Introduction
Bladder cancer is a malignant tumor that occurs on the bladder mucosa. It is the most common malignant tumor of the urinary system and one of the ten most common tumors throughout the body1,2. It accounts for the first place in the incidence of genitourinary tumors in our country, and its incidence is second only to prostate cancer in the West3. Bladder cancer is easy to recur, with the highest recurrence rate among solid tumors. After undergoing transurethral resection of bladder tumors, it requires regular reexaminations, and the recurrence rate of patients is as high as 50%4. It is precisely because of the high incidence and mortality of bladder cancer that the research on its mechanism has not stopped.
Hypoxia inducible factor-1 (HIF-1) was first discovered by Semenza and Wang in 1992, and the structure of HIF-1 was subsequently established and its cDNA coding sequence was proved5. HIF-1 is ubiquitous in human and mammalian cells. It can be stably expressed under hypoxic conditions, and is also expressed under normoxia. However, the synthesized HIF-1 protein is rapidly degraded through the intracellular oxygen-dependent ubiquitin protease degradation pathway6.
HIF-1 is a dimeric transcription factor composed of two protein subunits. It has a specific sensitivity to hypoxia, and its expression is tightly controlled by intracellular oxygen partial pressure. HIF-1 participates in the transcriptional regulation of many hypoxia-responsive genes in the body, and is a key factor in the body’s hypoxia response7. HIF-1 plays a key role in the reprogramming of cancer metabolism by activating the transcription of genes encoding glucose transporters and glycolytic enzymes. Many metabolic abnormalities in cancer cells increase HIF-1 activity.
Vascular endothelial growth factor A (VEGFA) is secreted by tumor cells, macrophages and fibroblasts, and is widely distributed in many tissues of the human body, such as glands, lungs, liver, kidneys, and myocardium8. However, its expression level is extremely low, and its role is only to maintain normal blood vessel density and Basic penetration function to maintain nutrients9. When tumor cells appear, their expression levels will generally rise greatly. Studies have shown that VEGFA is related to tumor invasiveness and tumor susceptibility. The structure of VEGFA protein is a homodimer composed of two peptide chains through disulfide bonds10. It is commonly found in the central nervous system, reproductive system and tumor tissues. Its main functions include electively enhance mitosis of vascular endothelial cells, stimulate endothelial cell proliferation, and promote blood vessel formation, increase the permeability of blood vessels, especially small blood vessels, and make plasma macromolecules extravasate and deposit in the extravascular matrix to provide nutrition for the growth of tumor cells and the establishment of new capillary network11.
Through protein function analysis, it is found that HIF-1α can target the regulatory gene VEGFA to promote tumor cell proliferation, migration, invasion, neovascularization, and lymph node metastasis.
This work aimed to investigate the molecular mechanism of the activation of hypoxia-inducible factors under different low oxygen partial pressures.
Materials and methods
Cell model construction
Cultivate the bladder cancer cell line UMUC3 in strict accordance with the requirements of in vitro aseptic culture, and then wait until the cells grow to the logarithmic phase. Place the cells in a Plexiglass box with volume fractions 5% CO2/95% O2, 5% CO2/95% N2, and 5% CO2/5% O2/90% N2 and continue to culture for 30 min, naming normal control (NC), hypoxia group-1 (Hy-1), and hypoxia group-2 (Hy-2), respectively. At the same time, continue to culture two groups of cells in 5% CO2/95% N2, 5% CO2/5% O2/90% N2 Plexiglass boxes, and transfect small interfering hypoxia-inducible factor 1α (si-HIF-1α) liposome plasmid, naming Hy-1 + si-HIF-1α group, and Hy-2 + si-HIF-1α group.
Cell scratch test
After the control group and the experimental group were digested and counted, 8 × 105 cells were divided into 35 mm2 culture dishes. Use a marker to draw a line on the bottom of the dish as a mark, aspirate the culture medium, and use a 10 mL pipette tip to mark the cells in the dish perpendicularly to the marker. Rinse with phosphate buffered saline (PBS) to remove the marked cells, add serum-free culture medium to continue culturing. Take pictures at 0 h and 24 h, and select the intersection of the line drawn by the marker and the cell scratch as the observation point, and then observe at a fixed point.
Cell cloning experiment
Take each group of cells in the logarithmic growth phase, digest them with 0.25% trypsin and pipette into single cells, and suspend the cells in a culture medium of 10% fetal bovine serum for later use. The cell suspension was diluted in gradient multiples, and each group of cells was inoculated into a dish containing 10 mL of pre-warmed culture medium at 37°C at a gradient density of 50, 100, and 200 cells per dish, and gently rotated to make the cells uniformly dispersed. Place it in a cell incubator at 37°C, 5% CO2 and saturated humidity for 2-3 weeks. It is frequently observed that when there are visible clones in the Petri dish, stop the culture. The supernatant was discarded and washed carefully with PBS twice. Add 4% paraformaldehyde to fix the cells with 5 mL and fix for 15 min. Then remove the fixative solution, add appropriate amount of GIMSA and apply the dye solution to dye for 10-30 min, and then slowly wash off the dye solution with running water. Turn the plate upside down and superimpose a transparent film with a grid. Count the clones directly with the naked eye, or count the clones with more than 10 cells on a microscope (low power). Finally, the clone formation rate was calculated with the following equation.
Cell migration
Twenty-four hours before the experiment, the cells of different groups were replaced with serum-free medium, and the culture was continued. Before inoculation, soak the 24-well plate and transwell chamber with 1 × PBS for 5 min to moisten the chamber. Digest the cells, wash the cells with serum-free medium, resuspended the cells in serum-free medium, count the cells and dilute to adjust the cell density to 5 × 105/mL. Inoculate 0.2 mL cell suspension (5 × 104 cells) into the transwell chamber, and then add 0.7 mL of RPMI-1640 medium containing 10% FBS to the lower 24-well plate, 3 replicate holes per group, and place them in a 37°C incubator for 24 h to terminate the culture. Add 1 mL of 4% formaldehyde solution to each well of the above cells, and fix them at room temperature for 10 min. Aspirate the fixative solution, wash once with 1 × PBS. Add 1 mL 0.5% crystal violet solution to each well, wash with 1 × PBS three times after dyeing for 30 min. Use a cotton swab to carefully wipe off the cells that have not migrated in the transwell, place them under a 200× microscope, and count the number of cells in each field of view.
Matrigel angiogenesis
Prepare matrigel and add it to the lower hole of the ibidi angiogenesis slide. Put the matrigel glue into the incubator to make it coagulate. Prepare a cell suspension with a density of 2 × 105 cells/mL, and add 50 μL of the cell suspension to the solidified ibidi angiogenesis slide. According to the growth rate of the cells, images are collected periodically for observation.
Western blot analysis
Collect tumor cells from each group, and add 200 mL of cell lysate to each six-well plate. After sonication, the cells were lysed on ice for 1 h. The lysed cell sample was centrifuged at 12,500 rpm for 15 min at 4°C. Then, transfer the supernatant in the centrifuge tube to a clean centrifuge tube. BCA protein quantification kit was used to quantify protein concentration. The measured protein samples were stored at -80°C. In Western blot electrophoresis, the protein loading concentration was 50 μg per well. After SDS-PAGE electrophoresis, the membrane was transferred and blocked. HIF-1α, VEGFA, and GAPDH (1: 500, anti-human, Abcam, USA) primary antibody were diluted to use concentration. The samples were incubated overnight on a shaker at 4°C. After washing with PBS, the samples were incubated with the secondary antibody (1: 1000, anti-human, Abcam, USA) for 30 min at room temperature in the dark. Finally, the developer was used for development and photography.
Results
Cell cloning experiment
Only cells that have both adherent and proliferative viability can form clones. As can be seen from figure 1, compared to the NC group, the number of cell clones in the other four groups is significantly reduced. The trend of the number of cell clones in the four groups of the experimental group is that Hy-2 group is larger than Hy-1 group, which indicated that cell hypoxia will negatively affect cell adhesion and proliferation activity. Hy-1 group is larger than Hy-2 + si-HIF-1α group, and Hy-1 + si-HIF-1α group is the least one. This showed that under hypoxic conditions, the inhibition of the expression of HIF-1α will make the situation worse, indicating that HIF-1α will increase cell adhesion and proliferation under hypoxia.
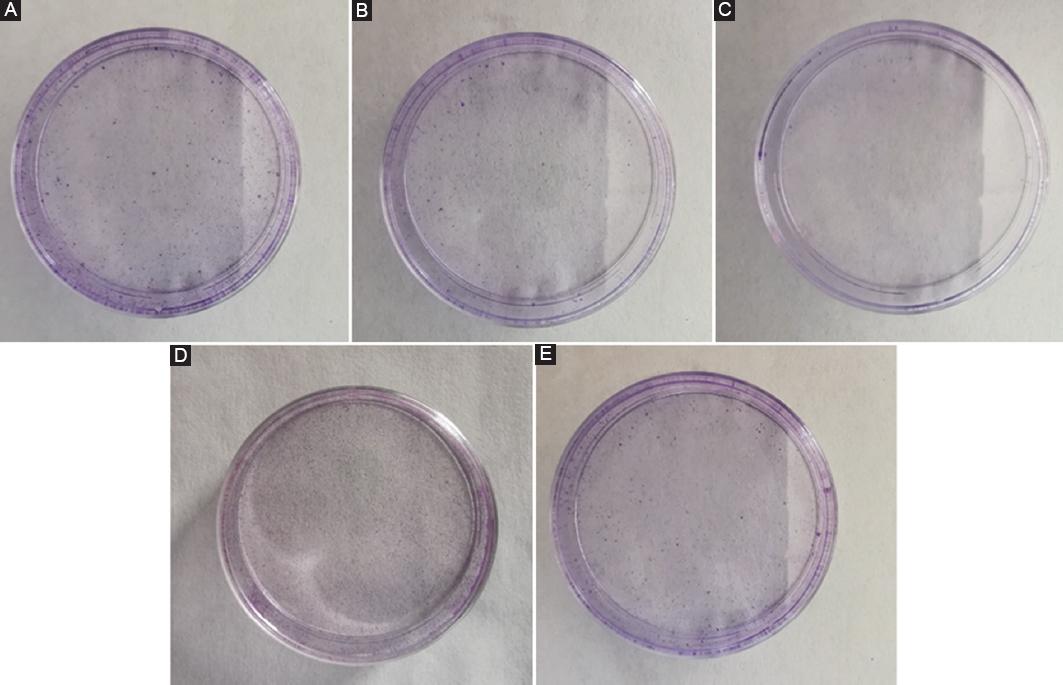
Figure 1 The results of cell cloning experiment. The result of normal control (NC) group is equivalent to that of healthy people. A: NC group. B: Hypoxia group-1 (Hy-1) group. C: Hypoxia group-2 (Hy-2) group. D: Hy-1 + small interfering hypoxia-inducible factor 1α (si-HIF-1α) group. E: Hy-2 + si-HIF-1α group. F: Statistical results of cell colony forming efficiency. The symbol * means p < 0.05, ** means p < 0.01 (compared to the NC group).
Matrigel angiogenesis experiment
Tumor cells themselves can secrete a variety of growth factors and induce angiogenesis. Angiogenesis plays an important role in the development and metastasis of tumors. The results shown in figure 2 demonstrated that the angiogenesis was obvious in the NC group, but not in the other groups. This shows that hypoxia has a negative impact on the angiogenesis of tumor cells. HIF-1α also participates in the regulation of this process.
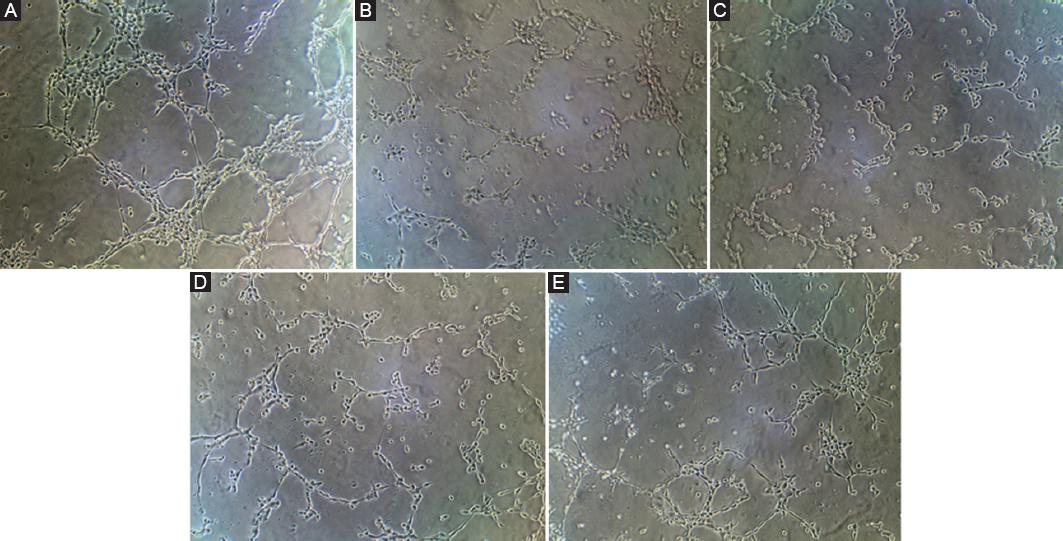
Figure 2 The results of matrigel angiogenesis experiment. The result of normal control (NC) group is equivalent to that of healthy people. A: NC group. B: Hypoxia group-1 (Hy-1) group. C: Hypoxia group-2 (Hy-2) group. D: Hy-1 + si-HIF-1α group. E: Hy-2 + si-HIF-1α group. F: Statistical results of angiogenesis number. The symbol * means p < 0.05, ** means p < 0.01 (compared to the NC group).
Cell scratch test
The cell scratch method is one of the simple and easy methods to detect the movement characteristics of tumor cells. It draws on the in vitro cell wound healing experimental model, in vitro cultured monolayer cells, scratches and wounds, and then adds drugs or overexpression or interference with its gene expression to observe its ability to inhibit tumor cell migration. It is shown from figure 3 that the cancer cell migration ability of the NC group is relatively strong, while the other four experimental groups are relatively weak. But relatively speaking, the migration ability is that Hy-2 group is more evident than Hy-1 group, which indicated that the lower the oxygen partial pressure, the weaker the migration ability of cancer cells.Hy-1 group is better than Hy-2 + si-HIF-1α group, and Hy-1 + si-HIF-1α group is the weakest one. This shows that HIF-1α gene will further inhibit the migration ability of cancer cells on the basis of low oxygen partial pressure.
Cell invasion test
The results of the transwell experiment are shown in figure 4. The NC group has strong invasive ability, while the other four groups have weak invasive ability. But for the remaining four groups, the tendency of invasion ability is that Hy-2 group is more evident than Hy-1 group, which showed that low partial pressure of oxygen reduces the invasion ability of cancer cells. Hy-1 group is better than Hy-2 + si-HIF-1α group, and Hy-1 + si-HIF-1α group is the weakest one. This indicated that the presence of the HIF-1α gene will further reduce the invasion ability of cancer cells on the basis of low oxygen partial pressure.
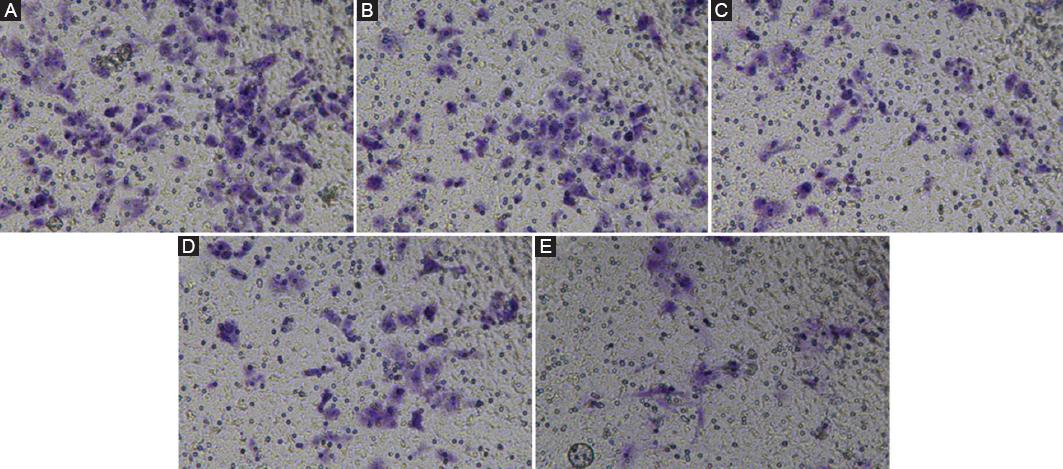
Figure 4 The results of cell invasion test. The result of normal control (NC) group is equivalent to that of healthy people. A: NC group. B: Hypoxia group-1 (Hy-1) group. C: Hypoxia group-2 (Hy-2) group. D: Hy-1 + si-HIF-1α group. E: Hy-2 + si-HIF-1α group. F: Statistical results of cell migration number. The symbol * means p < 0.05, ** means p < 0.01 (compared to the NC group).
Western blot
Figure 5 shows the up-regulation of HIF-1α expression in Hy-2 group and Hy-1 group relative to NC group. It can be seen that the hypoxic environment can cause the overexpression of HIF-1α. The HIF-1α expression of Hy-2 + si-HIF-1α group and Hy-1 + si-HIF-1α group was downregulated, indicating that the transfection of si-HIF-1α effectively inhibited the expression of HIF-1α. The results in figure 5 also show that, relative to the NC group, the expression of VEGFA in Hy-2 group and Hy-1 group also increased, in Hy-2 + si-HIF-1α group, and Hy-1 + si-HIF-1α group, the expression of VEGFA is significantly reduced. The trend of VEGFA is consistent with HIF-1α, so HIF-1α can target and regulate gene VEGFA to promote tumor cell proliferation, migration, invasion, and aniogenesis.
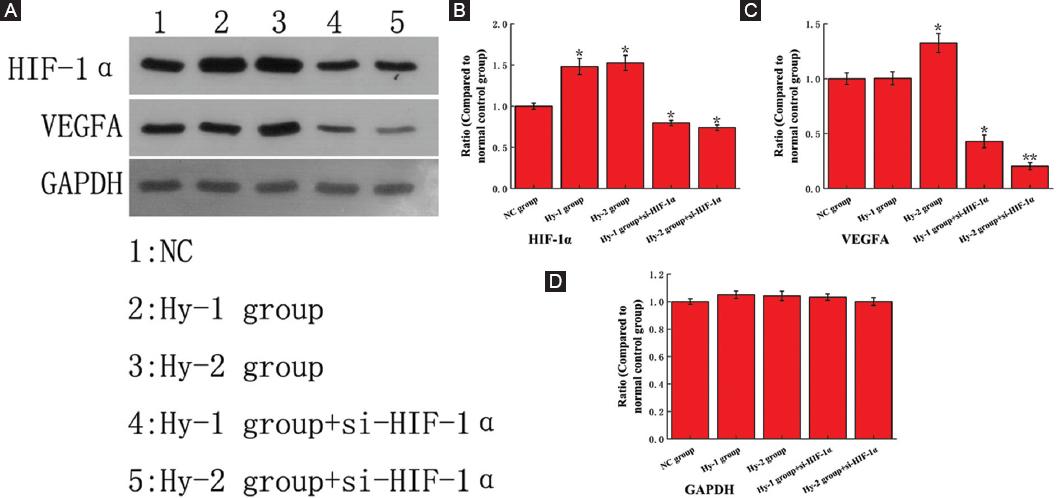
Figure 5 The Western blot analysis results of protein hypoxia-inducible factor 1α (HIF-1α), vascular endothelial growth factor A (VEGFA) and glyceraldehyde phosphate dehydrogenase (GAPDH). The data of normal control (NC) group are all equivalent to that of healthy people. A: Original gel electrophoresis image. B-D: The ratio of protein HIF-1α, VEGFA and GAPDH expression compared to NC group. The symbol * means p < 0.05, ** means p < 0.01 (compared to the NC group).
Discussion
Bladder cancer is a malignant tumor that occurs in the bladder mucosa. It is the most common malignant tumor of the urinary system and one of the ten most common tumors in the whole body. Symptoms include blood in the urine, painful urination and low back pain. The most common type of bladder cancer is transitional cell carcinoma. Other types include squamous cell carcinoma and adenocarcinoma12. Diagnosis is usually made by cystoscopy with tissue biopsy. The stage of cancer depends on transurethral resection and medical imaging. Treatment depends on the stage of the cancer and may include some combination of surgery, radiation therapy, chemotherapy, or immunotherapy. Surgical options may include transurethral resection, partial or complete bladder removal, or catheterization13. Our experiment has proved that HIF-1α gene can induce the recurrence and invasion of bladder cancer. Therefore, countermeasures to deal with HIF-1α should be considered during treatment.
HIF-1α is a subunit of the heterodimeric transcription factor hypoxia-inducible factor 1 (HIF-1) encoded by the HIF1A gene. It is a basic helix-loop-helix PAS domain that contains proteins and is considered to be the main transcriptional regulator of the hypoxia response of cells and development. The dysregulation and overexpression of HIF1A caused by hypoxia or genetic mutations is of great significance in cancer biology and many other pathophysiology, especially in angiogenesis and angiogenesis, energy metabolism, cell survival, and tumor invasion14,15. Two other alternative transcripts encoding different isoforms have been identified. The response mechanism of HIF-1α gene to intracellular oxygen partial pressure is still unclear, which can be a follow-up study of this project for more in-depth research.
VEGF-A is a dimeric glycoprotein that plays an important role in neurons and is considered to be the main and main inducer of blood vessel growth. VEGFA is essential for adults in organ remodeling and diseases involving blood vessels (such as wound healing, tumor angiogenesis, diabetic retinopathy, and age-related macular degeneration). During early vertebrate development, angiogenesis occurs, which means that the endothelium condenses into blood vessels. The differentiation of endothelial cells depends on the expression of VEGFA, if this expression is eliminated, it may lead to embryonic death. The result of alternative splicing is VEGFA produced by a set of three main isoforms. If any three isoforms (VEGFA120, VEGFA164, and VEGFA188) are produced, then this will not cause vascular malformations and intact VEGFA in mice. The death of gene knockout. VEGFA is very important in the role of neurons, because they also require vascular supply, and abolishing the expression of VEGFA in neural progenitor cells will lead to defects in cerebral vascularization and neuronal apoptosis16. Anti-VEGFA therapy can be used to treat patients with poor angiogenesis and vascular leakage in cancer and ocular diseases, but can also lead to inhibition of neurogenesis and neuroprotection17. VEGFA can be used to treat patients suffering from neurodegenerative diseases and neurological diseases, and can also increase vascular permeability, thereby terminating the blood-brain barrier and increasing inflammatory cell infiltration. Although this project has identified that HIF-1α gene can target and regulate VEGFA to promote tumor cell proliferation, migration, invasion, neovascularization and lymph node metastasis, the mediation mechanism is not fully understood, and further research is needed.
Early experimental results have shown that the effect of HIF-1α may affect the expression of VEGFA, thereby affecting some tumor behaviors18-20. The results of this experiment finally show that HIF-1α can target the regulatory gene VEGFA to promote tumor cell proliferation, migration, invasion, new blood vessel formation, and lymph node metastasis, which provide new references and alternative strategies for the diagnosis and treatment of bladder cancer in the future.











 text new page (beta)
text new page (beta)




