Introduction
Salivary gland neoplasms constitute approximately 1% of all tumors1. Pleomorphic adenoma is the most common benign tumor of the salivary glands, comprising 70% of all cases, which are often found in the parotid gland (80%), with 10% each in the submandibular and minor salivary glands2,3. Two European nationwide studies from Denmark and the Netherlands on the demographic investigation of pleomorphic adenomas have been reported. The annual incidence ranges from 0.2 to 4.9/100,000 person-year, with the median age of diagnosis being 50-60 years4,5. Women present at an older age than men, with painless swelling as the main chief complaint. In a report from Danish4, the recurrence rate was 2.7% and 3.3% of cases had malignant transformation. The Netherlands study5 reported a recurrence rate over 20 years of 6.7% and an overall risk of 0.15% of malignant invasion. In a recent study by 2020, Valstar et al.6 found that the first recurrence of pleomorphic adenoma was approximately 3.1%, of which 6.2% were malignant. The differences in recurrence and malignant change may result from the variable inclusion criteria adopted and the estimated risk cannot be compared with the data from a single center. Recurrent pleomorphic adenoma is associated with a risk of multiple distant metastases and may transform into carcinoma ex pleomorphic adenoma, the fourth most common malignant salivary tumor7. Consequently, determining the factors that influence recurrence and providing the correct treatment strategies are important to enhance prognosis.
Recent studies have suggested that the recurrence of pleomorphic adenoma may be related to an imbalance of matrix metalloproteinases (MMPs) and tissue inhibitors (TIMPs). Overexpression of MMP leads to excessive breakdown of the extracellular matrix. This occurs in many pathological conditions, such as cancer invasion and metastasis, arteriosclerosis or angiogenesis, and fibrosis8-10. However, some studies have revealed that TIMPs may increase the probability of malignant changes11,12. According to the model of cell-mediated MMP-2 activation, pro-MMP-2 secreted by fibroblasts binds to TIMP-2 and forms a complex with membrane-type-1 MMP on the cell surface. Therefore, the hypothesis of an imbalance between MMP and TIMP for the recurrence of pleomorphic adenoma is not firmly accepted.
Some pathologists believe that the capsular characteristics of pleomorphic adenomas influence the recurrence rate. Capsular characteristics include completeness of the capsule, formation of satellite nodules or pseudopodia, rupture of the tumor, and positive resection margins2,13,14. Multinodularity is generally found in pleomorphic adenoma after recurrence15,16. According to a study by Naeim et al.17, nodularity is due to infiltration of the stroma, leading to low cellularity. TIMP was saturated within the stroma, which implies a more invasive cellular property18. This study aimed to investigate the relationship between cellularity and the capsular characteristics of pleomorphic adenoma and its influence on operative considerations.
Materials and methods
Case selection
Cases were collected from May 2008 to April 2015 from the Pathological Department of the Hospital. This study followed the Declaration of Helsinki on medical protocol and ethics, and the regional ethical review board approved the study. All patients underwent excision, superficial parotidectomy, or total parotidectomy. Post-operative pathological reports confirmed a pleomorphic adenoma at the histological level. Cases with incomplete data were analyzed using a pairwise deletion. To observe cellularity and capsular characteristics under the microscope, specimens that were broken into several pieces or incomplete pathological sections were excluded from the analysis. In addition, immunohistochemistry of the cell proliferation index, Ki-67, was performed to investigate the invasive immunoreactivity among the groups.
Clinical data and characteristics
The recorded data included patient age, sex, location, chief complaints, duration of tumor presence, and type of surgery. Regarding location, pleomorphic adenoma was found in the parotid gland, submandibular gland, minor gland, and neck. The chief complaint was swelling accompanied by pain or painless swelling. Tumor size was calculated by multiplying the two largest measurements of the length, width, and height. The patients were divided into three groups based on tumor size: (1) <4 cm2, (2) 4-16 cm2, and (3) >16 cm2. The duration of tumor presence was grouped as <½ year, ½-3 years, 3-5 years, and more than 5 years according to the study of Suh et al.2. Regarding the type of surgery, excision, superficial parotidectomy, or total parotidectomy were performed according to the surgeon’s discretion.
Specimen preparation
Specimens were fixed in 10% formalin. Before dissecting the specimens for further processing, the surface was inked with four different dyes to mark the resection planes. The specimens were then dipped into Bouin solution (dye fixative) for 30 s before slicing in the direction perpendicular to the long axis of the nodule into 3 mm thick segments. Both ends of the tumor poles were sectioned horizontally along the long axis of the tumor to obtain transverse cuts of the capsule of the tumor vertex. The number of segments differed depending on the tumor size. Gradient concentrations of alcohol and xylene were applied to the tissue segments for dehydration before the segments were embedded in paraffin and stained with hematoxylin and eosin. Finally, the segments were cut to a thickness of 6 μm.
Cellularity grouping
The cellularity was categorized into four groups according to Seifert’s classification19 1976 (Fig. 1). The classic type includes a balanced amount of epithelial cells and stroma, while the myxoid type comprises abundant myxoid ground substance with interspersed spindle and stellate cells. The cellular type is composed of a predominance of ductal and solid trabecular structures and little intervening myxoid stroma, sometimes similar to monomorphic adenoma, when the epithelial component is uniformly differentiated. The cellularity was independently determined by two oral pathologists. In instances of equivocality, cellularity was assessed by a third oral pathologist.
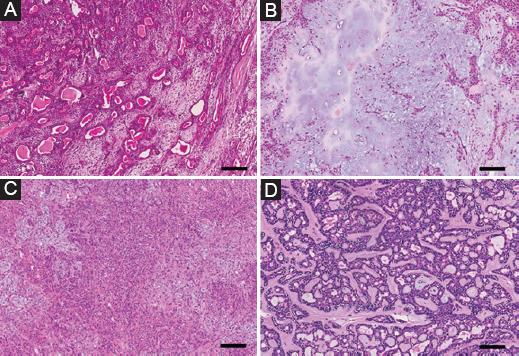
Figure 1 Cellularity of pleomorphic adenomas according to Seifert’s classification in 1976. A: classic type with a balanced amount of epithelial cells and stroma. B: myxoid type with abundant myxoid ground substance, interspersed spindle, and stellate cells. C: cellular type with a predominance of ductal and solid trabecular structures and little intervening myxoid stroma. D: cellular type with more uniformly differentiated cells like monomorphic adenoma (H&E stain, × 100, scale bar = 100 μm).
Capsular characteristics
Capsular characteristics included the completeness of the capsule, the existence of pseudopodia and satellite nodules, and a positive or negative resection margin (Fig. 2). The cellularity and capsular characteristics were observed using an inverted microscope (Olympus BX 53, Olympus Corporation, Shinjuku Monolith, 3-1, Nishi Shinjuku 2-chrome, Shinjuku-Ku, Tokyo, Japan). The observed images were then scanned using a slide scanner (ScanScope® CS, Aperio Technologies, Inc., USA) for analysis.
Immunohistochemistry of cell proliferation index: Ki-67
Paraffin-embedded tissues were sectioned (3 μm) on glass slides and coated with 2% 3-aminopropyltriethylsilane (Sigma Chemicals, St. Louis, MO, USA). The sections were deparaffinized by immersion in xylene, dried in alcohol, and incubated with 3% hydrogen peroxide for 40 min. The sections were immersed for antigen retrieval in citrate buffer (pH = 6.0) for 30 min at 95°C and blocked at room temperature for 20 min with 3% normal goat serum. The slides were then incubated overnight with the primary antibody (Ki-67, clone MM1, Novocastra, Newcastle, UK) at a 1:100 dilution at 4°C in a humidified chamber. After washing in Tris-buffered saline, the sections were treated with labeled streptavidin–biotin–peroxidase kits (K0492, DAKO, Denmark) and incubated in 3.3’-diaminobenzidine in a chromogen solution (DAKO, K3468) for 2-5 min. Finally, the sections were stained with Mayer’s hematoxylin and covered. Negative controls were obtained by excluding the primary antibody and substituting it with 1% PBS-BSA.
Immunoreactivity was evaluated at ×40 for at least 1000 cells on one pathological slide. The percentage of positive tumor cells was recorded and categorized by the semi-quantitative method20 as follows: (−) negative ≤ 5%, (+) low 6-25%, (++) moderate 26-50%, and (+++) high >50% of positive tumor cells.
Statistical analysis
The concordance between the two oral pathologists was 93.3%. The Krippendorff’s a value from the inter-rater comparison analysis was 0.89. All p-values were based on two-tailed statistical analyses. Pairwise deletion was used to handle missing data. Statistical analysis was performed using Fisher’s exact test for categorical variables and one-way ANOVA for continuous variables. Fisher’s exact test was applied because small samples were analyzed and more than 20% of cells had frequencies <5, which was inadequate to apply approximation with the Chi-squared test. Subgroup analyses were performed using paired comparisons. Statistical significance was set at p < 0.05. Microsoft Excel 2010 (Microsoft Corporation) was used for all the statistical calculations.
Results
Case selection
A total of 89 patients were enrolled in the study with two cases of malignant transformation. Clinical data and cellularity were observed. Five cases were excluded because they were broken, so the cellularity could not be determined. The remaining 84 cases were analyzed with pairwise deletions due to missing clinical information of 22 cases (Fig. 3). Regarding capsular characteristics, 11 were ruled because of incomplete pathological sections that could not be evaluated. Finally, 73 cases were preserved for analysis of the correlation between cellularity and capsular characteristics.
Association between clinicopathological factors and cellularity
There was no difference among the three groups in terms of age (p = 0.09), sex (p = 0.09), location (p = 0.75), size (p = 0.57) of the tumor, chief complaints (p = 0.63), or type of surgery (p = 0.24) (Table 1). The duration of tumor presence was associated with cellularity (p = 0.01). The significance resulted from the difference in duration between types 2 and 3 after subgroup analysis. In the group with <½ year, 13 of the 23 tumors were classic subtype (56.5%), two of the 13 tumors were myxoid subtype (15.4%), and 21 of the 41 tumors were cellular subtype (51.2%). In the group of tumors that existed for ½ - 3 years, seven of the 23 tumors were classic subtype (30.4%), three of the 13 tumors were myxoid subtype (23.1%), and 15 of the 41 tumors were cellular subtype (36.6%). In the group of tumors aged 3-5 years, two of the 23 tumors were classic subtype (8.7%), five of the 13 tumors were myxoid subtype (38.5%), and two of the 41 tumors were cellular subtype (4.9%). In the group of tumors over 5 years, one of the 23 tumors was classic subtype (4.3%), three of the 13 tumors were myxoid subtype (23.1%), and three of the 41 tumors were cellular subtype (7.3%).
Table 1 Clinicopathological analysis of patients with different cellularity
| Characteristics | No. of patients (%) | p* | ||
|---|---|---|---|---|
| Classic type | Myxoid type | Cellular type | ||
| Age, years | 0.09 | |||
| Median | 35 | 47 | 43.5 | |
| Range | 23-68 | 25-76 | 13-70 | |
| Sex | 0.09 | |||
| Male | 10 (38.5) | 5 (33.3) | 26 (60.5) | |
| Female | 16 (61.5) | 10 (66.7) | 17 (39.5) | |
| Location | 0.75 | |||
| Parotid gland | 16 (66.7) | 8 (61.5) | 19 (47.5) | |
| Submandibular gland | 4 (16.7) | 3 (23.1) | 9 (22.5) | |
| Minor gland | 2 (8.3) | 2 (15.4) | 7 (17.5) | |
| Neck | 2 (8.3) | 0 (0.0) | 5 (12.5) | |
| Chief complaint | 0.63 | |||
| Painless swelling | 18 (81.8) | 12 (92.3) | 34 (89.5) | |
| Pain and swelling | 4 (18.2) | 1 (7.7) | 4 (10.5) | |
| Duration of presence, year | 0.01 | |||
| ≤ 0.5 | 13 (56.5) | 2 (15.4) | 21 (51.2) | |
| 0.5-3 | 7 (30.4) | 3 (23.1) | 15 (36.6) | |
| 3-5 | 2 (8.7) | 5 (38.5) | 2 (4.9) | |
| ≥ 5 | 1 (4.3) | 3 (23.1) | 3 (7.3) | |
| Size, cm2 | 0.57 | |||
| ≤ 4 | 10 (41.7) | 3 (21.4) | 15 (40.5) | |
| 4-16 | 11 (45.8) | 8 (57.1) | 19 (51.4) | |
| ≥ 16 | 3 (12.5) | 3 (21.4) | 3 (8.1) | |
| Type of surgery | 0.24 | |||
| Excision | 16 (76.2) | 8 (66.7) | 28 (80.0) | |
| Superficial parotidectomy | 4 (19.0) | 2 (16.7) | 7 (20.0) | |
| Total parotidectomy | 1 (4.8) | 2 (16.7) | 0 (0.0) | |
*Categorical and continuous variables were analyzed using Fisher’s exact test and one-way ANOVA, respectively. Pairwise deletion was used to minimize loss during clinical data collection.
Capsular characteristics of the entire study and association with cellularity
Of the 73 patients, 14 (19.2%) had incomplete capsules. Regarding the histological subtype, an incomplete capsule was observed in seven of the 24 classic subtype tumors (29.2%), four of the 12 myxoid subtype tumors (33.3%), and three of the 37 cellular subtype tumors (8.1%) (Table 2). The incomplete capsule was significantly associated with histological subtype (p = 0.03). The relative risk of an incomplete capsule was 4.1-fold when comparing myxoid to cellular subtype, 1.1-fold for myxoid to classic subtype, and 3.6-fold for classic to cellular subtype. Satellite nodules were observed in 44 patients (60.3%). Fifteen of the 24 classic subtype tumors (62.5%), eight of 12 myxoid subtype tumors (66.7%), and 21 of the 37 cellular subtype tumors (56.8%) had satellite nodules. The presence of satellite nodules was not significantly associated with the histological subtype (p = 0.85). A positive resection margin was observed in 15 patients (20.5%). Six of the 24 classic subtype tumors (25.0%), two of 12 myxoid subtype tumors (16.7%), and seven of the 37 cellular subtype tumors (18.9%) had positive resection margins. The positive resection margin showed a statistically insignificant association with cellularity (p = 0.86).
Table 2 Relationship of cellularity and capsular characteristics (n = 73)
| Characteristics | No. of patients (%) | p* | ||
|---|---|---|---|---|
| Classic type (n = 24) | Myxoid type (n = 12) | Cellular type (n = 37) | ||
| Incomplete capsule | 0.03 | |||
| Absent | 17 (70.8) | 8 (66.7) | 34 (91.9) | |
| Present | 7 (29.2) | 4 (33.3) | 3 (8.1) | |
| Satellite nodule | 0.85 | |||
| Absent | 9 (37.5) | 4 (33.3) | 16 (43.2) | |
| Present | 15 (62.5) | 8 (66.7) | 21 (56.8) | |
| Resection margin | 0.86 | |||
| Negative | 18 (75.0) | 10 (83.3) | 30 (81.1) | |
| Positive | 6 (25.0) | 2 (16.7) | 7 (18.9) | |
*The variables were analyzed using Fisher’s exact test.
Percentage of cases distributed by staining scores of Ki-67
The marker Ki-67 showed scarce staining in most cases of pleomorphic adenoma, with negative or weak staining scores (p = 0.12) (Table 3). In the negative group, 19 of the 24 tumors were classic subtype (79.2%), eight of the 12 tumors were myxoid subtype (66.7%), and 34 of the 37 tumors were cellular subtype (91.9%). In the low group, four of the 24 tumors were classic subtype (16.7%), three of the 12 tumors were myxoid subtype (25.0%), and three of the 37 tumors were cellular subtype (8.1%). In the moderate group, one of the 24 tumors was classic subtype (4.2%), one of the 12 tumors was myxoid subtype (8.3%), and none was a cellular subtype. Although there was no difference in immunoreactivity among groups, we found that the Ki-67 of satellite nodules was relatively higher than that of the surrounding tissues (Fig. 4).
Table 3 Percentage of cases distributed by staining scores of Ki-67 (n = 73)
| Score | No. of patients (%) | p* | ||
|---|---|---|---|---|
| Classic type (n = 24) | Myxoid type (n = 12) | Cellular type (n = 37) | ||
| Negative (≤5%) | 19 (79.2) | 8 (66.7) | 34 (91.9) | 0.12 |
| Low (6-25%) | 4 (16.7) | 3 (25.0) | 3 (8.1) | |
| Moderate (26-50%) | 1 (4.2) | 1 (8.3) | 0 (0.0) | |
| High (>50%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
*The variables were analyzed using Fisher’s exact test.
Discussion
Pleomorphic adenomas are composed of myoepithelial and ductal cells arranged into different architectures, which intermingle with the mucoid, myxoid, and chondroid stroma4. With increasing duration of tumor presence, the stroma secreted by myoepithelial cells tends to increase, resulting in decreased cellularity and unique structural capsular characteristics. Capsular characteristics are believed to be related to recurrence and malignant changes in pleomorphic adenomas.
Satellite nodules, also termed daughter nodules, are specific capsular characteristics. Based on the study by Naeim et al.17 in 1976, the formation of satellite nodules was considered as capsular ingrowth. He proposed that capsular ingrowth was due to the infiltration of many small buds. With increasing stroma, the buds became small lobules, resulting in multinodularity. Some studies have revealed that the presence of satellite nodules may indicate recurrence of pleomorphic adenoma. In a retrospective study, Park et al.13 found that satellite nodules were observed in six of the 10 recurrent patients (60.0%) but in only 10 of the 100 non-recurrent patients (10.0%); therefore, the formation of satellite nodules is a risk factor for recurrence. However, Zbären and Stauffer and Paris et al.21,22 have opposing opinions. Paris et al.22 indicated that the stroma tended to be more abundant when the tumor existed for a longer duration, and cell islands were separated from the main tumor. Satellite nodules could not be a risk factor for recurrence, but were a reasonable phenomenon. Savera23 also proposed that since abundant TIMP is found in satellite nodules, the risk of recurrence risk would be reduced.
A positive resection margin is also supposed to be a capsular characteristic related to recurrence. In the early stages, surgeons prefer surgery with a broad safe margin to avoid any remaining capsule in the tissues. Some studies have shown that the recurrence of pleomorphic adenoma enucleation is 10-45% but 2-5% when superficial parotidectomy is performed24,25. Total parotidectomy or wide excision with neck dissection is rarely performed because of facial nerve paresthesia. The probability of paresthesia in patients after superficial parotidectomy is 61.7%26 while other studies have suggested that the resection margin is not a prognostic factor for recurrence. A negative respective margin does not guarantee that recurrence would not occur. Suh et al.2 found that the recurrence rate of pleomorphic adenoma was 21.4% even with a clear resection margin. In cases of malignant transformation, the margin of 80% of the cases was initially negative. Therefore, close follow-up is needed, even when a safe resection margin is achieved.
Another explanation for recurrence is the altered regulation of genes in the molecular aspects. Overexpression of MFAP4, DST, SLC35, and KCTD15 genes compared to normal tissues, and TP53 mutations are probably related to the tumor progression of pleomorphic adenoma27. MFAP4 is an extracellular matrix protein that mediates cell-to-matrix interactions. It seems to influence the overexpression of MMPs and their TIMPs in the stroma. DST is a junction protein of the plakin family and functions as an anchor unit for intermediate neural filaments to the actin cytoskeleton in epithelium or muscle tissue. The role of DST in tumor development remains unclear. Leick et al.28 reported that DST was overexpressed in human melanoma cell lines, but Shimbo et al.29 found that DST was downregulated in metastatic prostate cancer and invasive ductal cell carcinoma. SLC35 encodes nucleotide sugar transporters in the Golgi apparatus and endoplasmic reticulum. SLC35A2 mRNA was reported to be significantly increased in malignant colon cancer tissues, and was related to hematogenous metastasis30. The KCTD15 gene encodes a potassium channel tetramerization domain associated with obesity. The overexpression of KCTD15 in pleomorphic adenoma could suppress the Wnt/beta-catenin pathway, which is related to the regulation of cell proliferation and epithelial-mesenchymal transition27. TP53 mutations are also related to malignant transformation of pleomorphic adenoma. The identified mutations p.R248Q and p.Y220C are known to exert a dominant-negative effect on the complete inactivation of TP5331. Loss of TP53 function is the most common molecular initiation and progression in human malignancies. In addition to the genes described above, many genes (beta-catenin, calponin, EGF, EGFR, ErbB-2, FAS, FGF-2, p63, PDGF, REG I, TGF beta-1, and WT1) have been previously explored in the pathogenesis of pleomorphic adenoma32. If the mechanism of these genes can be found, we can expect a better prognosis in the treatment.
In this study, lower cellularity was related to a higher probability of incomplete capsule formation, implying the spilling out of tumor cells to the surrounding tissues. In such circumstances, a wider surgical margin can be considered to avoid recurrence. Since the duration of follow-up was <5 years, we could not evaluate the different histological subtypes between the recurrent and non-recurrent groups. The current surgical preference is excision, not enucleation and as recurrence has decreased over the past few years, the small sample size is not ideal. To further investigate the role of cellularity in prognosis, earlier cases could be traced in a future study.











 text new page (beta)
text new page (beta)