Introduction
On March 11, 2020, coronavirus disease 2019 (COVID-19) was declared pandemic by the World Health Organization, and the sudden increase of cases that needed hospital admission collapsed health systems. The high incidence of colorectal cancer places these patients as a group of special impact when faced with the impossibility of offering surgical treatment in a timely manner. Therapeutic delay has prognostic implications in colorectal cancer. In this context, prioritization and selection criteria had to be described to sort out patients for surgery selecting those patients with lower surgical risk and higher oncological priority for their priority scheduling.
Moreover, the scientific community was already concerned about the increased risk of morbidity-mortality due to COVID19 among oncologic patients and in those who had been operated on in the weeks before they developed symptoms. During the past few months, a multitude of protocols have been developed for the detection of COVID19 preoperatively (thoracic computed tomography [CT], severe acute respiratory syndrome coronavirus 2 [SARS-COV2]-polymerase chain reaction [PCR], both). Although it seems that preoperative PCR is the method of choice for screening asymptomatic COVID-19 patients, there are screening alternatives in case there is a lack of availability of SARS COV 2 PCR for this indication. It is important to know whether colorectal cancer surgery could have a worse outcome among those patients who are operated on during the COVID-19 incubation period.
Due to the possibility of future pandemic outbreaks or saturation of the health-care system, we believe it is important to know the results of perioperative multimodal management and patient selection in colorectal cancer in this context. Proper patient selection can help prioritize those patients with the lower surgical risk and more advanced tumor stage preoperatively, helping surgical planning when resources are limited.
The main objective of our study was to assess the prioritization capacity of patients with advanced colorectal cancer using a patient selection scale (PSS). As a secondary goal, we describe the results in terms of pre-operative and post-operative suspected and confirmed COVID infections during the period of highest incidence in the Community of Madrid using two different pre-operative protocols. We also present the short-term outcome results, morbimortality, hospital stay, and Enhanced Recovery After Surgery (ERAS) protocol compliance during this period.
Methods
We have carried out a prospective descriptive study that includes 69 colorectal cancer patients operated on from March 16 to June 3, 2020. We also carried out a comparative study between two periods of time to know the usefulness of PSS to detect patients with more advanced disease.
Around 300 patients pending oncological surgery were referred to our hospital, a monographic cancer center, to alleviate the saturation of the Madrid health-care system. Among Gastrointestinal Oncology patients, most of them had colorectal cancer, almost all diagnosed between 1 and 2 months before. Due to the structure of our hospital and the number of operating rooms and surgeons available, we were not able to operate on all of them immediately.
At the time we started to attend these patients, Surgical Oncology and Colorectal scientific societies had not published their recommendations yet so we developed our own criteria for selecting and prioritizing patients for surgery (table 1). Our goal was to detect preoperatively those patients with more advanced colorectal tumors and less surgical risk in order to schedule their surgical intervention with priority, ahead of those patients who could have less damage with the delay of surgery. The prioritization scale was created based on the experience of colorectal oncology surgeons, taking into account the parameters that could most influence the appearance of complications and long hospital stay as well as those clinical parameters that could make us suspect advanced or long-diagnosed and untreated oncological disease. Once the variables to be included were selected, they were provided with an increasing score for the creation of the scale. Taking into account that this scale was designed during the moment of highest incidence and highest saturation of the Madrid health system due to the COVID 19 pandemic, this prioritization algorithm could not be validated before its use nor designed with a statistical mathematical basis. The main objective of this simple scale is to have a tool that may be used in the preoperative consultation and that in a quick and objective way allows us to choose which patients should be treated first.
Table 1 Selection criteria for colorectal cancer surgery in COVID-19 pandemic
| - Polyps with adenoma | Defer 2-3 months |
| - Adenocarcinoma in resected polyps | |
| - Symptomatic local adenocarcinoma | Immediate Surgery |
| - Asymptomatic local adenocarcinoma | Surgery as soon as possible (stenotic first) |
| - Rectum T1 | Endoanal or defer. Avoid TaMIS |
| - Mid/low rectum T2 or higher | Neoadyuvant RT short course |
| Surgery up to 12 weeks later | |
| - Rectum postneoadjuvant | Surgery up to 12 weeks later |
| - Metastatic resectable | Neoadjuvant chemotherapy |
| - Metastatic unresectable | Chemotherapy under Oncologist criteria |
| - Metastatic postneoadyuvant | Continue chemo versus surgery up to 8 weeks |
| - Peritoneal carcinomatosis | Chemotherapy. Avoid HIPEC or PIPAC |
The left column summarizes common clinical situations for therapeutic decision making in colorectal cancer. In the column on the right, the changes or recommendations for treatment given by the major scientific societies in relation to the COVID19 pandemic. Those tumors that could be postponed or that have an alternative oncological treatment should be evaluated individually, in order to allow the prioritization of those pathologies that require non-deferrable surgical treatment.
The patient prioritization algorithm was used from April 6 to June 3. The first 2 weeks we operated on patients who had been diagnosed at our clinics and had been scheduled for surgery previously without the need of prioritizing patients with the same diagnosis and select the most urgent patients. Thereupon, the massive referral of oncological patients led to a saturation of our operating room availability, forcing us to prioritize. We developed a PSS to sort out patients with similar diagnoses (Fig. 1). First, we selected patients with obstructive or stenosing tumors with symptoms, then stenosing tumors without symptoms and after that non-stenosing tumors. We used the PSS to sort cases among patients of each group, to prioritize those patients with the worst oncological prognosis or risk of tumoral complications, along with the lower surgical risk. Those patients with lower score were scheduled before. Two periods were defined: first from April 6 to May 2, and second period thereafter. Two time periods were defined to compare patients, tumor characteristics complications and hospital stay to assess the capability of our patient selection algorithm to detect patients with more advanced tumors and lower surgical risk, who should be scheduled earlier. Qualitative variables were expressed in percentage and compared using Pearson's Chi-square test. Quantitative variables were expressed as mean or median and range. Statistical analysis comparing two periods of time was done with SPSS program.

Figure 1 Algorithm for prioritizing and scheduling patients. The Patient Selection Scale (PSS) was used to sort out patients with similar diagnoses.
In Madrid, 4.165 COVID-19 cases had been declared by March 16 and this figure rose to 69.685 cases by June 3. Since it was a pandemic infection, all patients had to be considered as potentially infected. During a first period (March 16 until April 15), screening for COVID-19 was done with clinical interview and chest X-ray due to the lack of availability of preoperative SARS-COV-2 PCR for asymptomatic patients. Patients were clinically assessed before surgery and were double-screened, first in the attending surgeon's visit and secondly in the pre-anesthesia visit. In case of X-ray suspicion in asymptomatic patient, a chest CT-scan was done. In case of clinical symptoms or close contact with a confirmed case, surgery was deferred at least 2 weeks, and assessment and image were repeated. In a second period (from April 15), due to the availability of preoperative SARS-COV-2 PCR, the screening protocol was modified and PCR 48 h before surgery was included in the study. We examined the asymptomatic patients detected preoperatively and the suspected COVID-19 infections during hospitalization and follow-up over these two periods.
Our center is an 87-bed hospital including 12 intensive care unit (ICU) posts and three operating rooms. During the period of the study, the number of COVID-19 patients admitted to our COVID-19 isolation ward ranged from 3 to 20, and three were admitted to the ICU. Extraordinary measures were taken to reduce possible transmissions between patients and health personnel. A double circuit was established in the hospital, isolating the COVID-19 hospitalization ward from the post-surgical hospitalization wards. Patients were required to be strictly isolated at home before surgery and to be accompanied by the same family member during the hospital stay.
Regarding surgery, minimally invasive surgery was indicated in all feasible cases and patients were pre-habilitated according to our hospital's ERAS protocol. During the weeks with the highest incidence of COVID in the Community of Madrid and the worst saturation of our surgical waiting list, open surgery was prioritized to attempt to reduce the time of anesthesia and surgery as well as the risk of transmission to healthcare professionals. All procedures were performed by 4 GI Senior Surgeons.
As for the measures taken in the operating room, patients were monitored and anesthetized by a team wearing an IPE suit, and intubation was performed with a video laryngoscope, avoiding manual ventilation, as well as the use of laryngeal masks and devices that could aerosolize particles. As for the possible aerosolization secondary to laparoscopy, it was handled with closed system aspiration after the end of the surgery. Both post-operative management and discharge criteria did not differ from the usual ones.
Pre-operative, surgical technique, and post-operative related variables were recorded and analyzed. The Clavien-Dindo classification was used to categorize morbidity-mortality. Patients with chest X-ray or symptoms consistent with COVID-19 in the post-operative period were tested with SARS-CoV-2 PCR.
Follow-up was carried out in two phases: first in the outpatient clinic for post-discharge follow-up. Subsequently, a telephone follow-up was carried out to ensure the absence of complications secondary to possible SARS-CoV-2 infection during the hospital stay.
Comparisons were made with Pearson's Chi-squared test for qualitative variables and T student test for quantitative ones. Quantitative variables were expressed as mean (range) and categorical variables as frequency and percentage.
Results
Sventy-nine colorectal cancer patients were considered for surgery in the study period, all of them lived in areas with a high incidence of COVID19. Ten patients were rejected and returned to their referring hospitals because they presented deferrable/non-priority surgeries in the current context of pandemic, they had an alternative treatment or high risk of complications in case of COVID-19. The remaining 69 patients were accepted for surgery and prioritized, following our criteria, in the study period.
Among patients operated on during the first period, 39% had advanced disease (Stage ≥ IIIA) and 39% had positive lymph nodes, compared to 26% (p = 0.320) and 21% (p = 0.179) in the second period, respectively. Mean stay and morbidity were similar. The main differences between these two periods are shown in table 2.
Table 2 Results after the application of the patient prioritization algorithm and the selection scale
| First period | Second period | Statistics (p) | |
|---|---|---|---|
| Dates | 4/6/2020-5/1/2020 | 5/2/2020-6/3/2020 | |
| Number of patients | 28 (54.9%) | 23 (45.1%) | |
| Score (PSS) | Mean 7.4, median 7 | Mean 8.4, median 8 | 0.015 |
| Age | Mean 62.8, median 62 | Mean 68.5, median 70 | 0.070 |
| Hospital stay | Mean 8.4, median 5 | Mean 6.17, median 5 | 0.254 |
| Complications | 10 (35.7%) | 8 (34.7%) | 0.945 |
| Advanced tumors (≥III) | 11 (39.2%) | 6 (26%) | 0.320 |
| N+ | 11 (39.2%) | 5 (21.7%) | 0.179 |
The following table shows the differences in patient characteristics, tumor stage, post-operative complications and hospital stay in the 2 time periods into which the study has been divided. The comparison of frequencies was made by the Pearson Chi-square test.
With regard to COVID19 screening, we differentiate between two periods with different protocols application. In the first period, in which screening was performed without preoperative SARS-CoV-2 PCR, three patients were postponed due to COVID-19 symptoms (cough, anosmia), recent epidemiological contacts or suspicious chest X-ray. There was a clinical suspicion (fever, and X-ray signs) of infection during the postoperative period in six patients, with only 1 confirmed with a positive PCR for SARS-CoV-2, but he did not develop any symptoms and had a satisfactory outcome. As for the second period, in which the protocol included the performance of preoperative PCR, we found one patient confirmed asymptomatic preoperatively, and there was a post-operative suspicion with negative SARS-CoV-2 PCR. In neither of the two periods were any symptoms detected during follow-up, and there were no deaths or readmissions secondary to this disease (Fig. 2). Overall there were four pre-operative deferrals (5.7%) and seven suspected infections during the post-operative period (10.1%) due to respiratory symptoms, bacterial pneumonia, and a chronic obstructive pulmonary disease exacerbation. Therefore, there was only one patient positive for SARS-CoV-2 among 69 operated on (1.4%).
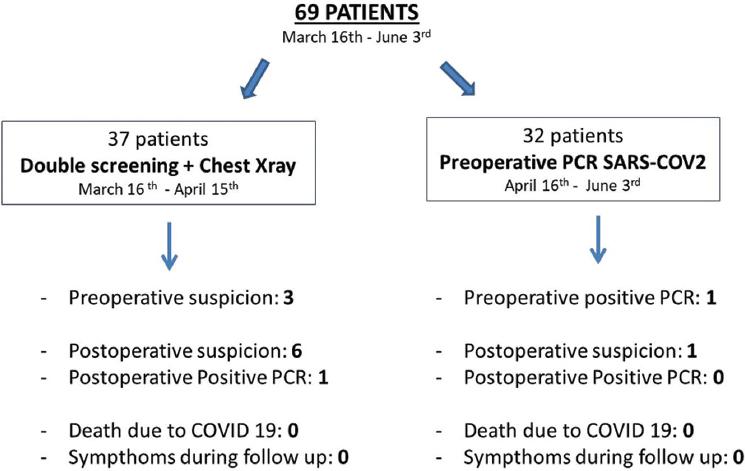
Figure 2 COVID19 preoperative screening and results. This figure represents the results according to the protocol used for preoperative COVID19 screening. Two periods are distinguished, both with a low incidence of preoperative COVID among the operated patients. Only one case was confirmed during the immediate postoperative period. There were no deaths or symptoms during follow-up.
Of the 69 patients operated on, we excluded two of them due to the diagnosis of peritoneal metastases at the beginning of the laparoscopy; the planned colonic resection procedure was cancelled and they were excluded from the results. Five out of the 67 patients (7.46%) who underwent surgery had two synchronous tumors (which meant that two resections were carried out simultaneously). Seventy-one colonic resections were performed on the remaining 67 patients. The main clinical aspects, surgical details, and information on complications and surgical stay are summarized in table 3.
Surgery was performed by minimally invasive approach in 53.6% of patients, the remaining 46.3% being done by open surgery. The stay in the Post Anesthesia Care Unit was 1 day per patient, with 1.4% of further readmissions due to surgical complications. 89.5% of patients completed ERAS protocol, with a median of 5 days of hospital stay (3-49). The hospital stay was extended in some patients due to difficulties in stoma management or perineal wound infections in cases of abdominoperineal resections. There was no mortality and overall morbidity was 35.8% (24 patients). There were four anastomotic leakages (5.9%) and three of them required reintervention (4.4%). About 19.4% of patients (13 of 67 patients with colonic resections) presented Clavien-Dindo I complications (5.9% of perineal infections and the remaining patients consisted of surgical wound infections). Seven percent patients developed Grade II complications (respiratory infection, and vascular catheter-associated infection) and 4.4% developed Grade III complications (three patients with anastomotic leakages with an onset as intra-abdominal collections). We only registered one patient (1.4%) with Grade IV complication due to multi-organ failure after evisceration and anastomotic dehiscence, requiring prolonged admission to ICU and hospitalization, with good subsequent outcome. Average clinic follow-up visit after discharge from hospital was at 8.45 days (4-25). Including subsequent post-discharge telephone contact, total follow-up was 45.7 days (17-86).
Five out of 67 patients (7.4%) went to the emergency department during the 1st post-operative week. Three patients (4.4%) were readmitted: two late anastomotic leakages and one patient on the 5th post-operative day after laparoscopic sigmoidectomy (having been discharged on the 3rd post-operative day), due to fever with neither symptoms nor X-ray signs of COVID-19.
Regarding pathological tumor staging, four patients presented Stage IV (5.7%), two of them known preoperatively (scheduled for colonic resection and simultaneous liver metastasectomy), and two patients with an incidental finding of carcinomatosis at the time of the initial surgical abdominal examination. Five patients presented non-invasive tumors (4 pTis, and one ypTis), representing 7.4% of the total. Metastatic regional node involvement was demonstrated in 32.8% of the operated patients. The rest of the anatomopathological details are reflected in table 3.
Discussion
In colorectal cancer, therapeutic delay, besides leading to a worse oncological prognosis, can lead to the appearance of locoregional complications with significant consequences for patients. 3-10 year survival is decreased in colorectal cancer if treatment is delayed more than 90 days from diagnosis1. Facing a scenario of saturation of the health system, it is mandatory to select those patients with higher risk of locoregional complications or worse oncological prognosis to prioritize their treatment against patients with less aggressive or advanced tumors, who could be deferred for some weeks.
COVID-19 pandemic has made us live a challenging unprecedented situation. Many surgeries were scheduled when the sudden need for mechanical ventilators forced hospitals to close operating rooms, leaving those patients waiting for surgery. Moreover, communications about the high risk of severe complications and worse outcomes in case of COVID-19 infection after surgery came up2 and Oncology Societies were advising about a higher mortality of COVID-19 among cancer patients3,4.
Therefore, in this context, eligible patients for surgery must be selected carefully due to the limits in hospital resources, risk for patients (SARS-CoV-2 infection and possible higher morbidity and mortality) and to avoid long surgeries and prolonged hospital stays in order to optimize the use of operating rooms, ICU and hospital beds5-8. The criteria we used for selecting patients were comparable to those published by Scientific Societies later9-12. We developed the PSS to help us prioritize patients with the same diagnosis for surgery13,14. To create that scale we considered the risk of developing tumor-related symptoms, the likelihood of tumor progression and its impact on survival15, the risk of morbidity-mortality related to surgery16-18 and the risk of a worse outcome in case of developing postoperative COVID-1919-22. The mean PSS was 8. It translates the urgent need of surgical treatment, the low surgical risk and the limited age of most of the patients. In fact, only patients older than 80 and/or ASA 3 can have a PSS higher than 10 among colorectal cancer patients.
As for comparing the results of the two periods of use of the prioritization algorithm, the average patient score was higher in the second period as was the mean age. Clinically, it seems interesting to observe how in the first period 39% of the patients demonstrated a tumoral stage higher than IIIA after the pathological report (although statistically not significant), with 39% of positive nodes in the lymphadenectomy performed, against 26% of advanced stage and 21% of pathological nodes found in the second period (Table 2). We find these differences interesting although they are not statistically significant probably due to the small sample size. The fact that we found tumors in more advanced stages in the first period indicates that our selection algorithm could allow us to identify these patients, without implying an increase in morbimortality. In terms of stay and complications, both have been similar. Statistically, the expected differences were not significant, possibly due to the small sample of both groups. We suggest the use of this algorithm and prioritization scale in studies with larger sample sizes.
During the study period, Madrid was one of the world's cities with a higher rate of COVID-19 among its population. In this setting, screening for COVID-19 with clinical interview and chest X-ray led to only 1.4% incidence of postoperative COVID-19. It is a low rate, probably explained by the low rate of patients with COVID-19 admitted to our center and the correct isolation of them, completely separated from surgical patients (23). Nowadays, most surgical guides recommend preoperative SARS-CoV-2 PCR and probably it is the best way to detect asymptomatic patients in the incubation period (24-26). In our case, although it is a small series of patients, pre-operative detection and postoperative infection data have been similar during the periods included in the different screening protocols (Fig. 2). Pre-operative careful screening for COVID-19 must be carried out and strict isolation of COVID-19 patients admitted to the hospital is needed if we want to keep these results. As the last condition could be impossible in some hospitals these days, given the overload of COVID-19 cases, other hospitals with a lower number of COVID-19 patients must be preserved as “clean-hospitals” and act as referral centers for oncological non-deferrable surgery. Larger studies would be needed to determine the cost benefit of preoperative performance of SARS-COV2 PCR.
Lei et al. published a 20.5% mortality among patients who develop COVID-19 shortly after surgery2. There was only one case of COVID-19 among our patients who was asymptomatic, so we cannot estimate and compare mortality among this group of patients.
Some authors have recommended open surgery to reduce the risk of viral transmission related to pneumoperitoneum evacuation24. On the other hand, some surgical scientific societies have recommended laparoscopy to diminish the contact with fluids27,28. Laparoscopic or robotic approach was our first preference, with safe evacuation of pneumoperitoneum through a closed system29. However, in order to optimize operating room utilization, we did not hesitate to convert to open surgery if we had difficulties or indicate open surgery from the beginning in case we predicted that laparoscopy would prolong surgical time. We also performed diverting ileostomy liberally to avoid complications which would result in a prolongation of the hospital length of stay.
Our results in terms of adherence to the ERAS protocol, surgical complications, fistula, postoperative stay and readmissions are comparable to series published before the pandemic30.
Conclusions
When faced with saturation of the health-care system, the use of algorithms and PSS can help select those cases that require a preference for surgical treatment over those that can be deferred. Although studies with larger numbers of patients would be needed, the use of rating scales to classify patients by oncological priority in colorectal cancer seems to identify these patients correctly preoperatively. In our series, given the low incidence of pre- and post-operative COVID-19, the performance of preoperative SARS-COV2 PCR does not appear to change the results in terms of surgical morbidity and mortality. Our results following colorectal cancer surgery are comparable with those published in no pandemic time.











 text new page (beta)
text new page (beta)


