Introduction
Surgical resection is the only treatment option that can offer long-lasting survival in patients with colorectal liver metastases (CRLMs)1. Modern chemotherapeutic agents have led to an increase of resectability in those patients with initially unresectable CRLM2. However, the advantages of the neoadjuvant treatment may be hampered by the side effects of chemotherapy on the non-tumoral liver. Different macroscopic and histopathological entities related to the use of these drugs have been documented. Oxaliplatin-based regimens are associated with hepatic sinusoidal congestion (“blue liver”), caused by the rupture of the sinusoidal membrane and the collagenization of perisinusoidal space3, while various degrees of liver steatosis and steatohepatitis (“yellow liver”) may follow irinotecan-based schemes4,5.
Sinusoidal obstructive syndrome (SOS) and chemotherapy-associated steatohepatitis (CASH) have been associated with a higher post-operative mortality, morbidity, and in-hospital stay after major hepatectomy6,7.
An adequate pre-operative evaluation of the liver functional reserve and, hypothetically, of parenchymal injury, should be the cornerstone of prevention from the risk of post-operative liver-related complications. Indocyanine green (ICG) retention rate at 15 min (ICG-R15) is a validated test of hepatic function, useful to calculate the security volume threshold before hepatic resection in patients with underlying cirrhosis8,9 and in other different settings10.
The primary goal of this study is to study the relationship between pre-operative ICG-R15 and the severity of chemotherapy-associated liver injury (CALI). The secondary objective is to investigate the predictive value of pre-operative variables on the development of post-operative complications (POCs).
Materials and methods
Patient’s selection
For the purposes of this study, we selected 69 patients out of our prospectively maintained database of consecutive patients who underwent curative resection for CRLM at our hospital over a 7-year period, on the basis of the following selection criteria: (1) availability of sufficient non-tumoral liver parenchyma for pathological analysis; (2) preoperatively recorded ICG test values; (3) no chronic underlying liver disease, and (4) known data about pre-operative chemotherapy (adjuvant post-colectomy and/or neoadjuvant before hepatic surgery). We discarded patients submitted to synchronic liver and bowel surgery (who could show morbidity not related to the liver procedure) and with previous portal embolization (who could present parenchymal and vascular alterations not related to chemotherapy). The study was conducted according to the principles of the Declaration of Helsinki and was approved by the local ethics committee.
Pre-operative chemotherapy
Chemotherapy protocols were simplified into three groups: (1) oxaliplatin group (who received FOLFOX); (2) irinotecan group (who received FOLFIRI); and (3) sequential group (patients who switched from FOLFOX to FOLFIRI or vice versa). In about two-thirds of the patients, a monoclonal antibody (cetuximab or bevacizumab) was included in the chemotherapeutic regimen. Clinical decision-making of each case went throughout a weekly discussion at the Multidisciplinary Committee of our Hospital devoted to digestive cancer.
Pre-operative planning
Number, size, and location of lesions together with liver vascular inflow and outflow were evaluated with a pre-operative computed tomography (CT) scan performed 4-6 weeks before surgery; a magnetic resonance was realized in all the cases of ill-defined lesions. A 3D liver reconstruction and a volumetric study based on the pre-operative CT were performed. In major hepatectomies, a future liver remnant volume of at least 30% of the total liver volume was considered adequate.
From serum aspartate aminotransferase (AST) level and platelet count recorded before surgery, we calculated the AST-to-platelet ratio index (APRI) score11, to be correlated with pathologic findings.
ICG test was performed the day before surgery, injecting intravenously a bolus of 0.5 mg/kg of body weight of the fluorescent dye ICG (ICG-PULSION, Germany) and recording the retention rate at 15 min (ICG-R15 as a percentage) during the hepatic clearance time by means of pulse spectrophotometry (PULSION Medical System, Germany). An impaired ICG clearance did not preclude the performance of major resections (≥ 3 Couinaud segments). However, whenever feasible, parenchymal sparing resections were preferred, trying to assure an oncological tumor-free margin of at least 1 mm12.
Surgical procedure
In all patients, we performed a thoroughly intraoperative restaging ultrasound (US) (MyLab™70 XVG, Esaote Platform, Italy). A contrast-enhanced US (SonoVue, Bracco, Italy) was realized in the cases of doubtful or isoechoic lesions.
Liver transection was generally realized with the complementary use of Cavitron US surgical aspirator (CUSA, Tyco Healthcare, USA) and LigaSure (Covidien, UK). Low central venous pressure (< 4 mmHg) was maintained during the transection phase to minimize venous bleeding; intermittent pedicle clamping (Pringle maneuver) was used on demand. Low-intensity radiofrequency (TissueLink, Medical Inc., USA) was used to cauterize the parenchymal transection surface.
Pathological analysis
Histopathological injury in the non-tumoral liver parenchyma was assessed reviewing archival pathological specimens (previously formalin-fixed, paraffin-embedded, and stained with hematoxylin/eosin and Masson’s trichrome), selecting non-neoplastic areas distant from the tumor. All the specimens were reviewed independently by two pathologists (I.P. and E.S.) who were unaware of any clinical data related to pre-operative chemotherapy.
SOS (Fig. 1A) was punctuated using the pathological score reported by Rubbia-Brandt et al.3 and considered pathological when it reached a Grade 2 or 3 over 3. The presence of nodular regenerative hyperplasia (NRH) (Fig. 1B) and centrilobular vein fibrosis (CVF) (Fig. 1C) was also assessed13. CASH (Fig. 1D) was evaluated by means of the non-alcoholic fatty liver disease (NAFLD) activity score, as reported by Kleiner et al.14, and classified as pathological with a score of 4 or superior. To simplify the analysis, CALI was considered to be present if the non-tumoral parenchyma showed at least a pathological SOS, a pathological NAFLD, or the association of CVF and NRH.

Figure 1 A:low-power examination of the liver reveals sinusoidal dilatation of centrilobular and mediolobular areas. B: at ×4 is shown nodular hyperplasia areas delimited by portal tracts and atrophic hepatocytes. C: high-power examination reveals fibrous tissue (arrow) around centrilobular vein. D: low-power photomicrograph shows severe macrovesicular steatosis. (A, B, and D on hematoxylin and eosin stain; C on Trichrome Masson stain).
Post-operative evaluation
Post-operative morbidity was classified using the Clavien-Dindo scale15, and major complications were classified as Clavien-Dindo III-V. An abdominal contrast-enhanced CT was realized in every case of suspected surgical complication. Post-hepatectomy liver failure (PHLF) was defined using the “50-50” criteria (prothrombin time < 50% and total bilirubin 50 micromol/L at post-operative day 5)16. Bile leakage was defined as bile-stained liquid in the abdominal drainage at any moment after hepatectomy, after percutaneous puncture, or found during relaparotomy. Post-operative mortality was considered in the 90 days following surgery.
Statistical analysis
The statistical analysis was performed using SPSS software (version 25.0, IBM, USA). To compare variables between groups, Student’s t, two-tailed Mann–Whitney, Chi-square, and Fisher’s exact tests were used when appropriate. All predictors with p < 0.10 by univariate analysis were considered in the multivariate model. A multivariate logistic regression analysis was performed to identify independent factors. Prediction accuracy was evaluated with the area under the receiver operating characteristic curve. p < 0.05 was considered to indicate a statistically significant difference.
Results
Patient’s characteristics
The clinicopathological characteristic and surgical procedures of the entire cohort are listed in table 1. Of the 69 patients analyzed, 58.6% were male and 41.4% were female. Mean age was 59.6 (± 12.1) years. Liver metastasis was synchronous with the primary tumor in 63.8% of cases, and patients presented an average of 4.9 (± 5.5) liver nodules. Fifty-nine patients (85.5%) received FOLFOX, 2 patients (2.9%) received FOLFIRI, and 8 patients (11.6%) received both regimens sequentially. A median of nine chemotherapy cycles (interquartile range 5.0-14.0) were delivered in each patient with a median interval before surgery of 6.4 weeks (interquartile range 5.0-11.6 weeks). Major resections represented 47.8% of the total procedures, minor resections (1 or 2 Couinaud segments) 37.7%, and non-anatomical wedge resections represented 14.5%. Radiofrequency ablation (RFA) was associated to liver resection in 19 cases (27.5%).
Table 1 Patient’s characteristics
| Characteristic | Value |
|---|---|
| Total no. of patients | 69 |
| Sex (male/female) | 40/29 |
| Age, years | 59.6 (12.1) |
| Synchronous metastases (%) | 44 (63.8) |
| Extrahepatic disease (%) | 14 (20.3) |
| No. of liver metastases | 4.9 (5.5) |
| Pre-operative CEA ≥ 10 ng/ml (%) | 20 (29.0) |
| Maximum size of metastases, mm | 32.2 (20.0) |
| Chemotherapeutic agents | |
| Oxaliplatin group (%) | 59 (85.5) |
| Irinotecan group (%) | 2 (2.9) |
| Sequential group (oxaliplatin and irinotecan) (%) | 8 (11.6) |
| Use of bevacizumab (%) | 35 (50.7) |
| Use of cetuximab (%) | 15 (21.7) |
| No. of chemotherapy cycles, median (IQR) | 9.0 (5.0-14.0) |
| Chemotherapy washout period, weeks, median (IQR) | 6.4 (5.0-11.6) |
| Type of hepatic resection | |
| Major resection (e 3 Couinaud segments) (%) | 33 (47.8) |
| Minor resection (1 or 2 Couinaud segments) (%) | 26 (37.7) |
| Wedge resection/s (%) | 10 (14.5) |
| Associated RFA (%) | 19 (27.5) |
Data are expressed as mean (standard deviation), median (interquartile range, IQR), or number (percentage), when indicated
CEA: carcinoembryonic antigen; RFA: radiofrequency ablation.
Pathological analysis and prediction of CALI
Pathological analysis of non-tumoral liver detected CALI in 44 liver specimens (63.8%), while the remaining 25 (36.2%) showed a normal parenchyma or did not fulfill the criteria of CALI. The subtypes of pathological injuries described are shown in figure 2.
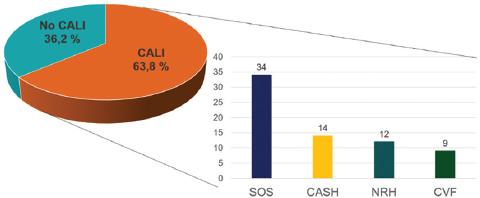
Figure 2 Proportion of patients with and without CALI. In the histogram, individual pathological features and their frequencies are specified. CALI: chemotherapy-induced liver injury; CASH: chemotherapy-associated steatohepatitis; CVF: centrilobular vein fibrosis; NRH: nodular regenerative hyperplasia; SOS: sinusoidal obstructive syndrome.
Patients with CALI were older and of male sex in a higher proportion compared to patients without CALI, although not significantly (p = 0.071 and p = 0.076, respectively) (Table 2). The presence of dyslipidemia was strongly associated with the development of CALI (p = 0.033), which, in turn, showed a dose-dependent onset, being significantly more frequent after 12 cycles of chemotherapy (p = 0.049).
Table 2 Comparison of pre-operative characteristic between patients with and without chemotherapy-associated liver injury (CALI)
| Pre-operative characteristics | Non-CALI (n = 25) | CALI (n = 44) | p |
|---|---|---|---|
| Age, years | 56.2 (11.2) | 61.6 (12.2) | 0.071 |
| Age ≥ 65 years (%) | 8 (32.0) | 21 (47.7) | 0.203 |
| Male sex (%) | 11 (44.0) | 29 (65.9) | 0.076 |
| Diabetes (%) | 2 (8.0) | 2 (4.5) | 0.460 |
| Dyslipidemia (%) | 2 (8.0) | 13 (29.5) | 0.033 |
| BMI ≥ 28 kg/m2 (%) | 3 (12.0) | 5 (11.4) | 0.611 |
| Alkaline phosphatase, U/L | 104.6 (37.4) | 98.4 (40.5) | 0.543 |
| Alanine aminotransferase, U/L | 27.1 (11.4) | 30.0 (27.4) | 0.619 |
| Aspartate aminotransferase, U/L | 30.3 (14.7) | 30.0 (21.7) | 0.950 |
| ICG-PDR, %/min | 19.3 (6.2) | 17.7 (4.2) | 0.203 |
| ICG-R15, % | 7.5 (4.8) | 8.9 (5.0) | 0.267 |
| ICG-R15 ≥ 10% (%) | 7 (28.0) | 16 (36.4) | 0.479 |
| Platelet count (103/mm3) | 198.1 (48.4) | 209.3 (75.3) | 0.505 |
| APRI score | 0.52 (.27) | 0.51 (.33) | 0.879 |
| No. of chemotherapy cycles | 9.6 (8.2) | 11.2 (7.2) | 0.426 |
| ≥ 0.426hemotherapy cycles (%) | 10 (40.0) | 27 (61.4) | 0.087 |
| ≥ 12 chemotherapy cycles (%) | 6 (24.0) | 21 (47.7) | 0.049 |
| Chemotherapy washout period, weeks | 10.7 (14.3) | 15.6 (19.7) | 0.292 |
| Use of both oxaliplatin and irinotecan (%) | 4 (16.0) | 4 (9.1) | 0.312 |
| Use of irinotecan (%) | 4 (16.0) | 6 (13.6) | 0.525 |
| Use of bevacizumab (%) | 11 (44.0) | 24 (54.5) | 0.400 |
| Use of cetuximab (%) | 7 (28.0) | 8 (18.2) | 0.342 |
Data are expressed as mean (standard deviation), or number (percentage), when indicated. P values refer to Student’s t or two-tailed Mann–Whitney for continuous variables and Chi-square or Fisher’s exact tests for categorical variables
CALI: chemotherapy-associated liver injury; BMI: body mass index; ICG-PDR: indocyanine green plasma disappearance rate; APRI: aspartate aminotransferase-to-platelet ratio index; ICG-R15: indocyanine green retention rate at 15 min.
None of the pre-operative routine laboratory tests (aminotransferases, alkaline phosphatase, and platelet count) reflected the presence of CALI. APRI score was similar between patients with and without CALI (p = 0.879). ICG-R15 showed a trend to be more pathological in patients with CALI (8.9 ± 5.0%), compared to patients without CALI (7.5 ± 4.8%) but without statistical significance (p = 0.267).
Considering separately each pathological injury described, a significant relationship could be found between ICG-R15 and the presence of CVF, but only when the pathological cutoff was set at 16% (Table 3).
Table 3 Comparison of pathological features of chemotherapy liver toxicity and patients with two cutoffs (at 10% and at 16%) of indocyanine green retention rate (ICG-R15)
| Pathological injury | ICG-R15 < 10% (n = 46) | ICG-R15 ≥ 10% (n = 23) | p | ICG-R15 < 16% (n = 62) | ICG-R15 ≥ 16% (n = 7) | p |
|---|---|---|---|---|---|---|
| Sinusoidal obstructive syndrome (Grade 2 o 3) (%) | 23 (50.0) | 11 (47.8) | 0.865 | 31 (50.0) | 3 (42.9) | 0.517 |
| Centrilobular vein fibrosis (%) | 4 (8.7) | 5 (21.7) | 0.129 | 6 (9.7) | 3 (42.9) | 0.042 |
| Nodular regenerative hyperplasia (%) | 7 (15.2) | 5 (21.7) | 0.500 | 9 (14.5) | 3 (42.9) | 0.095 |
| CASH (NAFLD activity score ≥ 4) (%) | 8 (17.4) | 6 (26.1) | 0.397 | 12 (19.4) | 2 (28.6) | 0.436 |
Data are expressed as number (percentage). P values refer to Chi-square or Fisher’s exact tests
CASH: chemotherapy-associated steatohepatitis; NAFLD: non-alcoholic fatty liver disease; ICG-R15: indocyanine green retention rate at 15 min.
CALI, ICG-R15, and post-operative outcomes
Patients with CALI experienced a higher incidence of severe POC (25.0%), compared to the group without CALI (4.0%, p = 0.024), being the two groups comparable with respect to tumoral burden, proportion of major resections, association with RFA, and use of laparoscopic technique (Table 4). Furthermore, in-hospital stay was significantly longer in patients with CALI (a mean of 11.0 ± 10.1 days vs. 7.3 ± 4.4 days in patients without liver injury, p = 0.039).
Table 4 Comparison of intraoperative and post-operative outcomes in patients with and without CALI and between those with a normal versus ICG-R15
| Characteristic | Non-CALI (n = 25) | CALI (n = 44) | p | ICG R15 < 10% (n = 46) | ICG R15 < 10% (n = 23) | p |
|---|---|---|---|---|---|---|
| Tumor maximum diameter, mm | 28.4 (16.5) | 34.3 (21.6) | 0.241 | 31.8 (21.7) | 33.0 (16.5) | 0.817 |
| No. of metastases | 6.2 (7.1) | 4.1 (4.2) | 0.116 | 4.7 (5.7) | 5.2 (5.1) | 0.702 |
| Major resections ( ≥ 3 Couinaud segments) (%) | 11 (44.0) | 22 (50.0) | 0.632 | 20 (43.5) | 13 (56.5) | 0.307 |
| Laparoscopy (%) | 1 (4.0) | 6 (13,6) | 0.199 | 5 (10.9) | 2 (8.7) | 0.571 |
| Pringle maneuver, minutes | 18.7 (9.4) | 17.9 (11.7) | 0.879 | 17.3 (10.1) | 21.5 (11.3) | 0.490 |
| Associated RFA (%) | 9 (36.0) | 10 (22.7) | 0.235 | 12 (26.1) | 7 (30.4) | 0.703 |
| Minor complications (Clavien-Dindo I-II) (%) | 5 (20.0) | 6 (13.6) | 0.488 | 6 (13.0) | 5 (21.7) | 0.352 |
| Severe complications (Clavien-Dindo III-V) (%) | 1 (4.0) | 11 (25.0) | 0.024 | 5 (10.9) | 7 (30.4) | 0.043 |
| Bile leakage (%) | 2 (8.0) | 4 (9.1) | 0.625 | 4 (8.7) | 2 (8.7) | 0.686 |
| Post-operative mortality (90 days) (%) | 0 (0) | 1 (2.3) | 0.453 | 0 (0) | 1 (4.3) | 0.333 |
| Liver failure (%) | ||||||
| All resections (n = 69) | 2 (8.0) | 5 (11.4) | 0.501 | 3 (6.5) | 4 (14.4) | 0.161 |
| Major resections (n = 33) | 2 (18.2) | 4 (18.2) | 0.671 | 2 (10.0) | 4 (30.8) | 0.147 |
| RBC transfusion (%) | 2 (8.0) | 7 (13.6) | 0.294 | 3 (6.5) | 6 (26.1) | 0.032 |
| In-hospital stay, days | 7.3 (4.4) | 11 (10.1) | 0.039 | 8.9 (9.1) | 11.3 (7.2) | 0.279 |
Characteristics related to tumoral burden and surgical technique are also compared. P values refer to Student’s t or two-tailed Mann–Whitney for continuous variables and Chi-square or Fisher’s exact tests for categorical variables. Data are expressed as mean (standard deviation) or number (percentage), when indicated
CALI: chemotherapy-associated liver injury; ICG-R15: pathological indocyanine green retention rate; RFA: radiofrequency ablation; RBC: red blood cell.
Mortality, bile leakage, red blood cell (RBC) transfusion rate, and incidence of PHLF were not different between patients with and without CALI. Patients with a pathological ICG-R15 showed higher incidence of severe POC (p = 0.043) and a higher rate of perioperative RBC transfusion (p = 0.032) compared to those with a normal ICG-R15 (Table 4). In a sub-analysis of the 33 major liver resections realized, PHLF developed in four patients among 13 with ICG-R15 ≥ 10% (30.8%) and only in two out of the 20 with ICG-R15 < 10% (10.0%, p = 0.147). Meanwhile, no significant differences in the liver tumor status and operative procedure were seen between these two groups.
Prediction of major POC
Among all the pre-operative and surgical variables analyzed, only a pathological ICG-R15 (≥ 10%) was found to be an independent predictor of severe POC, including death (OR = 4.075, 95% C.I.: 1.077-15.422, p = 0.039, AUC 0.738) (Table 5). Use of RFA (vs. non-use) seemed to be a protective factor against POC (p = 0.094 at univariate analysis) and was included in the multivariate model, but did not show significance as an independent factor (p = 0.106).
Table 5 Univariate and multivariate analysis of the influence of pre-operative factors and surgical technique on the development of severe post-operative complications and mortality (Dindo-Clavien Grades III-V) in the study cohort (n = 69)
| Variables | Univariate analysis (p) | Odds ratio | 95% CI | p |
|---|---|---|---|---|
| Age ≥ 65 years | 0.161 | - | - | - |
| Male sex | 0.368 | - | - | - |
| Dyslipidemia | 0.715 | - | - | - |
| Diabetes | 0.137 | - | - | - |
| BMI ≥ 28 kg/m2 | 0.137 | - | - | - |
| Synchronic metastases (vs. metachronic) | 0.294 | - | - | - |
| Four or more nodules | 0.803 | - | - | - |
| More than 12 chemotherapy cycles | 0.121 | - | - | - |
| Tumor major diameter ≥ 30 mm | 0.178 | - | - | - |
| APRI score > 0.5 | 0.200 | - | - | - |
| ICG-R15 ≥ 10% | 0.043 | 4.075 | 1.077- 15.422 | 0.039 |
| Major hepatectomy | 0.131 | - | - | - |
| Non-use versus use of RFA | 0.094 | 0.167 | 0.019- 1.463 | 0.106 |
| Open surgery (versus laparoscopy) | 0.649 | - | - | - |
APRI: aspartate aminotransferase-to-platelet ratio index; BMI: body mass index;
RFA: radiofrequency ablation.
Discussion
CALI may play an important role in the morbidity and mortality after hepatic resection for CRLM. Since the first description of SOS by Rubbia-Brandt et al.3, additional features of morphological change in non-tumoral liver due to oxaliplatin have been documented, such as centrilobular and perisinusoidal fibrosis, peliosis, and nodular hyperplasia13. Subsequently, other authors pointed out the usefulness of semi-quantitative grading of anti-CD34 antibodies (a marker of sinusoidal capillarization)17, and of nuclear proliferation markers18 as measurable hallmarks of CALI.
Irinotecan-induced steatohepatitis has been associated with higher post-operative mortality7, while the presence of SOS can be the cause of higher morbidity19, transfusion rate20, and liver dysfunction (ascites and liver failure)21. Despite other studies could not show a clear association between CALI and a worse post-operative outcome22,23, a recent review and meta-analysis that include eight retrospective Eastern and Western publications, gathering more than 700 patients24, strongly suggest that sinusoidal dilatation and steatohepatitis are associated with severe post-operative morbidity and liver dysfunction, respectively.
In this context, it seems to be very useful to have a non-invasive diagnostic tool to predict the grade of CALI to better select and prepare especially those patients requiring a major hepatectomy. ICG clearance determination is a trustful bedside test largely employed, especially in Eastern countries, to assess hepatic functional reserve in patients with known liver disease8,9. ICG is an anionic dye which, following intravenous injection, almost completely binds to plasma proteins with no extravascular distribution. Its elimination is a carrier mediated process through the biliary canaliculi and no enterohepatic circulation takes place25. ICG clearance reduction in cirrhosis may be explicated by the reduction of hepatic blood flow and by a reduced uptake of the dye from the sinusoids to the hepatocytes, as a consequence of microvascular intrahepatic changes (portovenous shunts or capillarization of sinusoidal space)26. A decade ago, Krieger et al. documented the influence of chemotherapy for CRLM on ICG clearance value27 but these data were not confirmed by Wakiya et al.28 Recently, also, Wang et al.29 pointed out the relation between pre-operative ICG-R15 value and the use of chemotherapy, although in their work, no histological study of CALI was performed on liver specimens.
In our series of 69 patients, a significant relationship could be found between ICG-R15 and the presence of CVF, which is a late-onset oxaliplatin-related injury, but only when the cutoff was set at 16%. SOS, CASH, and NRH could not be efficiently detected by ICG clearance test.
Interestingly, the presence of dyslipidemia seemed to be a predisposing factor for the development of CALI. An altered free fatty acid and cholesterol metabolism and storage, as seen in dyslipidemia, could boost the toxic effect of 5-fluorouracil (a component of both FOLFOX and FOLFIRI schemes) on hepatocyte mitochondria30, and possibly contribute to a pro-inflammatory environment leading to steatohepatitis.
The present study shows, on the one hand, the high prevalence of CALI (almost 64%) in patients submitted to chemotherapy for CRLM and, on the other, confirms the notion that CALI is associated with a higher risk of post-operative severe complications and prolonged hospital stay.
Surprisingly, although ICG-R15 was only marginally correlated to the severity of CALI, it showed to be the best predictor of severe POC. Furthermore, perioperative RBC transfusion rate was significantly higher in patients with an impaired ICG-R15.
In our usual practice for CRLM, we do not consider a pathological ICG-R15 as a contraindication to perform major resections. In a sub-analysis performed considering exclusively major hepatectomies, we found a trend toward a higher incidence of liver failure among patients with a pathological ICG-R15 compared to those with a normal one, but without statistical significance.
These data should be interpreted with caution due to the limited number of events in these groups and a possible problem of underpower, but suggest that parenchymal sparing procedures and a meticulous technical execution should be advisable in patients with a pre-operative ICG-R15 ≥ 10%.
In a retrospective series of 161 patients with CRLM who received pre-operative chemotherapy, the group of Makuuchi31 performed 37 major hepatectomies in patients with an ICG-R15 ≥ 10%, although theoretically assuring at least 60% of the future liver remnant volume by means of portal vein embolization. In this subgroup of patients with marginal liver functional reserve, the authors observed significantly poorer blood test values associated with liver dysfunction, higher amount of blood loss, and significantly higher total morbidity.
We acknowledge some limitations of our study, mainly caused by the retrospective design: first, the rather wide variability between the last chemotherapy cycle and surgery. This could be explained by the fact that more than one-third of our patients presented liver metachronic disease and, for many of them, the last chemotherapy cycle dated back to the end of adjuvant treatment after colonic surgery. It has been documented that, after 2-4 weeks of chemotherapy cessation, ICG values improve gradually32, while histological injury may persist months afterward33.
Another relative drawback is due to the fragmentation of pathological features of chemotherapy toxicity that corresponds to various and often unrelated histological changes, dependent on specific drugs. The synthesis of these features in a single variable (CALI) is arbitrary but, we believe, useful to interpret the impact of liver injury in a clinical setting.











 text new page (beta)
text new page (beta)


