Introduction
Acute portal vein thrombosis (PVT) is an infrequent entity, usually consequence of an oncological disease or liver cirrhosis. Occasionally, it may appear associated with hematological alterations, laparoscopic surgery, or local intra-abdominal inflammatory processes (pancreatitis, cholangitis, cholecystitis, etc.), especially when they reach a medium-high degree of severity.
During the last year, the entire world has dealt with the pandemic caused by coronavirus disease 2019 (COVID-19). Affected patients develop respiratory symptoms that in some cases progress to more severe and systemic disease. It has been observed that a significant proportion of them also develop different coagulopathies such as disseminated intravascular coagulation or thrombotic events, which worsen the course of the disease.
This is the case presentation of a patient without any risk factors, who developed acute PVT within the context of mild cholecystitis, and whose only relevant antecedent was past previously an asymptomatic COVID-19 infection. The aim of presenting it is to investigate the possible role COVID-19 infection could have on the development of acute PVT and if we should have special considerations in the follow-up and treatment of this kind of patient to avoid the appearance of thrombotic complications.
BRIEF clinical report
We present the case of a 51-year-old male patient with no previous history of interest. He came to the Emergency Department in our Hospital with mild abdominal pain in the right upper quadrant, nausea without vomiting, and no fever. The blood test showed slight leukocytosis (12,100 leuc/mm3), neutrophilia (74%), and C-reactive protein of 1.2 mg/dl. Liver, pancreatic, and biliary profile was normal.
An abdominal computed tomography (CT) was performed, showing gallbladder with no solid content, a hyper enhanced 4 mm wall, with slight trabeculation of the surrounding fat tissue, suggestive in the clinical context of incipient cholecystitis (Fig. 1).

Figure 1 First computed tomography scan of the patient. Incipient cholecystitis with hyper enhanced wall and slight trabeculation of the surrounding fat tissue.
With these findings and being our hospital in a critical situation, with limited beds and operating rooms because of the coronavirus pandemic, due to the good clinical situation of the patient, we decided treatment with ciprofloxacin and metronidazole at home to perform early cholecystectomy as soon as possible.
Follow-up was carried out in outpatient consultation at 7 days, with good evolution. The pain and accompanying symptoms had disappeared and the control test was rigorously normal. However, 4 days later, the patient returned to the emergency department for sudden onset abdominal pain in the right upper quadrant, general discomfort, and fever. In blood tests, he presented 21300 leuc/mm3, neutrophilia, CPR 5.5 mg/dl, GOT 577 U/l, GPT 472 U/l, GGT 749 U/l, and the rest at normal levels.
An abdominal CT was performed with evidence again of a minimal thickening of the gallbladder wall with no content inside, with few inflammatory signs around it, suggesting mild cholecystitis without changes comparing with the previous CT scan (Fig. 2A). However, the patient presented left PVT as a new appearance (Figs. 2B and C).
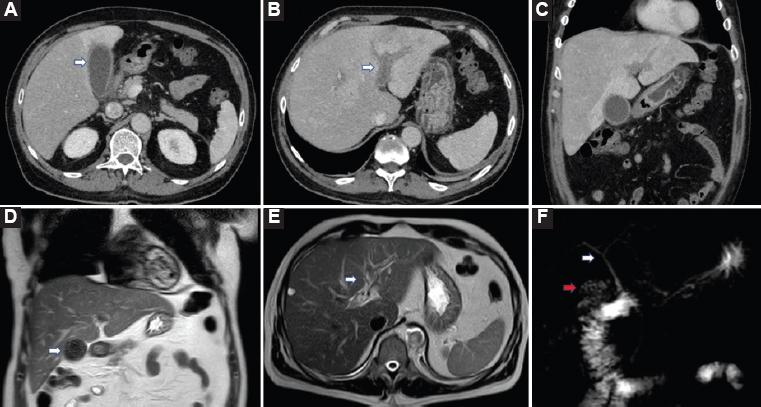
Figure 2 Computed tomography (CT) scan and magnetic resonance imaging (MRI) during hospitalization. A: CT scan with mild cholecystitis without any changes in the gallbladder comparing the previous CT scan. B-C: same CT scan showing left portal vein thrombosis (PVT) and alteration of perfusion in the affected liver. D: MRI demonstrating gallbladder with cholesterolosis, with no surrounding inflammation. E: left PVT. F: normal biliary tract.
Hospital admission was decided before which serology and polymerase chain reaction (PCR) against COVID-19 was performed. The result was positive for IgG, negative for IgM, and negative for PCR. The patient did not report respiratory symptoms at any previous time.
Anticoagulant treatment was established, as well as broad-spectrum antibiotics. Given the persistence of elevation of transaminases and elevation of bilirubin up to 5 mg/dl, cholangiography magnetic resonance imaging was performed, demonstrating a gallbladder with cholesterolosis, normal bile duct, without evidence of choledocholithiasis, cholangitis, or other alterations, except left PVT (Figs. 2D-F). ERCP was also performed with the same result.
Subsequently, the patient presented a good clinical evolution with normalization of analytical parameters, so discharge was decided. He was studied by hematology without evidence of coagulation disorders, and laparoscopic cholecystectomy was carried out correctly after 6 weeks.
In a post-operative CT scan control performed 4 weeks after surgery, the signs of PVT had disappeared (Fig. 3).
Discussion
This article describes the development of acute PVT in a patient with mild cholecystitis with the only antecedent of having developed antibodies against COVID-19. The pathogenesis of PVT is multifactorial. In most cases, it is related to cirrhosis or liver malignancies, and only in a third of cases is it attributable to another origin, such as hematological diseases or local intra-abdominal inflammatory processes (pancreatitis, cholangitis, hepatitis, etc.)1.
The association of acute cholecystitis with PVT is considered infrequent and appears in the literature as a clinical case reports2-4. When it appears, it is usually in multipathological patients or in severe cholecystitis, where an intense local inflammatory response occurs and triggers this process5,6. Some authors observed no etiological factors associated with PVT and speculated that the occurrence of PVT post-acute cholecystitis could be due to local infectious processes involving the cystic vein7.
However, this was not the situation in our patient. The cholecystitis diagnosed in the first CT scan was incipient cholecystitis, with a slight analytical and general condition alteration, without local abscesses or collections, with a correct evolution with antibiotics during the first 7 days. We also observe during the imaging control tests that the gallbladder inflammatory reaction remained at a minimum at all times.
In addition, we did not found any signs of thrombosis in the anatomical pathology of the specimen.
During the current global pandemic, patients affected by the severe acute respiratory syndrome coronavirus 2 virus have developed different acute respiratory symptoms to a greater or lesser severity, with a significant morbidity and mortality rate. Furthermore, clotting disorders, with organ dysfunctions and coagulopathy resulting in higher mortality, have been observed in some of these patients. Excessive activation of the immune system such as that observed against COVID-19, especially in late stages of the disease, plays a decisive role in endothelial dysfunction, damage to microvascular permeability, and thrombosis8.
The exact pathophysiology of the increased prevalence of thromboembolism events in COVID-19 positive patients remains unclear9. In some ill patients, the increase in inflammatory cytokines such as interleukin-6 (IL-6), IL-10, and tumor necrosis factor Alfa is the possible mechanisms10-12. Other authors suggest that the attachment of the viruses to the endothelial surfaces via angiotensin-converting enzyme receptor leads to lymphocytic endotheliitis, interferon-1, and prothrombotic genes overexpression13.
These thrombotic phenomena have been described mainly at pulmonary level, where thromboembolism has been evidenced in up to 67% of patients with COVID-19 infection. However, there are many articles based on imaging tests and autopsies in patients who died from COVID-19, which show thrombotic involvement at many other levels of the organism such as brain, heart, bowel, kidney, and even liver, as in our case14-18.
When PVT occurs acutely, the predominant symptoms are abdominal pain, fever, and in laboratory tests, we can find leukocytosis, elevated acute phase reactants, liver enzymes, and bilirubin. This was what we found in our patient at the time of diagnosis, and whenever we are faced with this clinical situation, we must take it into account and include PVT in our differential diagnosis.
The optimal strategy for the treatment of acute cholecystitis complicated by PVT remains to be investigated. The treatment goal is to prevent extension of the thrombus and to attain portal vein permeability. The various treatment approaches include the management of the underlying disease, use of antibiotics, hydration, anticoagulation therapy, and (infrequently) thrombolytic therapy or surgical embolectomy2. The early recognition of this complication with appropriate management can improve outcomes and shorten hospital stay. Prompt recognition is also critically important to avoid life-threatening complications such as mesenteric ischemia and infarction19.
In our case, we opted for medical treatment with anticoagulation at therapeutic doses and antibiotherapy, with good evolution of the clinical status. In agreement with a hematologist, we waited 6 weeks after the establishment of anticoagulation to perform laparoscopic cholecystectomy, with no complications.
Conclusion
Considering all the above mentioned, we believe that in our patient, dysregulated immune response as a late consequence of coronavirus infection could influence the development of acute PVT, extremely rare within the context of incipient cholecystitis in a patient without risk factors.
Despite the fact that this is an exceptional case and we cannot draw reliable conclusions, we believe that the relationship between COVID-19 infection and the increased risk in the development of this complication must be considered to implement special diagnostic and treatment measures in these patients.











 text new page (beta)
text new page (beta)



