The Lamiaceae family contains 7,139 species (Harley et al. 2005), and the genus Salvia groups nearly 1,000 of them. From these species, 306 are distributed in Mexico. Salvia urica Epling is a perennial herbaceous plant native to Mexico, with a restricted distribution in the state of Chiapas, as well as in Guatemala and Belize (Martínez-Gordillo et al. 2017). It grows at elevations from 400 to 2,600 meters above mean sea level, in seasons ranging from summer to winter (Clebsch 2003). This plant is commonly known as “pendolita morada”, “canastillas”, “tutzunún”, “Salvia bretónica”, and “chichingua azul” (Chicago Natural History Museum 1973, Padilla-Gómez 2007). Some traditional medicinal uses given to S. urica are to treat “bilis”, diabetes mellitus, stomachache, and diarrhea (Padilla-Gómez 2007). However, no phytochemical and pharmacological reports of the constituents derived from this plant species support its ethnomedical uses in Mexico. To date, there is just a report describing the antibacterial effect of a methanolic extract of the plant against Escherichia coli (Sakagami et al. 2001). Originally, Salvia urica and Salvia amarissima Ortega were classified together in section Uricae Epling, due to their morphological resemblance (Epling 1939). Later, they were moved to a broader section: Scorodonia Epling (Epling 1941). Phylogenetic studies of Salvia subgenus Calosphace have shown that both these sections are artificial groupings (Fragoso-Martínez et al. 2018). However, Salvia amarissima and S. urica are closely related, forming a clade with Salvia leucochlamys Epling, Salvia ozolotepecensis J.G.González & Fragoso, Salvia perlonga Fernald, and Salvia praestans Epling (González-Gallegos et al. 2019). The lack of phytochemical and pharmacological studies, and the phylogenetic closennes between S. urica and S. amarissima prompted us to study the first species; in order to explore if it produces secondary metabolites of diterpenoid-type that supports this relationship since a chemotaxonomic approach, and if it shares pharmacological properties with S. amarissima.
As part of our ongoing efforts to study the phytochemical composition of Mexican medicinal plants for drug discovery and agrochemical purposes (Ortega et al. 2020, Bautista et al. 2022, García-Nava et al. 2022), herein, we describe the isolation and identification of the major metabolites from an acetone extract of Salvia urica, as well as the subsequent determination of the anti-hyperglycemic and anti-propulsive effects of these constituents.
Materials and methods
General experimental procedures. 1D NMR experiments were performed on an Agilent Mercury NMR spectrometer 500 MHz, equipped with a gradient probe for variable temperature experiments. Chemical shifts were referred to TMS, and J values are given in Hertz (Hz). Column chromatography (CC) was performed on silica gel 60 (Merck G). Thin-Layer Chromatography (TLC) was carried out on precoated Macherey-Nagel Sil G/UV254 plates of 0.25 mm thickness, and spots were visualized by spraying with 3 % Ce(SO4)2 in H2SO4 2 N, followed by heating. The GC-MS analyses were obtained on an Agilent gas chromatograph, model GC-7890b coupled to an Agilent mass spectrometer EM-5877A, using a GC capillary column DB 5ht (30 m × 0.320 mm i.d. × 0.1 μm, Agilent). The HPLC analyses were obtained on a 1200 HPLC Agilent chromatograph equipped with a G1315D Agilent PDA detector and a G1315A Agilent fluorescence detector. HPLC separation was performed in a Synergi Polar RP column (Phenomenex, 5 mm, 4.6 × 250 mm) with a gradient of acetic acid 1 % (A) and acetonitrile (B) at the following times: 0 min, 80 % A; 1 min, 80 % A; 31 min, 40 % A, 33 min, 20 % A; 43 min, 20 % A. The chromatograms were obtained at 30 °C; the standards used were amarissinin A (1) (> 98 %, HPLC-PDA) and 5,6-dihydroxy-7,3’,4’-trimethoxyflavone (2) (> 98 %, HPLC-PDA); and the calibration curves of the standards were made using different concentrations 0.25-0.00625 mg/mL.
Plant material. The aerial parts were collected at the flowering stage in Teopisca, Chiapas, Mexico, in December 2021. A voucher specimen was deposited at the Herbarium of Facultad de Ciencias, UNAM (FCME-180582), and determined by Dr. Martha Martínez Gordillo.
Extraction and isolation. The dried and grounded aerial parts of Salvia urica (218 g) were subjected to extraction by maceration with acetone (3 × 2 L). The extract was filtered, and the solvent was recovered by distillation at reduced pressure to obtain 11 g of dried residue. The S. urica acetone extract (SUE) was submitted to silica gel CC (8.5 × 4.5 cm, 125 mL), eluting with hexane-EtOAc, EtOAc, and EtOAc-MeOH mixtures in increasing polarity. The grouping fractions was conducted by their likeness observed in the TLC analyses as follows: Fraction A (frs.1-7, eluted with hexanes-EtOAc 9:1), fraction B (frs. 8-22, eluted with hexanes-EtOAc 8:2), fraction C (frs. 23-27, eluted with hexanes-EtOAc 3:2), fraction D (frs. 28-38, eluted with hexanes-EtOAc 3:2), fraction E (frs. 39-43, eluted with hexanes- EtOAc 1:1), fraction F (frs. 44-52, eluted with hexanes-EtOAc 3:7), fraction G (fr. 53, eluted with EtOAc), fraction H (fr. 54, eluted with EtOAc), fraction I (frs. 55-58, eluted with EtOAc-MeOH 9:1). Fraction D (1.6 g) was submitted to silica gel CC (10.0 × 2.0 cm, 25 mL), eluting with CHCl3-MeOH mixtures in increasing polarity to give five fractions (D1-D5). Fr. D2 (eluted with CHCl3-MeOH 97:3) yielded 62 mg of amarissinin A (1) by sequential crystallizations from acetone/hexanes, EtOAc/hexanes, and acetone/EtOAc. Fr. E yielded 7 mg of 5,6-dihydroxy-7,3’,4’-trimethoxyflavone (2), which was filtrated, and crystallized from EtOAc/hexanes.
Compound 1.- yellowish crystals, mp 219-224 °C; 1H NMR (500 MHz, CDCl3-DMSO-d6, Figures S1 and S2): δH 7.82 (br s, H-16), 7.39 (t, J = 1.8 Hz, H-15), 6.69 (ddd, J = 10.1, 4.9, 2.2 Hz, H-2), 6.54 (br d, J = 1.8 Hz, H-14), 6.12 (br s, H-11), 5.92 (dt, J = 10.1, 1.4 Hz, H-1), 4.72 (dd, J = 8.7, 1.5 Hz, H-19 Pro R), 4.35 (d, J = 8.7 Hz, H-19Pro S), 2.71 (dd, J = 19.3, 4.9 Hz, H-3a), 2.61 (dt, J = 19.3, 2.2 Hz, H-3b), 2.47 (ddd, J = 13.1, 12.0, 5.1 Hz, H-7a), 2.21 (ddd, J = 13.5, 11.6, 5.4 Hz, H-7b), 2.03 (s, H3-20), 1.61 (m, H-6a), 1.56 (m, H-6b); 13C NMR (125 MHz, DMSO-d6, Figure S3): δC 197.39 (C, C-10), 173.89 (C, C-18), 161.75 (C, C-17), 151.47 (C, C-12), 150.37 (C, C-9), 143.96 (CH, C-15), 143.04 (CH, C-2), 141.48 (CH, C-16), 127.39 (CH, C-1), 120.72 (C, C-8), 119.31 (C, C-13), 106.62 (CH, C-14), 104.60 (CH, C-11), 75.81 (C, C-4), 69.49 (CH2, C-19), 57.18 (C, C-5), 29.84 (CH2, C-3), 28.79 (CH2, C-6), 21.95 (CH2, C-7) and 18.55(CH3, C-20).
Compound 2.- yellowish crystals, mp 240-241 °C; 1H NMR (500 MHz, DMSO-d 6, Figure S4): δH 12.62 (s, OH-5), 8.72 (s, OH-6), 7.70 (d, J = 8.5 Hz, H-5’), 7.59 (br s, H-2’), 7.13 (dd, J = 8.5, 1.6 Hz, H-6’), 6.98 (s, H-8), 6.94 (s, H-3), 3.88 (OCH3-7), 3.85 (OCH3-3’), 3.80 (OCH3-4’); 13C NMR (125 MHz, DMSO-d 6, Figure S5): δC 182.26 (C, C-4), 163.34 (C, C-2), 154.41 (C, C-7), 152.04 (C, C-4’), 149.03 (C, C-3’), 146.16 (C, C-5), 129.98 (C, C-6), 123.07 (C, C-1’), 119.98 (CH, C-6’), 111.7 (CH, C-5’), 109.39 (CH, C-2’), 103.45 (CH, C-3), 91.26 (CH, C-8), 56.16 (CH3, OCH3-7), 55.73 (CH3, OCH3-3'), 55.58 (CH3, OCH3-4').
Identification of the volatile constituents by GC-MS. A sample of 5 g of plant material was macerated with acetone (3 × 60 mL) for 48 h to obtain residues ranging from 490 to 503 ± 9.9 mg (5.5 ± 0.08 % yield dry weight). Then, 10 mg of extract were dissolved in 2 mL of acetone using sonication, and a volume of 0.2 μL containing this solution was injected into the gas chromatograph (splitless). The temperature of the port injection was maintained at 220 °C, and the oven was 80 °C for 2 min, hereafter increased at 3, 5, and 10 °C/min until 100, 150, and 330 °C, respectively. The final temperature was maintained for 9 min. Each analysis was repeated in triplicate, and the volatile compounds were identified by deconvolution, using the W10N11 database (Wiley10Nist11) and the % area normalized (Sepúlveda-Cuellar et al. 2021).
High Performance Liquid Chromatography. A sample of plant material (10 g) was macerated with acetone (3 × 60 mL) for 48 h. This procedure was repeated with three independent samples to obtain residues between 490-503 ± 9.9 mg. Then, a solution of each extract at 2.5 mg/mL was prepared in methanol. The injection volume of standards was 5 μL, and 10 μL for samples, using a flow rate of 0.8 mL/min. The temperature of the equipment was 30 °C. The PDA detector acquires the chromatograms at 240 and 330 nm, and the FL detector operates with an excitation longwave of 250 nm, and an emission longwave of 410 nm. All experiments were repeated in triplicate according to Sepúlveda-Cuellar et al. (2021).
Animals. Balb/c male mice of 8-10 weeks of age weighing 25 ± 5 g, and with glucose level values of 161 ± 5 mg/mL were used for the antihyperglycemic test. Sprague-Dawley male rats (200-250 g) for the antipropulsive test were used. The animals were obtained and raised in the Animal House of the National Medical Center “Siglo XXI” at Instituto Mexicano del Seguro Social (IMSS). The in vivo experiments were conducted following the Official Mexican NOM-0062-ZOO-1999 (SEMARNAT 1999) for Animal Experimentation and Care. The room temperature was 22 ± 2 °C with a 12-h light-dark natural cycle to maintain the animal care. The animals were fed with a standard diet and water ad libitum. All in vivo experiments were conducted with the approval of the Specialty Hospital Ethical Committee of the National Medical Center “Siglo XXI” at IMSS (register: R-2019-3601-004).
Induction of experimental type 2 diabetes in mice. The diabetes was induced in male Balb/c mice using the streptozotocin/nicotinamide model, according to a minor modification of the method described by Valdés et al. 2019. The mice fasted for 16 h before being treated with an intraperitoneal solution of streptozotocin (100 mg/kg). After 30 min of the streptozotocin administration, the mice were treated with nicotinamide (240 mg/kg in cold saline solution) via intraperitoneal. The mice were fed with sucrose solution (10 %) ad libitum over three days at the end of the third day. The sucrose solution was retired and substituted with water on the fifth day. After 24 h, blood glucose levels were measured by the glucose oxidase method (ACCU-CHECK® Instant Blood Glucose System, Roche, DC®, Mexico).
Antihyperglycemic effect. Balb/c mice were randomly divided into 8 groups (n = 6 per group). The groups were separated by treatment as follows: Normoglycemic (NM control) and Diabetic (SID2 control) mice treated with vehicle (2 % tween 20 in water); NM mice and SID2 treated with SUE (300 mg/kg), compound 1 (50 mg/kg) and compound 2 (50 mg/kg), respectively. The collection of blood samples from the tail vein was at 0, 2, and 4 h. The analysis of blood samples was done by the glucose oxidase method (Valdés et al. 2019). The results were expressed as mean values ± standard error of the mean (SEM). The statistical analyses were performed by GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). The statistical evaluation was conducted by Bonferroni test for multiple comparisons with a P < 0.05 of significance.
Antipropulsive effect. The rats were fasted for 12 h before starting the experiment, but with water access ad libitum. The method reported by Calzada et al. (2010) was followed with minor modifications. The groups were divided into control and test groups (n = 6 per group). The control group was treated with vehicle (1 mL 2 % DMSO in water) or loperamide hydrochloride (10 mg/kg, 1 mL in 2 % DMSO, positive control), and the test groups were divided into SUE (12.5 - 50 mg/kg), compound 1 (0.125 - 1.5 mg/kg) and compound 2 (0.125 - 1.5 mg/kg). After 20 min, the animals were administered with 1 mL of charcoal meal ENT#091;10 % charcoal suspension in 5 % aqueous gum ArabicENT#093; by oral route. After 30 min, the animals were sacrificed, and their stomach and small intestine were removed and extended on a glass surface. The distance from the pylorus to the caecum was measured and expressed as a percentage. All results were expressed as mean ± S.E.M. and evaluated by Student’s t-test with a P < 0.05 of significance.
Results
The GC-MS analysis of the volatile constituents from the acetone extract of Salvia urica indicated the presence of 16 metabolites, which include six triterpenes, seven alkanes and three aromatic metabolites such as anthraquinone and naphthol derivatives (Supplementary material, Table S1). The relative amounts of the volatile compounds, by class, were: 76.61, 15.39, and 7.99 %, respectively. The HPLC-PDA profiling of the non-volatile constituents in the acetone extract of S. urica, followed by the subsequent quantification of the isolated compounds (Supplementary material, Figures S1-S5) by chromatographic methods, indicated that amarissinin A (1, rt = 24.24 min, 330 nm) and 5,6-dihydroxy-7,3',4'-trimethoxyflavone (2, rt = 24.58 min, 330 nm) are the major constituents in the extract (Figure 2, Table 1).
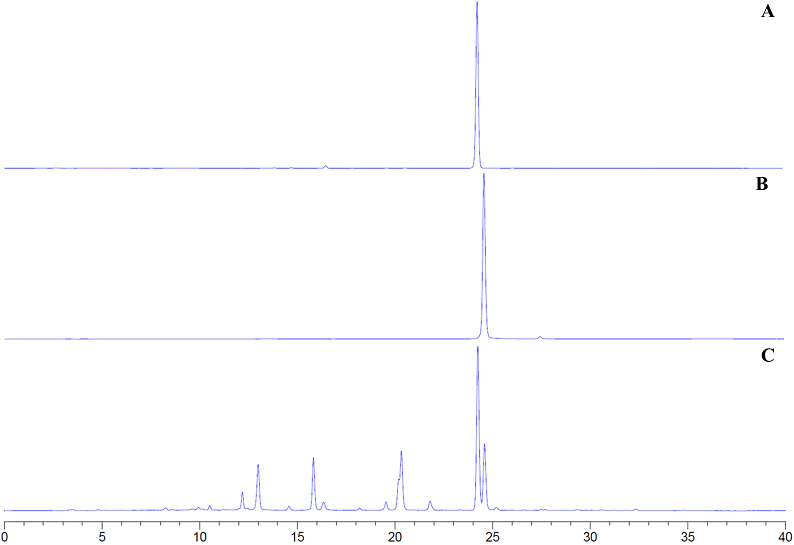
Figure 2 (A) HPLC-PDA profiling chromatogram of amarissinin A (1). (B) HPLC-PDA profiling chromatogram of 5,6-dihydroxy-7,3',4'-trimethoxyflavone (2). (C) HPLC-PDA profiling chromatogram of the acetone extract of Salvia urica at 330 nm.
Table 1 Quantification of amarissinin A (1) and 5,6-dihydroxy-7,3',4'-trimethoxyflavone (2) by HPLC-DAD (at 330 nm) in the total extract from Salvia urica.
| Acetone extract (yield in % w/w) | Compound 1 (mg/g extract) | Compound 2 (mg/g extract) |
|---|---|---|
| 5.17 ± 0.09 | 163.36 ± 3.17 | 141.98 ± 4.59 |
Note: Data expressed as average ± SD. Compound 1 = -46.4x +25.36; Compound 2 = -66.109x +25.305
In the normoglycemic mice, the extract and the metabolites isolated from Salvia urica did not significantly affect glucose levels (Table 2). However, in streptozotocin-induced type 2 diabetes mice, the extract and the metabolites showed an antihyperglycemic effect at 2 and 4 h after their administration (Table 2). Compound 2 showed a dose-dependent decrement in glucose levels; and, the acetone extract showed the lowest glucose level after 4 h of treatment. A synergic effect between the constituents of the extract is possible.
Table 2 Blood glucose levels of male normoglycemic mice (NM) and streptozotocin-induced type 2 diabetes mice (SID2) at 0, 2 and 4 h, on the acute antihyperglycemic test.
| Treatment | Blood glucose levels (mg/dL) | ||
|---|---|---|---|
| 0h | 2h | 4h | |
| NM control | 137.5 ± 5.6 | 131.3 ± 3 | 133 ± 3.5 |
| SID2 control | 300 ± 32.7 | 356.3 ± 21.5 | 351.3 ± 25.2 |
| SID2 + SUE (300 mg/kg) | 308.2 ± 7.6 | 285.2 ± 19 | 173 ± 31.7*, ( |
| SID2 + 1 (50 mg/kg) | 297.3 ± 6.9 | 337.8 ± 32.6 | 302.3 ± 45.4 |
| SID2 + 2 (50 mg/kg) | 289.8 ± 8.5 | 252.3 ± 5.4*, ( | 237.3 ± 5.6*, ( |
| NM + SUE (300 mg/kg) | 134.5 ± 3.7 | 128.3 ± 5.2 | 134.5 ± 3.1 |
| NM + 1 (50 mg/kg) | 136 ± 4.9 | 136.5 ± 5.3 | 133.8 ± 5 |
| NM + 2 (50 mg/kg) | 134.8 ± 2.5 | 126.8 ± 2.2 | 130.3 ± 2 |
The data are expressed as mean ± S.E.M, n = 6; * P < 0.05 compared to the initial value; αP <0.05 compared to DM2 control 2h, β P <0.05 compared to DM2 control 4h. SID2: Streptozotocin-induced type 2 diabetic mice, SUE: Acetone extract of Salvia urica; 1: Amarissinin A; 2: 5,6-dihydroxy-7,3',4'-trimethoxyflavone.
On the charcoal-gum Arabic-induced hyperperistalsis model in rats, the extract, and their major metabolites of Salvia urica were tested at different doses. All showed a dose-dependence effect. Among them, amarissinin A (1) showed the best inhibitory effect on the peristalsis at 0.125 mg/kg, and the 5,6-dihydroxy-7,3',4'-trimethoxyflavone (2) showed a half maximal effective concentration of 0.79 mg/kg, in comparison with the positive control (1.003 mg/kg, Table 3).
Table 3 Antipropulsive effect Salvia urica and loperamide products on charcoal activated-gum Arabic induced hyperperistalsis model in rat.
| Treatment | Hyperperistalsis (cm) | Antipropulsive Effect (%) | CE50 (mg/kg) | |
|---|---|---|---|---|
| 2 h | ||||
| AC | 75.3 ± 2 | - | - | |
| AC-GA | 91.6 ± 2.7 | - | - | |
| AC-GA + SUE | 50 mg/kg | 78 ± 1 | 83.6 ± 6.6 | 16.65 |
| 25 mg/kg | 80.3 ± 1 | 69.3 ± 6.2 | ||
| 12.5 mg/kg | 85.3 ± 0.6 | 38.7 ± 3.8 | ||
| AC-GA + 1 | 1.5 mg/kg | 79.3 ± 1.6 | 75.5 ± 10.4 | 0.81 |
| 0.5 mg/kg | 83.6 ± 1.6 | 48.9 ± 10.1 | ||
| 0.25 mg/kg | 86 ± 1.4 | 34.6 ± 9 | ||
| 0.125 mg/kg | 91 ± 0.4 | 4 ± 2.4 | ||
| AC-GA + 2 | 1.5 mg/kg | 63.1 ± 1.19 | 100 | 0.79 |
| 0.5 mg/kg | 86.3 ± 0.2 | 32.6 ± 1.4 | ||
| 0.25 mg/kg | 90 ± 0.4 | 10.2 ± 2.4 | ||
| 0.125 mg/kg | 97.3 ± 1.3 | 0 | ||
| AC-GA + C | 5 mg/kg | 39.6 ± 6.5 | 100 | 1.003 |
| 2.5 mg/kg | 77.6 ± 6.1 | 85.7 ± 5.2 | ||
| 1.25 mg/kg | 83 ± 0.4 | 46.9 ± 2.4 | ||
Antipropulsive effect calculated after administration of the treatments. Values expressed as means ± SEM, n = 6, CE50: half maximal effective concentration. AC: Activated charcoal; GA: Gum Arabic; SUE: Acetone extract of Salvia urica; 1: Amarissinin A; 2: 5,6-dihydroxy-7,3',4'-trimethoxyflavone; C: Loperamide chloride.
Discussion
The species of the Salvia genus have broad traditional medicinal uses in Mexico, which include treating digestive and gynecological problems, and affections of the nervous system (Jenks & Kim 2013). Previous reports have explored the phylogenetic relationships of species from Salvia subgenus Calosphace based on nuclear and plastid markers (ITS, trnL-trnF, and trnH-psbA) (Fragoso-Martínez et al., 2018; González-Gallegos et al. 2018), and nuclear loci from massive sequencing data (Lara-Cabrera et al. 2021). Using the phylogeny of Salvia subgenus Calosphace as a tool to target specific metabolites as proposed by Ortiz-Mendoza et al. (2022), we decided explore the phytochemistry of key species; since we analyzed a species that was closely related to S. amarissima, which belongs to a taxon that has shown a wide variety of metabolites, which include neo-clerodanes, 9,10-seco-neo-clerodanes, and amarissanes (Bautista et al. 2016). The major metabolites found in S. urica: amarissinin A (1) and 5,6-dihydroxy-7,3',4'-trimethoxyflavone (2), also are produced in S. amarissima, supporting their phylogenetic closeness. In addition, the occurrence of compounds 1 and 2 supports the hypothesis that S. urica has similar pharmacological effects like to its congener. A previous study analyzed the inhibitory effect of methanolic extracts of plants on the production of verotoxin by the enterohemorrhagic strain of Escherichia coli O157:H7 (VEc) (Sakagami et al. 2001). In that study, the extract from S. urica showed an inhibitory effect on VEc. Likewise, Calzada et al. (2020) reported that compounds 1 and 2, isolated from S. amarissima, showed antiprotozoal activity against Entamoeba histolytica and Giardia lamblia with IC50 values ranging from moderate to good activity (62.1-101.1 and 0.05-0.13 μM, respectively), compared with positive control (0.23-1.22 μM, metronidazole). In the present study, the compounds 1 and 2, but now isolated from S. urica, showed an antipropulsive effect on charcoal-gum Arabic-induced hyperperistalsis model in rats, providing new insights to understand underlying ways to stop diarrhea; and provided evidence-based support for the traditional uses of both plant species. Concerning to antidiabetic effect, previous reports have shown that the antihyperglycemic activity of a total extract from S. amarissima is associated with an α-glucosidase inhibitory activity. The subsequent phytochemical analysis of the extract led to the isolation of compound 2, as the more active constituent (IC50 1 800 μM) of the extract. This activity was even higher than those for diterpenes (IC50 > 10 000 μM) also isolated, and close near to positive control (IC50 100 ± 0.3 μM, acarbose) (Flores-Bocanegra et al. 2017, Solares-Pascacio et al. 2021). Considering the above, it is plausible that the presence of compound 2 contribute significantly to the antihyperglycemic effect of S. urica, through the inhibition of α-glucosidase. In addition, it is worthy to note that was not detected the presence of oleanolic and ursolic acids, and stigmasterol in the extract of S. urica; which in turn, have been identified as the bioactive constituents, responsible for the antihyperglycemic properties of Salvia species (Santos et al. 2012, Bakrim et al. 2022, Ortega et al. 2022). The triterpenoids α- and β-amyrins were also detected in significant amounts in the acetone-soluble extract from S. urica by GC-MS (Supplementary material, Table S1). The relative amount (%) of each triterpenoid in the volatile portion of the extract was: 17.38 ± 8.70 and 25.84 ± 0.98, respectively. Both compounds have been reported as α-glucosidase inhibitors, and are involved in the improving of the insulin plasm levels and exert a protective effect of β-cells; and prevent the resistance to insulin via AMPK signaling induction (Mabhida et al. 2018, Dirir et al. 2022, Entezari et al. 2022). Due the above, amyrins are considered as multi-target compounds, therefore additional studies with the acetone extract of S. urica and their individual constituents are necessary to explore other action mechanisms that led to understand the antihyperglycemic effect of the plant.
In conclusion, the phytochemical study of an acetone-soluble extract from the aerial parts of S. urica led to the identification of its major constituents, their quantification through HPLC-PDA, and the evaluation of their antihyperglycemic and antiperistaltic effects, providing evidence-based support for the traditional medicinal uses of the plant. Finally, the pharmacological effect displayed by the diterpenes derived from Salvia amarissima as multidrug-resistance modulators in cancer cells (Bautista et al. 2016), could prompt exploring these properties in Salvia urica.
Supplementary material
Supplemental material for this article can be accessed here https://doi.org/10.17129/botsci.3368.











 nueva página del texto (beta)
nueva página del texto (beta)




