There are nearly 300 to 350 species of Magnolia L. (Magnoliaceae Juss.) occurring worldwide, in tropical, subtropical, and warm temperate climates (Rivers et al. 2016, Vázquez-García et al. 2016). The genus is distributed across Southeast and East Asia, Eastern Canada, the United States of America, Mexico, the Caribbean, and Central and South America. Unfortunately, half of its species (48 %) are at risk of extinction (Rivers et al. 2016). Mexico is one of the two major centers of diversity for this genus in the Neotropics with 38 species (Sánchez-González et al. 2021, Vázquez-García et al. 2021a, b). About 80 % of these species are under threat or endangered, and the conservation status for the remaining 20 % is not determined due to a lack of enough information (Rivers et al. 2016).
Magnolia pugana (Iltis & A.Vázquez) A.Vázquez & Carvajal is an endangered species endemic to western Mexico. It has been categorized as Endangered in the IUCN Red List of Threatened Species since 2014 (Gibbs & Khela 2014, IUCN 2022). It thrives in riparian forests with intermittent streams and ravines of southern Zacatecas and central Jalisco (Vázquez-García et al. 2002). Despite the high fruit and seed production of Magnolia pugana in wild populations, germination without scarification is low (Jacobo-Pereira et al. 2016), and predation by rodents is high (Vázquez-García 1994). Thus, populations of this species consist of isolated individuals or small groups in tributary creeks within riparian forests, where natural recruitment is limited (Rivers et al. 2016). Multiple anthropic disturbance factors affect Magnolia populations, including livestock, habitat fragmentation, and illegal wood extraction (He et al. 2009, Kundu 2009, Vásquez-Morales et al. 2017, Serna-González et al. 2019). High fragmentation and isolation of populations and habitats, high deforestation rate, low regeneration, forest conversion to pasture lands and agriculture, forest fires, and expansion of urban and rural human settlements are the major threats to M. pugana populations (Linsky & Muñiz-Castro 2022). Furthermore, it is estimated that precipitations will decrease (up to 10 %) and temperatures will increase (2 to 4 °C) in western Mexico, under the most severe climate change scenario (Durán 2010, Ibarra-Montoya et al. 2011, IPCC 2014, Ruiz-Corral et al. 2016), which may affect, to a great extent, seed germination and survival of seedlings occurring in natural populations, thus, increasing their vulnerability to extinction (Donohue et al. 2010, Vásquez-Morales et al. 2014).
Germination is a critical stage in the plant life cycle that modulates population and community dynamics as it depends on numerous potentially adverse biotic and abiotic conditions such as increased temperature and low water availability (Harper 1977, Dürr et al. 2015). Germination of Magnolia seeds has been studied in Mexico, particularly M. pugana (Jacobo-Pereira et al. 2016) and other species of the genus (Vovides & Iglesias 1996, Saldaña-Acosta et al. 2001, Corral-Aguirre & Sánchez-Velásquez 2006, Vásquez-Morales & Sánchez-Velásquez 2011, Toledo-Aceves 2017, Vásquez-Morales & Ramírez-Marcial 2019, Gallardo-Yobal et al. 2022). However, the combined effect of temperature and water potential was not evaluated. Reductions in percentage and delayed germination under stress provoked by alterations caused by global warming (higher temperatures and decreased humidity) have already been documented for various ecosystems. Daws et al. (2008) found that 14 pioneer species of the Neotropical semideciduous forest in Panamá, exhibited reductions and delays in germination at water potentials ≤ -1 MPa. On the other hand, Flores & Briones (2001) and Flores et al. (2017) reported, in arid and semiarid environments, a probable increase in temperature > 4 °C and decrease in humidity, which would inhibit seed germination of some species, however, if the tolerance threshold of soil water potential above -0.4 MPa and high temperature is not exceeded, germinability could be increased and made faster. Furthermore, in Atlantic rainforests, Braz et al. (2014) reported that germination of Arecaceae species is reduced and mean germination time is prolonged at water potentials ≤ -0.4 MPa, however, they were able to observe a low germinability at -0.8 MPa. In contrast, Ooi et al. (2009) reported that germination percentage was not affected by the increase in temperatures from 60/20 to 70/25 °C (soil diurnal temperature range), in most ephemeral species in the arid region of the western interior of Australia, due to a possible adaptation in their germination ecology to a greater temperature range that facilitated the breaking of physical seed dormancy. Therefore, it is evident that temperature and humidity fluctuation due to global warming can approach or exceed tolerance thresholds and lead to seed death and thereby influence species distributions (Donohue et al. 2010, Dürr et al. 2015).
However, these phenomena have been understudied in species thriving in warm temperate mesic forests (Siegel & Brock 1990, Falleri et al. 2004). On the surface soil of such environments, low water potentials (drought stress) are rarely present, but if they decrease, seed germination of some species, such as Magnolia pugana, may be affected (Evans & Etherington 1990, Daws et al. 2008, Walck et al. 2011). Studies conducted on different species of this genus warn us about sensitivity and vulnerability to drought during the stages comprising their growth and development (Nash & Graves 1993, Sjöman et al. 2018). Thus, the present study aims to evaluate the combined effects of higher temperatures and water stress on the germination of M. pugana seeds. We test the hypothesis that the interaction of increased temperature and decreased water potentials harms seed germination of M. pugana by reducing its germination rate and germination percentage.
Materials and methods
Study species. Magnolia pugana is an evergreen tree species of high longevity that grows up to 25 m tall and could reach over 1.5 m in dbh (Vázquez-García et al. 2021b). It occurs naturally in western Mexico between 1,100 to 1,569 m asl., with mean annual temperatures from 20 to 26 °C, and annual precipitation from 900 to 1,000 mm (Jacobo-Pereira et al. 2016). The reproductive age of the species has not been reported; however, based on cultivated plants, it starts at seven years of age. This species exhibits a flowering period between March and June, with the possibility of observing flowers throughout the year (Dahua-Machoa 2018). Additionally, it produces white fragrant flowers and has narrow oblong or elliptic coriaceous leaves (Figure 1A). Their fruits are dehiscent oblongoid polyfollicles that usually stay joined together, with dehiscence occurring in April or May of the following year (Figure 1B). The polyfollicles contain 21 to 47 seeds with a size of approx. 3-7 mm covered with a scarlet aril (Figure 1C). The species is used for medicinal purposes, where its petals, prepared as tea, are thought to have properties beneficial for the treatment of the heart (Osorio-Muñoz 2020).

Figure 1 Magnolia pugana: A) Flower, B) fruit (oblongoid polyfollicles), and C) seeds (with or without aril).
Sampling sites. To obtain current environmental conditions and sampling heterogeneity, two populations of M. pugana located in tributary creeks that drastically reduce their flow in dry seasons were selected, one from Palo Verde, in the municipality of Mezquital del Oro, southern Zacatecas, Mexico (21° 15´ 38. 4” N, 103° 18´ 22.3” W; 1,530 m asl), and the other near San Nicolás, in the municipality of Zapopan, central Jalisco, Mexico (20° 48´ 53.4” N, 103° 34´ 49.8” W; 1,445 m asl). Its mean annual temperatures are 22.1 and 20.6 °C, while annual precipitation is 803.5 and 1,007.1 mm, respectively (CONAGUA 2019).
Seed collection. Mature polyfollicles of the two populations were collected in April 2019, from at least 10 M. pugana parental trees which were separated by distances of 10-100 m from each other. Approximately 2,100 seeds were sorted and then treated with a 3 % sodium hypochlorite solution for 30 min to remove fungal contamination and later dried with absorbent paper (Saldaña-Acosta et al. 2001). Seeds were stored in a plastic container in a conventional refrigerator at 4 °C to avoid dehydration (Jacobo-Pereira et al. 2016).
Viability test. A sample of thirty seeds was randomly selected from a mixture from the two locations. Aril was removed via manual scarification and then seeds were placed in sterile plastic Petri dishes (90 × 15 mm). The seeds were washed in running water, dissected transversely with a scalpel, submerged in a 1 % tetrazolium solution, and then placed in a drying oven (JISICO Co., Ltd. J-DECO) at a temperature of 30 ºC for 24 hours in complete darkness (Yaklich & Kulick 1979, Jacobo-Pereira et al. 2016). Finally, the seeds were examined under a stereoscope and classified according to the coloration of the embryo. Embryos dyed red were considered viable and those presenting no coloration were considered non-viable (Baskin & Baskin 2014).
Germination tests. The experimental design was a 3 × 5 factorial arrangement, comprising three constant temperatures (24, 28, 37 °C) and five water potentials (Ψw of 0, -0.3, -0.6, -0.9, -1.2 MPa). The temperature of 24 °C was selected because it is the optimum temperature for the germination of this species (Bonner & Karrfalt 2008) and represents the average of the month when seeds are dispersed for germination, whereas 28 °C is the maximum temperature in the warmest month in the habitat. We consider that 37 °C could represent the maximum extreme temperature to which M. pugana seeds could be exposed in western Mexico under the climate change scenario RCP 4.5 projected for future years (2050-2100) in groups G3 and G5, which includes levels of severe warming (2 to 3 °C) and severe drying (-50 to -10 mm of seasonal precipitation), and levels of moderate continentalization (0-1.5 °C) (Ibarra-Montoya et al. 2011, IPCC 2014, Ruiz-Corral et al. 2016, CONAGUA 2019).
The five different water potentials (Ψw of 0, -0.3, -0.6, -0.9, -1.2 MPa) were chosen to simulate the diverse levels of water stress that seeds would experience under global climate change scenarios, and are within the range of values described for different soil types worldwide (Dürr et al. 2015). The different water potentials were calculated and prepared with polyethylene glycol (PEG 8000) following Michel (1983). PEG 8000 was dissolved in distilled water and placed in a magnetic stirrer for 16 hours at 20 °C. Each treatment combination for five water potentials and the three temperatures comprised five repetitions of 20 randomly selected seeds. Seeds were placed separately in sterile plastic Petri dishes (90 × 15 mm) containing 20 ml of the respective PEG 8000 solution and sealed with plastic wrap to prevent evaporation, while 20 ml of distilled water (Ψw = 0 MPa) in a temperature of 24 °C was used as control. The experiment used a 12 hours’ photoperiod in a germination chamber (Lumistell ICP-19 d-c/iv), Treatments at different temperatures and water potentials were evaluated over 45 days the time suggested by Barbour (2008) necessary for Magnolia seeds to germinate. Seeds showing an emerged radicle were considered germinated (Baskin & Baskin 2014). The number of germinated seeds was counted, and the germination percentage (GRP) and mean germination time (MGT) were calculated. The MGT of germinated seeds per experimental unit was calculated using the following formula according to Ranal et al. (2009):
Where n i is the number of seeds germinated in the i th time; k is the last day of germination evaluation; t i is the time from the beginning of the experiment to the i th observation, given in the corresponding experimental unit expressed in the number of days.
The GRP was transformed with Arcsine square root, to ensure homogeneity of variances (Ranal & Santana 2006). Shapiro-Wilk test was used to test for normality and a Bartlett test was used to test for equal variances (Crawley 2012). A two-way analysis of variance (ANOVA) was performed to study the effects of the factors: temperature, water potential, and its interaction on GRP and MGT. Environmental chambers were nested to the temperature factor. All statistical analyses were performed with the GerminaR package (Lozano‐Isla et al. 2019) in R. When statistical differences were observed, means were compared using multiple comparisons Student-Newman-Keuls (SNK) (α = 0.05). All statistical analyses were carried out in the R software v. 3.5.2 (R Core Team 2018).
Results
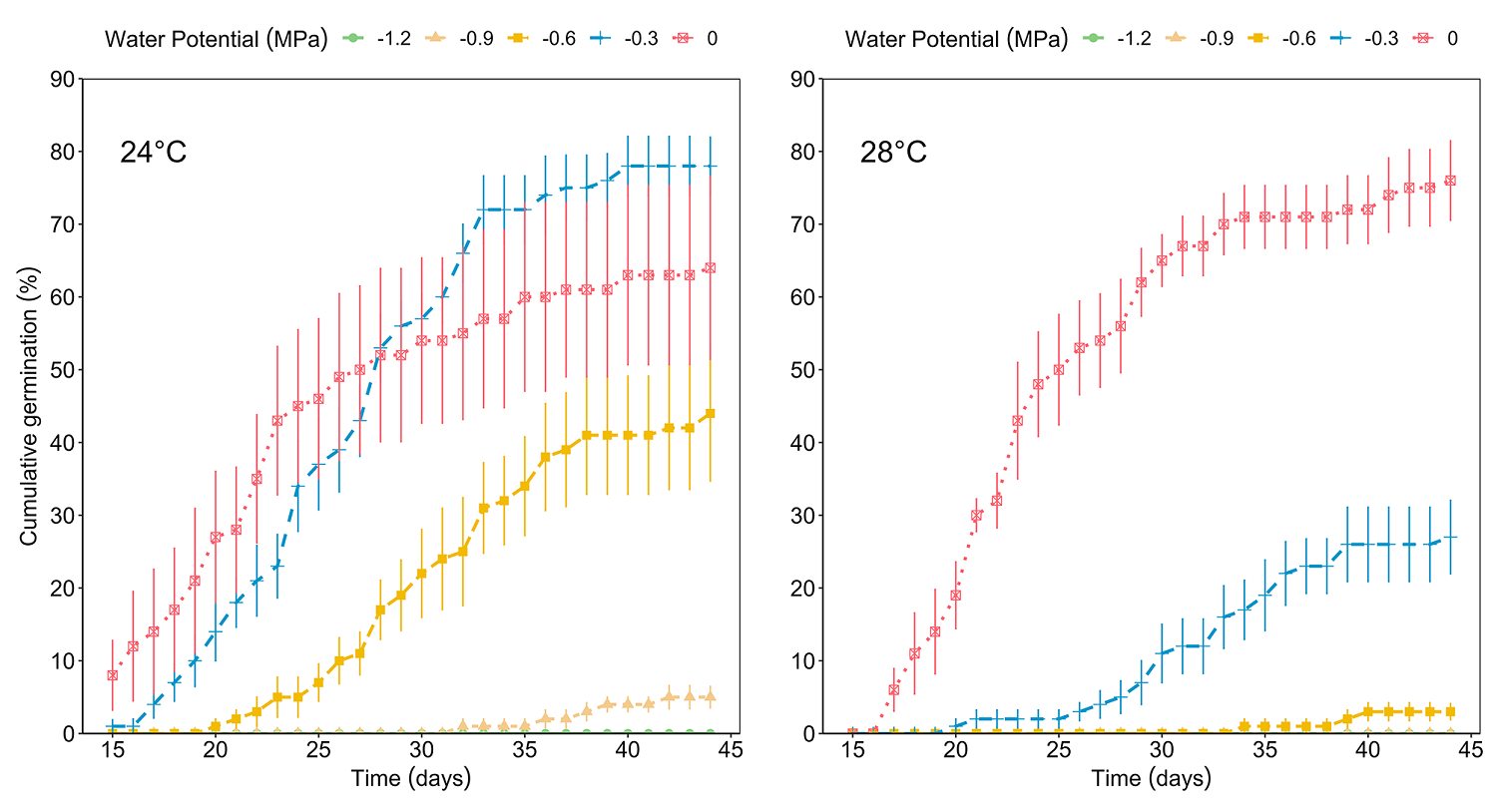
The viability test showed positive results for 24 viable seeds out of 30 (80 %). As no germination was observed in the seeds placed at 37 °C, this temperature was excluded from the analysis. The ANOVA test for GRP revealed significant effects of both factors, temperature (F = 24.49, P < 0.001) and water potential (F = 68.75, P < 0.001), and of its interaction (F = 13.32, P < 0.001) (Figure 2). The GRP decreased significantly as the temperature increased and water potential decreased. On the other hand, the effect of the interaction between temperature and water potential on MGT was significant (F = 3.86, P < 0.001). The MGT was delayed due to the effect of decreasing water potentials (F = 24.00, P < 0.001) and increasing temperature (F = 20.01, P < 0.001) (Figure 3). The highest GRP was 78 % at 24 °C and water potential Ψw = -0.3 MPa, while the lowest was 5 % at 24 °C and water potential Ψw = -0.9 MPa (Table 1).
Table 1 Effects of temperature and water potential (Ψw) on germination percentage (GRP) and mean germination time (MGT) of Magnolia pugana.
| Temperature (°C) / water potential (Ψw, MPa) |
GRP (%) | MGT (days) |
|---|---|---|
| 24/0 | 64 ± 12.6 a | 23.9 ± 1.5 d |
| 24/-0.3 | 78 ± 4.0 a | 26.4 ± 0.4 cd |
| 24/-0.6 | 44 ± 9.4 b | 30.16 ± 1.2 bc |
| 24/-0.9 | 5 ± 1.6 c | 36.62 ± 1.8 a |
| 24/-1.2 | 0 ± 0 c | 0 ± 0 a |
| 28/0 | 77 ± 13 a | 24.79 ± 0.9 d |
| 28/-0.3 | 28 ± 4.6 b | 33.08 ± 1.2 ab |
| 28/-0.6 | 3 ± 1.22 c | 37.6 ± 1.8 a |
| 28/-0.9 | 0 ± 0 c | 0 ± 0 a |
| 28/-1.2 | 0 ± 0 c | 0 ± 0 a |
Significant differences between treatment means are indicated by lower-case letters next to the standard error according to the SNK test (α = 0.05).
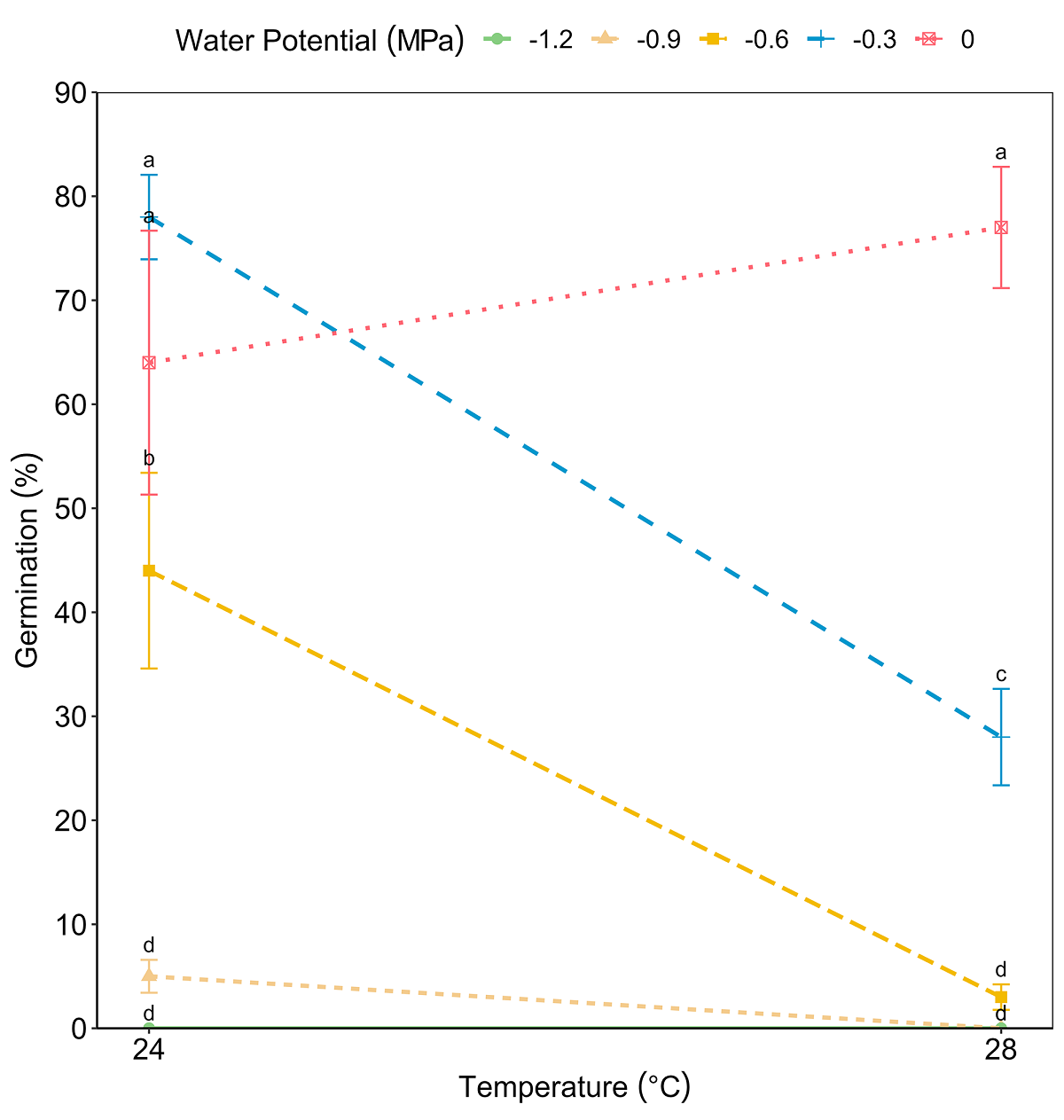
Figure 2 Interaction of factors temperature and water potential on the germination percentages of Magnolia pugana seeds, different letters mean statistical differences between averages by multiple comparisons (Student-Newman-Keuls) (α = 0.05).
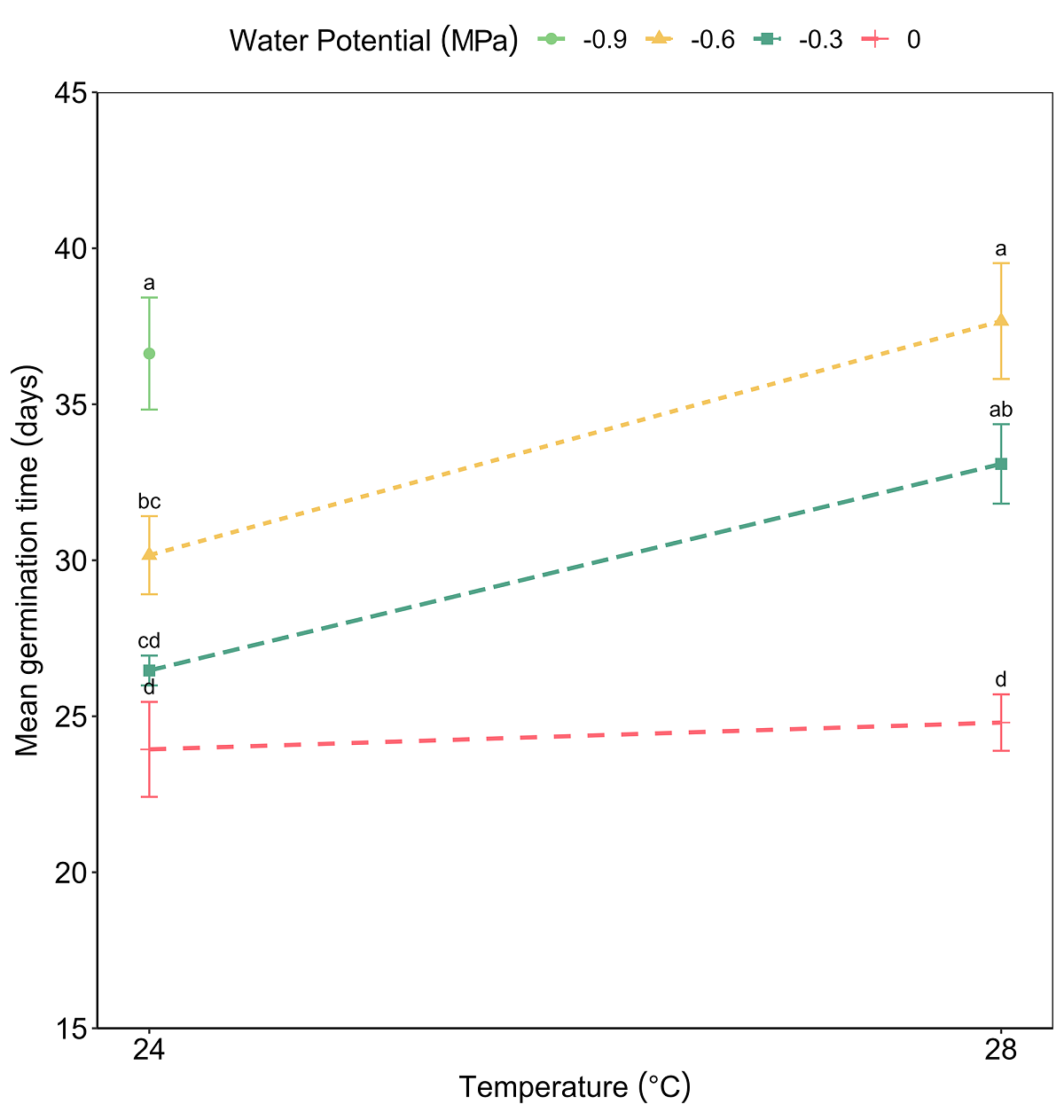
Figure 3 Effects temperature, water potential, and interaction between factors, on the mean germination time of Magnolia pugana seeds, different letters mean statistical differences by multiple comparisons (Student-Newman-Keuls) (α = 0.05).
Additionally, when water potential Ψw = 0 MPa was applied at 28 °C resulted in 77 % of germination, but with the same temperature and Ψw = -0.6 and -0.9 MPa, resulted in a significant decrease in the number of germinated seeds (3 and 0 %, respectively). Finally, a water potential Ψw = -1.2 MPa resulted in no germination at both temperatures.
The water potential Ψw = 0 MPa facilitated germination in a shorter time at temperatures of 24 °C (MGT = 23.93 ± 1.52 days), and at 28 °C (MGT = 24.79 ± 0.90 days), while the Ψw = -0.9 MPa treatment registered a delay for MGT at 24 °C (36.62 ± 1.79 days) and it produced any germination at 28 °C. In the combination of 24 °C and water potential Ψw = 0 MPa (control), seeds began to germinate on day 16, on the other hand, with 28 °C and Ψw = 0 MPa germination initiated the day 17th. At 24 °C and Ψw = -0.3 MPa germination began on day 17, but at 28 °C and -0.3 MPa, the beginning of germination was delayed until day 20 (Figure 4). At the other lower water potential treatments and the higher temperature combinations, germination was delayed even more or was zero. MGT increased due to the effect of decreased water potential and high temperature.
Discussion
This study represents the first report about the effects of temperature and water potential as combined factors on the germination of Magnolia seeds. Our results support our hypothesis that germination of Magnolia pugana is negatively affected by the combined effect of increasing temperature and water stress, thus confirming that germination in this species would be adversely affected under the predicted conditions of global warming. Interactions between low water potential due to decreases in precipitation (up to 10 %), and temperature increases (between 2 and 4 °C), are conditions that may be present in habitats within the natural distribution of M. pugana under climate change scenarios (Durán 2010, Ibarra-Montoya et al. 2011, IPCC 2014, Ruiz-Corral et al. 2016). We infer that a temperature increment of 4 °C (up to 28 °C) only affects germination if there is water stress, but temperatures higher than 28 °C inhibit germination in drier conditions.
Our results showed that GRP decreased and MGT increased at the highest temperature (28 °C) in combination with lower water potentials (Ψw of -0.3, -0.6, -0.9, and -1.2 MPa). At water potential Ψw = 0 MPa GRP was not different between 24 and 28 °C treatments but decreased to 0 % at the highest temperature (37 °C). Temperatures higher than 30 °C have been reported to decrease the germinability of Magnolia wilsonii, M. sinica, and other tropical woody species (Han & Long 2010a, Buttler et al. 2014, Lin et al. 2022). This could be explained by the fact that increasing temperatures and desiccation unbalance endogenous abscisic acid concentrations by decreasing auxins, gibberellins, and cytokinins to inhibit germination (Baskin & Baskin 2014, Liu et al. 2019). Similar results have been observed for some species in other temperate and humid environments: the effect of temperature (5 to 25 °C) and water potentials (Ψw = -0.10 to -0.30 MPa) on germinability for riparian species (Salix alba, S. triandra, S. viminalis, and Populus nigra) resulted in 80 to 100 % germination at all temperatures for all species (Van Splunder et al. 1995), at this temperature range, thermal variation was not a restrictive factor for the cited species as it was not for M. pugana; however, germination percentages for all these species were from approximately 100 % at Ψw = -0.10 MPa and decreased to 0 % at Ψw = -0.26 MPa.
The later results agree with those reported for two riparian species, Populus euphratica, and P. pruinosa, which showed considerably lower germination percentages with decreasing water potentials, to almost 0 % at Ψw = -0.6 MPa (P. pruinosa), and up to 5 % at Ψw = -0.9 MPa (P. euphratica) (Li et al. 2006), whereas both species had 100 % germination at Ψw = 0 MPa. Although Li et al. (2006) took into account the factors included in the present study, they did not evaluate their interactions.
The reduction in germinability with increasing temperatures has been observed for other temperate mesic tree species such as Magnolia officinalis (Zhou et al. 2012), M. sinica (Lin et al. 2022), and Acer saccharum (Solarik et al. 2016). M. officinalis experienced the highest germination percentage between 15 and 25 °C, which coincides with the results in the present study, as well as the finding that temperatures higher than 30 °C also considerably decreased germination percentage (Zhou et al. 2012). Similarly, Lin et al. (2022) reported a M. sinica germinability ca. 87 % at 25/15 °C but poorly at 30 °C. A possible explanation for these responses could be related to the environmental conditions of their habitats (Han & Long 2010a, b, Fernando et al. 2013, Iralu & Upadhaya 2016, Aragón-Gastélum et al. 2018). Germinability and germination speed represented by MGT may be affected by seed quality (e.g., seed size, mass, and nutrients), which are strongly related to environmental factors such as temperature, light, and precipitation of the seed provenances (De Frenne et al. 2011, Chamorro et al. 2013, Carón et al. 2014). Thus, the sensitivity of germination to climatic variability could be a function of the phenotypic plasticity of the species, its local adaptation, and its geographic distribution (Nicotra et al. 2010, Cochrane et al. 2015).
Therefore, we infer that germination of Magnolia pugana seeds is likely to be manifested by decreasing germination percentages and delayed mean times, due to the future warmer climate and lower precipitation predicted for the geographical distribution area of this species (IPCC 2014, Ruiz-Corral et al. 2016). A similar effect was found with temperature increase for M. sinica and M. wilsonii, for which climate warming could hurt their germinability (Han & Long 2010a, Lin et al. 2022). In addition, Vázquez-García et al. (2021b) predicted a loss of 66 % of the suitable environmental area of M. pugana by the end of the present century under a scenario of high emissions of greenhouse gases (SSP3-7.0), which may increase its vulnerability to extinction, since not only its germination could be affected, but also the survivorship of seedlings, saplings, and adult trees.
Germination percentages in Magnolia pugana decreased and MGT increased with reducing water potential, as reported in other tropical woody plant species showing faster germination time and the highest percentage at a water potential of Ψw = 0 MPa and the lowest at Ψw = -1.0 MPa (Daws et al. 2008). However, these authors did not evaluate the interaction with temperature, since they focused on ten species at a single temperature (26 °C). Generally, in subtropical species, such as eucalypts, germination does not occur in water potentials of less than Ψw = -0.25 MPa, with greater germination typically occurring at Ψw = 0 MPa (López et al. 2000). In contrast, it has been reported that in Cercidium praecox and Prosopis laevigata, from semi-arid environments, a 0 MPa treatment resulted in the lowest germination 77 and 64 % respectively, while a Ψw = -0.41 MPa resulted in 100 % for both species (Flores & Briones 2001). In general, for these xerophilous species, the germination percentage increased with increasing temperature, in combination with low water potentials, while the MGT was shorter, as reported for seeds associated with drier habitats which present higher germination percentages in a reduced time (Evans & Etherington 1990).
The viability of seeds obtained in the present study was 80 %, higher than that previously reported for the same species (Jacobo-Pereira et al. 2016). Various authors have reported different viability percentages in Magnolia species: 78 % for M. iltisiana (Saldaña-Acosta et al. 2001), 100 % for M. dealbata (Corral-Aguirre & Sánchez-Velásquez 2006), 80 % for M. schiedeana (Vásquez-Morales & Sánchez-Velásquez 2011), 92 and 87.5 % for M. perezfarrerae and M. sharpii, respectively (Vásquez-Morales & Ramírez-Marcial 2019). Habitat characteristics, longevity during storage, seed moisture content, and the morpho-anatomical features of recalcitrant seeds explain this variability (Ibrahim & Roberts 1983, Vaz et al. 2018). Our main conclusion in this experimental study on the effect of increased temperature and drought stress on the germination of Magnolia pugana seeds is that the interaction of simulated drought and temperature increases affected decreasing germination percentages and delaying the mean germination time. Such conditions are expected under climate change scenarios where severe warming (> 3 °C) and levels of severe drying -300 to -150 mm (< 10 % of seasonal precipitation) in the species distribution zones, which will, in turn, determine the dynamics of natural populations, increasing their medium to long-term vulnerability. A temperature increment of 4 °C (at a germination temperature of 28 °C) only affects germination if there is drought stress. At drier conditions, temperatures higher than 28 °C inhibit germination. The observed effects of temperature increase and water potential reduction to the germination of M. pugana seeds will help predict the fate of this critically endangered endemic species under future climate change.











 nova página do texto(beta)
nova página do texto(beta)