test was used when over 20% of the cells' expected count was <5. Continuity correction was used when the expected value over 20% of the cells was between 5 and 25, and Pearson Chi-square were used if the expected value over 20% of the cells was >25. p < 0.05 was accepted as statistically significant.
Results
According to the endothelial injury classification, no significant injury was observed in four specimens, Type 1 injury was detected in seven specimens, Type 2 injury was observed in seven specimens, and Type 3 injury was detected in two specimens in the control group. In the FG treatment group, endothelial damage was not observed in 13 samples, Type 1 damage was observed in five samples and Type 2 damage was observed in two samples. No Type 3 endothelial damage was observed in the FG treatment group.
There was no significant change in saphenous vein diameter in the group treated with FG. Endothelial damage secondary to intraluminal pressure developed less. The increase in SVG diameter and endothelial damage due to severe distension in the SVG occurred in the control group compared to the treatment group. While no serious damage was observed in the histopathological examination of SVG taken from the FG treatment group, severe endothelial damage and tunica media defects were observed in the control group. Endothelial damage was significantly less in the FG treatment group, as demonstrated in the evaluation of results between groups (Table 1).
Table 1 Distribution of saphenous vein injury in the groups as per classification
| Vascular damage class | Group 1 (FG ) (n = 20) | Group 2 (control) (n = 20) | p value |
|---|---|---|---|
| No injury n (%) | 13 (65) | 4 (20) | 0.004 |
| Type 1 endothelial damage n (%) | 5 (25) | 7 (35) | 0.490 |
| Type 2 endothelial damage n (%) | 2 (10) | 7 (35) | 0.127 |
| Type 3 endothelial damage n (%) | 0 (0) | 2 (10) | 0.487 |
Discussion
Coronary artery bypass grafting continues to be an important surgical treatment to increase the quality and duration of life in patients with coronary artery disease affecting more than one vessel. Long-term success is determined by graft success. Neointimal hyperplasia may develop in a short time, especially in venous grafts, and early occlusions may occur.
In this study, which was carried out by creating an ex vivo model; human SVG was used. This study shows that perivenous support with FG and exposed to arterial pressure can reduce endothelial damage in SVG.
FG was used as a topical surgical adhesive. It consists of human fibrinogen and bovine thrombin activated with calcium chloride. It is an agent that can be absorbed without the development of foreign body reaction and extensive fibrosis by increasing wound healing as well as its hemorrhage-stopping effect8.
Fibrin and fibrin degradation products are chemotactic agents on vascular tissue. FG applied around the venous graft increases smooth muscle and fibroblastoid cell migration. In this way, it promotes outward remodeling or adhesion formation between the vessel and the underlying tissue. An intact endothelial layer prevents thrombus formation within the vessel and thus limits the migration of smooth muscle cells into the intima. Besides external vascular support, the protection of the endothelial layer and its modulating effect on smooth muscle cell migration may further reduce the stimulus for intimal hyperplasia9. Due to the stretching of the SVG wall due to increased intraluminal pressure, phosphorylation of p38 mitogen-activated protein kinase is induced and has been shown to cause apoptosis10.
Therefore, as a result of mechanical stress, it causes destruction of alpha actin filament in venous smooth muscle cells11. Loss of endothelial integrity due to mechanical stress causes activation of coagulation factors and endothelial growth factors, resulting in platelet aggregation12. Venous smooth muscle cells differentiate. Matrix metalloprotease activity is increased, leading to increased expression of cytoskeleton-associated proteins that promote migration and proliferation of smooth muscle cells13.
The protective effect of FG backing on human SVG segments is comparable to our previous findings of perivenous cyanoacrylate adhesive backing. We have shown that perivenous cyanoacrylate adhesive support provides external support to the graft without any chemotactic effect in SVGs and provides primary protection of the graft against damage from excessive stress14.
Stooker et al. performed a similar study on the saphenous vein under lower intraluminal pressure (60 mmHg) and explained that by providing adequate external vein support with FG, they prevented excessive stretching and prevented endothelial damage at low pressures15. The difference of our study from this study is; We demonstrated the protective effect of FG against changes in SVG under 120 mmHg pressure. The results of our study are shown in table 1 in detail. Ip et al. divided coronary artery endothelial injury into three types7.
Endothelial damage was defined as: Type 1 injury: normal morphology despite functional changes in the endothelial layer; Type 2 injury: endothelial layer detachment, local peeling, and preservation of the inner elastic lamina and medial layer despite intimal damage; and Type 3 damage: peeling of the endothelial layer followed by the formation of lower endothelial tissue and intimal and medial damage in the corresponding classification. He reported that especially Type 3 injury may result in stenosis and occlusion in the coronary artery. No Type 3 endothelial injury was observed in our FG treatment group (Table 1).
Okazaki et al. classified endothelial injury into five stages16. The classification includes Stage 1: normal morphology; Stages 2 and 3: minor or diffuse adhesion of blood cells (corresponding to Type 1 injury); Stage 4: rare isolated separation in endothelial cells (corresponding to Type 2 injury); and Stage 5: generalized lack of endothelial cells (corresponding to Type 3 injury). In particular, development of Type 3 (Stage 5) injury and widespread formation of a sub-endothelial layer will lead to platelet aggregation and the formation of thrombus as a result of contact between blood components and this layer. This will trigger smooth muscle proliferation and migration with mitogen factors; hence, this may result in early or late stenosis or occlusion in the anastomosis area.
When endothelial damage was classified in our study; while no significant injury was observed in four specimens in the control group, Type 1 injury was detected in seven specimens, Type 2 injury in seven specimens, and Type 3 injury in two specimens. In the FG treatment group, endothelial damage was not observed in thirteen samples, while Type 1 damage was observed in five samples and Type 2 damage was observed in two samples. No Type 3 endothelial damage was observed in the FG treatment group. As shown in the evaluation of the results between groups, endothelial damage was found to be significantly less in the FG group (Table 1).
In this study, we showed that the application of FG on the perivenous SVG provides extravascular support and also prevents endothelial damage due to increased intraluminal pressure and mechanical stress.
Conclusion
Perivascular application of fibrinogen glue on the saphenous vein showed a protective effect against endothelial damage secondary to high intraluminal pressure. External vein graft support with FG may provide adequate protection against SVG injury in the early period and may promote remodeling of the vein graft in line with arterial wall properties.
In this study, FG application showed that it provided external support to the graft without any chemotactic effect and provided primary protection of the graft against endothelial damage caused by high pressure. For this reason, we believe that graft patency rates will be better thanks to the effect of preventing the endothelial damage caused by mechanical and chemical means, as well as the hemorrhage-stopping effect of FG. However, more in vivo studies are needed to investigate the effects on the graft in the long-term.











 text new page (beta)
text new page (beta)