Introduction
Total knee arthroplasty (TKA), is a procedure commonly performed by orthopedic surgeons across the globe, and it is expected that the demand for primary and revision TKAs will rise exponentially in our country. Only at our hospital, Unidad Médica de Alta Especialidad «Dr. Victorio de la Fuente Narváez», a total of 1,720 primary TKAs and 254 revision TKAs were performed in 2017, which is a 19% increase compared to 2016, when a total of 1,443 primary TKAs and 218 revision TKAs were performed. Acute kidney injury (AKI) is a serious complication following surgery. It is now recognized that even mild AKI can develop into chronic kidney disease (CKD) in a previously normal kidney and can also cause rapid disease progression in patients with chronic kidney failure, and it is also associated with an increase in hospital length of stay, costs and mortality.1
The incidence of AKI following elective and emergency orthopedic procedures has been reported to be up to 9%.2 The incidence of AKI following elective joint replacement goes from < 2 to 14.6%.3,4,5
The Acute Kidney Injury Network (AKIN) defines AKI as an increase of serum creatinine concentration of > 26.5 μmol (> 0.3 mg/dl) or 1.5 times its baseline value at 48 hours postoperative, as a well as a decrease in urinary output < 0.5 ml/kg/h.6
Tourniquet application is almost indispensable in the orthopedic practice. A tourniquet is often used in total knee arthroplasty resulting in improved visualization of structures, reduced intraoperative bleeding and better cementation.7,8,9 However, a series of complications can be related to tourniquet use, and these complications may be related to and excessive tourniquet time or pressure. The local and systemic physiological effects and the anesthetic implications are reviewed. Localized complications result from either tissue compression beneath the cuff or tissue ischemia distal to the tourniquet. Systemic effects are related to the inflation or deflation of the tourniquet.10,11,12,13
The purpose of this study was to evaluate the incidence of AKI following primary TKA, and to identify whether tourniquet application is a risk factor for developing AKI.
Material and methods
ClinicalTrials.gov Identifier: NCT03795805
We conducted the trial in Mexico City, with subjects recruited from the Department of Joint Replacements at Unidad Médica de Alta Especialidad «Dr. Victorio de la Fuente Narváez». The protocol was approved by the Instituto Mexicano del Seguro Social ethics committee. The study was performed in accordance with the protocol, and all subjects provided written informed consent.
Subjects older than 18 years old, who presented at the consult with a diagnosis of knee osteoarthritis, had failed a trial of nonoperative treatment and where schedule to undergo TKA were included. Major exclusion criteria were patients who did not sign informed consent or were schedule to undergo unicompartmental knee arthroplasty or osteotomies.
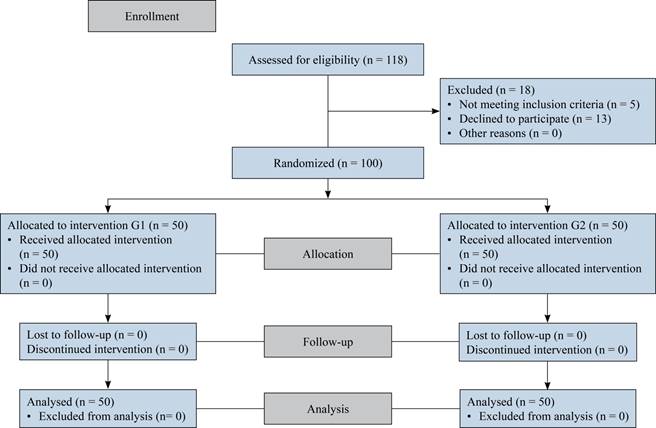
Patients were randomly assigned to undergo into two groups by computer generated random numbers. Group 1 (G1) underwent TKA with tourniquet application and group 2 (G2) underwent TKA without tourniquet application. All patients in G1 were operated on by the same surgeon (MS-A) as did the patients in G2 (FV-C) (Figure 1).
Serum creatinine and hemoglobin levels were obtained as part of preoperative evaluation and at 48 hours following surgery. Blood loss was quantified as both the bleeding during surgery as well as blood collected in the surgical drain. Range of motion was evaluated 48 hours after surgery using a goniometer. Skin-to-skin surgical time was obtained from the anesthetic record sheet. All measures were recorded in a data collection sheet.
A single injection of antibiotic prophylaxis was given. In G1 a pneumatic tourniquet was used after exsanguination using an Esmarch bandage. In G2 a combination of 50 cm3 normal saline, 0.5 mg epinephrine and 246.25 ropivacaine was injected intra articular to the knee joint 5 minutes before surgery started. Subcutaneous thromboprophylaxis was started in the evening before surgery. The anterior approach was used with a midline incision and a standard parapatellar arthrotomy. Soft tissues were released in a stepwise manner to achieve ligamentous balance in extension. Thus, flexion and extension gaps were approximately equal. Enough laxity was achieved to enable full extension and flexion and anterior translation, but not too loose to cause abnormal anteroposterior motion, impingement, or bearing spin out. Tensioning devices were not routinely used. The proximal tibia was resected with the anterior cruciate ligament sacrificed. The posterior cruciate ligament was preserved with a bone block. Both the femoral and tibial components were cemented. Primary patellar resurfacing was not performed. A surgical drain was placed prior to skin closure.
Postoperatively, physiotherapy (walking exercises aided with a walker and continuous passive motion exercises) was started on day one. Manual lymphatic drainage was performed by the physiotherapist on day two. Surgical drain was removed on day two.
Results
A total of 100 patients were included in the study (50 in each group). Patients were predominantly women (54%), with a mean age of 67.5 years (41-86 ± 8.18). In G1, patients were predominantly women (58%), with a mean age of 66.82 years (range 41-84, SD 8.93). In G2, half the patients were men and the other half women, with a mean age of 68.48 years (range 47-86, SD 7.34). Comorbidities by group are shown in Table 1.
Table 1: Comorbidities by group.
| Group 1 (n = 50) | n (%) |
|---|---|
| • Arterial high blood pressure | 41 (82) |
| • Diabetes mellitus | 16 (32) |
| • Rheumatoid arthritis | 11 (22) |
| • Chronic kidney disease | 3 (6) |
| • Hypothyroidism | 3 (6) |
| • No comorbidities | 4 (8) |
| Group 2 (n = 50) | |
| • Arterial high blood pressure | 43 (86) |
| • Diabetes mellitus | 14 (28) |
| • Rheumatoid arthritis | 3 (6) |
| • Chronic kidney disease | 1 (2) |
| • Hypothyroidism | 1 (2) |
| • Parkinson’s disease | 1 (2) |
| • Dyslipidemia | 1 (2) |
| • Mixed cardiomyopathy | 1 (2) |
| • No comorbidities | 6 (12) |
Mean surgical time was 83.7 ± 14.2, mean surgical blood loss was 528 cm3 (528 ± 119.4 cm3), differences between groups are shown in Table 2. Differences between Hemoglobin levels in each group and differences between range of motion and pain in each group are shown in Table 2. All patients started walking with the help of a walker at 48 hours postoperative. Fourteen percent of patients required a blood transfusion, 11 in G1 and 3 in G2 (p = 0.021).
Table 2: Differences in outcomes between groups.
| G1 | G2 | p‡ | |
|---|---|---|---|
| Time (min)* | 85.2 (15.3) | 82.2 (12.9) | 0.291 |
| Blood loss (cm3)* | 581 (100.9) | 475 (113.5) | < 0.001 |
| Flexion (o)* | 59.6 (17.3) | 82.2 (12.9) | < 0.001 |
| Extension(o)* | 0 (0) | 0.2 (1.4) | 0.320 |
| Pain (VAS)* | 7.02 (1.2) | 5.08 (0.6) | < 0.001 |
| Mean (g/dl) | p§ | ||
| G1 | |||
| • Preoperative hemoglobin | 14.1 | < 0.001 | |
| • Postoperative hemoglobin | 10.4 | ||
| G2 | |||
| • Preoperative hemoglobin | 14.7 | < 0.001 | |
| • Postoperative hemoglobin | 10.5 | ||
| G1 | |||
| • Preoperative serum creatinine | 0.74 | < 0.001 | |
| • Postoperative serum creatinine | 0.91 | ||
| G2 | |||
| • Preoperative serum creatinine | 0.84 | 0.472 | |
| • Postoperative serum creatinine | 0.82 |
* Values are expressed as mean (SD).
‡ Student’s t-test for independent samples was used.
§ Student’s t-test for paired samples was used.
Min = minutes, ° = Degrees, VAS = visual analogue scale.
Regarding serum creatinine level difference, we found mean preoperative levels of 0.79 ± 0.21 mg/dl and mean postoperative levels of 0.87 ± 0.33 mg/dl (p = 0.001), differences between groups are shown in Table 2.
Acute kidney injury rate was found to be 22% when strict definition was applied (elevation of > 0.3 mg/dl compared with baseline). Risk factors identified are shown in Table 3.
Table 3: Risk factors for acute kidney injury following total knee arthroplasty.
| AKI (n = 22) | No AKI (n = 78) | OR (IC 95%) | p | |
|---|---|---|---|---|
| Tourniquet application | 16 | 34 | 2.667 (1.137-6.254) | 0.014 |
| Age older than 65 years | 17 | 48 | 1.831 (0.738-4.541) | 0.132 |
| Male | 13 | 33 | 1.696 (0.798-3.602) | 0.125 |
| Surgical time > 90 minutes | 5 | 14 | 1.254 (0.529-2.972) | 0.409 |
| Blood loss > 500 cm3 | 17 | 29 | 3.991 (1.597-9.978) | 0.001 |
| Postoperative hemoglobin < 10 g/dl | 13 | 22 | 2.683 (1.275-5.645) | 0.008 |
| Blood transfusion | 7 | 7 | 2.867 (1.428-5.756) | 0.012 |
| High blood pressure | 18 | 66 | 0.857 (0.334-2.199) | 0.448 |
| Diabetes mellitus | 12 | 18 | 2.800 (1.360-5.764) | 0.006 |
| Rheumatoid arthritis | 5 | 9 | 1.807 (0.794-4.109) | 0.161 |
AKI = acute kidney injury, TKA = total knee arthroplasty.
Discussion
The main finding of the present study was to describe a 22% incidence of acute kidney dysfunction in patients who underwent total knee arthroplasty, as well as to prove that the use of tourniquet in total knee arthroplasty significantly increases serum creatinine levels 48 hours after surgery, and also increases the risk of suffering acute kidney dysfunction by 2.6 times. Likewise, the use of the tourniquet is related to an increase in total postoperative bleeding, lower postoperative flexion capacity and an increase in the postoperative pain scores. It was observed that, in addition to the use of the tourniquet, the presence of diabetes mellitus, blood loss greater than 500 cm3, postoperative Hb less than 10 g/dl, or the need for transfusion, also increases the risk of presenting subsequent acute kidney dysfunction to a total knee arthroplasty.
Hassan and colleagues,14 in their retrospective study, described an incidence of acute renal dysfunction of 9.7%, compared with 22% of our study, which allowed them to identify risk factors such as age over 65 years and the presence of arterial high blood pressure, which were not identified in our study, and on the contrary, they did not identified the presence of Diabetes mellitus as a risk factor, which was identified in our study.
Similarly, in a study conducted by Wu et al.,15 a relatively low incidence of acute renal dysfunction (3.3%) is described in comparison with our study, and, like us, they did not identified arterial high blood pressure, BMI greater than 30, surgical time over 90 minutes, and the patient’s gender as risk factors for the development of acute kidney dysfunction, however, unlike our study, they did not identified blood loss > 500 cm3, the need for transfusion or the presence of diabetes mellitus to be risk factors for acute kidney dysfunction.
In the study by Kimmel et al.,3 an incidence of acute renal dysfunction secondary to prosthetic joint replacement of 15% was reported, as well as, among the multiple risk factors listed, the use of a pneumatic tourniquet, but an Odds Ratio is not provided. Likewise, Diabetes mellitus is listed as a risk factor for acute kidney dysfunction together with blood transfusions, similar to our study.
Conclusion
The use of tourniquet should be indicated with caution and should not be used routinely in patients with other risk factors for the development of acute kidney dysfunction, other measures to achieve trans-surgical hemostasis should be implemented in our environment to reduce the incidence of acute kidney dysfunction related to the use of the tourniquet. Likewise, factors such as diabetes mellitus, systemic arterial hypertension, transurgical bleeding and BMI should be studied due to conflicts in the international literature.











 text new page (beta)
text new page (beta)