Located at the convergence of North and South America, Mesoamerica is one of the most biodiverse regions on our planet (i.e., a hotspot sensuMyers et al. 2000). This region ranks third in the list of 34 global biodiversity hotspots (Myers et al. 2000, Miller et al. 2001). Several explanations have been suggested for such high biological diversity. A prominent one is related to the great biological mixing that resulted from the southward and northward migrations of numerous elements from the Nearctic and Neotropical biotas, respectively, after the formation of the Central American bridge (Raven & Axelrod 1974, Graham 2011). Paradoxically, the Mesoamerican hotspot has also been relatively isolated from areas located north and south of it, as it is enclosed by the Isthmus of Panama to the south (Raven & Axelrod 1974, Stehli & Webb 1985), and by the mountains of central and northern Mexico, particularly the Trans-Mexican Volcanic Belt, to the north (Myers et al. 2000). Additionally, there is evidence that multiple taxa occurring in Mesoamerica have undergone particularly high speciation rates, leading to the explosive radiation of some groups (Jaramillo-Correa et al. 2009, Gutiérrez-Rodríguez et al. 2011). From an ecological perspective, the heterogeneous geographical setting of the region also contributes to its biological wealth, as it comprises a large variety of environments including extensive coastal plains and complex mountain systems with assorted geological bedrocks and broad elevational gradients (DeClerck et al. 2010). Currently, the floristic richness of the entire Mesoamerican region is estimated at over 24,000 vascular plant species, of which about one quarter are regional endemics (Myers et al. 2000). Given its large size, along with its outstanding biodiversity and the presence of relatively well-preserved ecosystems, this region has been highlighted among the highest priorities for biodiversity conservation worldwide (Miller et al. 2001). Notwithstanding its relevance for both the global and continental biological diversity, and despite significant efforts made over the last two centuries to contribute to the botanical knowledge of the Mesoamerican hotspot, it is remarkable that our understanding of its flora is still scattered and uneven. A sensible way to move forward in this regard would be through the preparation of thorough floristic checklists for naturally defined regions within the hotspot.
The Usumacinta River Basin (URB), centrally located in Mesoamerica, is a large naturally defined region stretching over seven million hectares that includes a broad gamut of landscapes, ranging from the cold Guatemalan Highlands in the Cuchumatanes region where the headwaters of this complex fluvial network are located, down to the discharge zone in the Gulf of Mexico (de la Maza & Carabias 2011, Meave et al. 2021). In the URB, three sectors can be distinguished according to topographic, geomorphological and hydrological features (Figure 1). The highest elevations correspond to the upper Usumacinta basin (hereafter, upper basin), which represents the main catchment sector of the basin; in the upper basin is located the Sierra de los Cuchumatanes, an area characterized by the highest levels of plant endemism in Mesoamerica (Véliz Pérez 2008) and the presence of large cloud forest tracts; unfortunately, these forests are not only being rapidly cleared at present, but they likely represent the most susceptible forest ecosystems to increased temperatures under future scenarios of global change (Hulme & Sheard 1999, Ornelas et al. 2013). Further downriver is the middle Usumacinta basin (hereafter, middle basin), a sector mainly characterized by processes of water transport and transference, which hosts the largest tracts of moist tropical forest within the Maya lands (part of the Guatemalan Petén department and the Lacandona region in Mexico; Myers et al. 2000). Finally, where the river approaches the Mexican coastline of the Gulf of Mexico is the lower Usumacinta Basin (hereafter, lower basin); this sector is predominantly a discharge zone that encompasses extensive continental wetlands of the San Pedro River in Guatemala, along with a large wetland zone across the deltaic plain of the Usumacinta in Mexico; this area boasts the largest diversity of aquatic plants in Mesoamerica (Lot & Novelo 1988, Méndez-H. et al. 2001, Novelo & Ramos 2005, López-Jiménez et al. 2020).
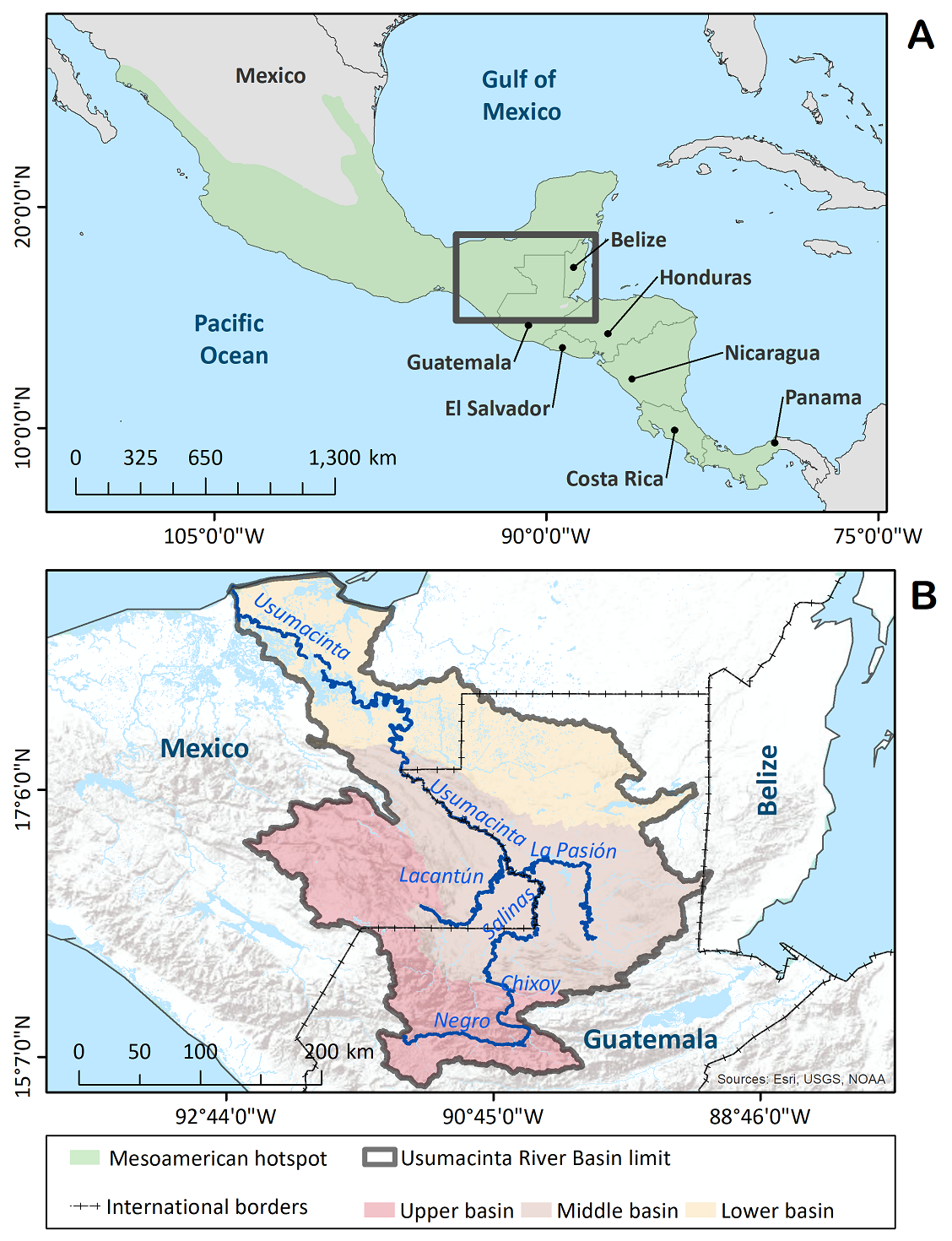
Figure 1 (A) The Mesoamerican hotspot in Central America and Mexico, sensuMyers et al. (2000). (B) Location of the Usumacinta River Basin in Guatemala and Mexico. In (B), the different colors indicate the elevational sectors into which the basin was divided.
The earliest efforts to document the vascular plant diversity in the URB correspond to the botanical surveys conducted by José Mariano Mociño in Guatemala and the Mexican state of Chiapas between 1795 and 1799 (CONABIO 2012); however, the first plant collections were not made until the 19th century, by Jean Linden in the lower basin (Ceulemans & Viane 2006). In the 20th century, several large projects significantly advanced our floristic knowledge of the URB including the studies of Faustino Miranda (1953, 1961) in Chiapas; Paul C. Standley and Julian A. Steyermark, who led the colossal project Flora of Guatemala between 1947 and 1977 (Standley & Steyermark 1946); Dennis E. Breedlove (1981) with the Flora of Chiapas project, which included many areas located within the URB; Cyrus L. Lundell (1942), who explored the wetlands of the deltaic plain of the Usumacinta and the Petén department in Guatemala; and Clark P. Cowan (1983), who published the first checklist of vascular plants for Tabasco state, Mexico, including numerous species from the extensive wetlands within the URB. Efforts at the sub-regional level have also resulted in significant progress in the study of the region’s flora over the years: West et al. (1987) explored the flora of the Tabasco lowlands; Martínez et al. (1994) prepared a checklist of vascular plants for the Lacandona region; and more recently, Ochoa-Gaona et al. (2018) studied aquatic and riverine plants for the Mexican sector of the URB. In addition, several studies have focused on particular areas within the URB, which have provided valuable insights into its biodiversity: Carlson (1954) in the Montebello Lakes region in Chiapas; Véliz Pérez (1998) in the Sierra de los Cuchumatanes (Guatemala); Meave et al. (2008) in Yaxchilán (Chiapas); Nesheim et al. (2010) in the lowland forests of the Maya Biosphere Reserve in Petén; and more recently Jiménez-López et al. (2018) and López-Jiménez et al. (2020) in the Centla wetlands of Tabasco. In addition, the floristic knowledge of the URB has indirectly benefited from the Flora Mesoamericana project (e.g., Davidse et al. 1994, 2018). Despite all this work and considering the relevance of the biodiversity it hosts, no comprehensive account of the vascular plant species richness has been produced to date for the URB.
A review of the botanical exploration conducted in the URB over the last two centuries reveals a long albeit highly fragmented history. Most published accounts are restricted to political units located within the basin (states or lower territorial units). Given this scenario, it became evident that there was an urgent need to focus on the study of the floristic diversity of the URB by viewing it as a single natural region, defined by the drainage area of the Usumacinta River and its multiple tributaries. Considering the differences in the history of floristic knowledge acquisition between these two countries, performing a cross-border integration of the floristic knowledge of this region as a whole became a task of utmost importance, particularly in the context of lowland tropical and montane forest conservation in Mesoamerica. We addressed the following research questions: What is the magnitude of the vascular plant species richness in the Usumacinta River Basin? What are the peculiarities of this flora regarding the most speciose families and genera? Are there noteworthy floristic elements in this flora? Finally, how uniform is the spatial distribution of records of vascular plants in this region?
Materials and methods
Study region. The Usumacinta River Basin is delimited by the watershed divide that separates the waters flowing to the mouth of the Usumacinta from waters flowing elsewhere. Extreme coordinates framing the URB are 14( 53’ 33” - 18( 42’ 01” N, and 89( 07’ 10” - 92( 42’ 51” W (Figure 1). Despite its irregular shape, the limits of the URB in different directions are readily identifiable: the volcanic mountain ranges of Guatemala represent its southern limit; to the east, the region abuts the Maya Mountains of Belize; the western limit is mainly provided by the mountain system that delineates the Central Chiapas Highlands, which separates the URB from the Grijalva River Basin; and to the north, the limit is represented by the Gulf of Mexico coastline. The URB so defined encompasses a total surface of 77,435.9 km2, an area slightly larger than the Republics of Panama or Ireland. More than half (55.8 %) of the URB is located in Guatemala, with the remaining 44.2 % in Mexico; there is also a very small portion of the basin in Belize (0.04 %), which was not considered in this study (Meave et al. 2021).
The river after which the basin is named —the Usumacinta— starts in the middle basin, at the point where the Lacantún, La Pasión and Chixoy rivers converge. Before this point and along more than 360 km the fluvial system flows through Guatemalan territory, and thereafter it becomes the fluvial border between Chiapas, Mexico, and Petén, Guatemala; over the following 386 km the river flows mostly through the lower basin in Tabasco, Mexico. Finally, the Usumacinta joins the Grijalva River some 24 km from the coast and their waters are discharged through a common mouth into the Gulf of Mexico (de la Maza & Carabias 2011, Gallardo-Cruz et al. 2019). According to the volume of water discharged into the Gulf, the Usumacinta is second only to the Mississippi River in North America, and the seventh worldwide (de la Maza & Carabias 2011, Kolb & Galicia 2012).
The URB encompasses a broad elevational gradient that begins at the highest elevations of the Sierra de Los Cuchumatanes in Huehuetenango department, Guatemala (ca. 3,700 m asl), and drops to sea level at the Tabasco and Campeche coastline. Accordingly, there is a remarkable reduction of mean annual temperatures with increasing elevation, ranging from 28 °C in the lower basin, through 24 °C in the middle basin, to 10 °C in the upper basin (de la Maza & Carabias 2011). Kolb & Galicia (2012) reported a total annual precipitation of 2,068 mm on average for the entire basin; this single figure conceals the enormous variation in rainfall across the different elevational sectors of the URB. Although dry regions are almost completely absent within the URB (except for some intermontane valleys in Huehuetenango and Quiché departments in Guatemala, and apparently along the Jataté river in Chiapas, where vegetation is tropical dry forest), the lowland areas of the coastal plain receive much less precipitation than areas located higher up in the mountains. Indeed, perhaps the most striking feature of the basin’s climate is the extremely high precipitation in some highland areas in Guatemala (ca. 8,000 mm), which explains the large discharge of the rivers draining the basin into the Gulf of Mexico (Meave et al. 2021).
Database construction and nomenclatural and taxonomic curation. The construction of the vascular plant database for the URB was done in three phases: (1) initial information compilation, (2) geographical filtering, and (3) taxonomic and nomenclatural curation. Figure 2 illustrates the workflow that led to the construction of the database. We used Microsoft Excel to integrate the database. Herbaria acronyms mentioned in the complete text follow Thiers (2016).
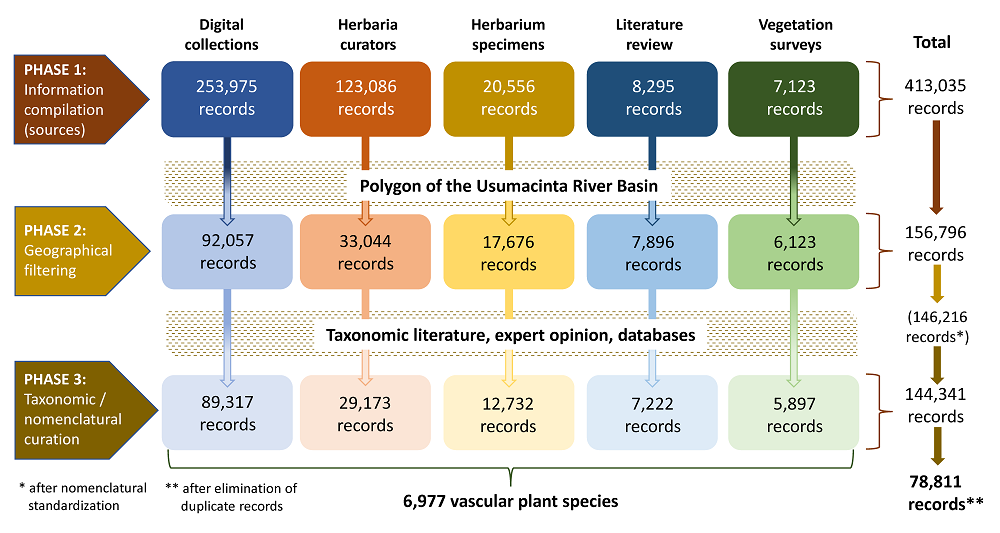
Figure 2 Workflow diagram depicting the three phases involved in the construction of the database of vascular plant species from the Usumacinta River Basin in Guatemala and Mexico. The figures in the boxes are the number of records corresponding to each source of information at each phase. The nomenclatural standardization step is described in the text.
Phase 1 consisted in the compilation of information from different sources, which can be broadly classified into five categories:
(i) National digital collections. First, we were granted permission to download 115,831 records from Tabasco, Chiapas and Campeche states in Mexico, and from Guatemala, from the SNIB-REMIB, which is Mexico’s National Biodiversity Information System, under the management of the National Biodiversity Comission (Sistema Nacional de Información sobre Biodiversidad de México, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). Next, we downloaded 138,144 records from Tabasco, Chiapas and Campeche states from the digital repository of the National Herbarium of Mexico (MEXU), Universidad Nacional Autónoma de México. These two sources produced a total of 253,975 records.
(ii) Information obtained from herbarium databases. We approached the curators of three herbaria (MO, CH, and AMO) hosting significant collections for the study region and adjacent areas to request information on plant collections with this geographical scope. Thanks to their kindness, this resulted in 101,594 records from MO for Guatemalan departments and Mexican states with territories in the URB, 4,078 records from CH for Chiapas municipios, and 7,332 records of orchid species from Tabasco, Chiapas and Campeche from AMO. In addition, we retrieved 10,082 records of tree species from Chiapas from CAS through the Global Biodiversity Information Facility (GBIF) (see González-Espinosa 2019). In total, this procedure contributed 123,086 records to the database.
(iii) Herbarium specimens. Information was also acquired through direct examination of specimens deposited in four herbaria. From BIGU we obtained ca. 15,000 records for plants from Guatemalan departments within the URB, and smaller numbers from CHIP and HEM (records from Chiapas), and MEXU (records from Tabasco and Chiapas). Specimen examination resulted in 20,556 records for the database.
(iv) Literature review. We conducted a review of literature hosted digitally in three Mexican academic institutions: the Universidad Nacional Autonóma de México (Mexico City), El Colegio de la Frontera Sur (San Cristóbal de Las Casas, Chiapas), and the Universidad Juárez Autonóma de Tabasco (Villahermosa, Tabasco). Additionally, we conducted searches in four international databases, namely the Web of Science, ScienceDirect, SCOPUS, and Google Scholar, focusing on scientific publications reporting checklists of native vascular plants from areas located within the URB. This stage included studies at the local (i.e.,Véliz Pérez 1998, Meave et al. 2008) and regional levels (Martínez et al. 1994, Méndez-H. et al. 2001, Véliz Pérez et al. 2014, Villaseñor 2016), floristic lists, but more importantly, taxonomic treatments (i.e.,Miller & Seigler 2012, Karremans & Vieira-Uribe 2020) (See Supplementary Material, Table S1). This procedure resulted in a total of 8,295 records.
(v) Regional vegetation surveys. Between 2014 and 2018 some authors of this work took part in vegetation surveys in 560 plots located across the Mexican portion of the URB. The database was supplemented with a total of 7,123 records of vascular plant species gathered through fieldwork.
The second major phase in the construction of the database consisted in the application of a geographical filter to select those records whose provenance could be unambiguously ascribed to the URB. This action was required because it was clear that the large number of records obtained from the five above-described sources (413,035) included numerous records whose provenance did not correspond with the URB. We strictly applied the criterion that any record with geographic coordinates outside the URB polygon had to be eliminated, while any record with geographic coordinates inside the polygon was retained, regardless of the proximity to the URB limit. This task was done on ArcGIS 10.4 ESRI (ESRI 2016). For those records lacking geographic coordinates, only those extracted from the literature review for studies unambiguously conducted within the URB were retained in the database (7,896 records). The filtering process resulted in considerable reductions in the number of records from the different sources: 92,057 records from digital collections, 33,044 from the information provided by curators, 17,676 from the examination of herbarium specimens, 7,896 from the literature review, and 6,123 from vegetation surveys. Geographical filtering resulted in a total of 156,796 records in the database (Figure 2).
The final phase in the construction of the database, and by far the most critical one, consisted in the curation of the taxonomic and nomenclatural information, including standardization and update procedures. To this end, we first standardized the scientific names and authors according to the The Plant List v. 1.1 database (www.theplantlist.org). The homogenizing process was performed automatically in R version 3.5.2 (R Core Team 2018), with the Taxonstand package (Cayuela et al. 2012). Thereafter, each scientific name was reviewed thoroughly to determine if the name was valid, "or if it was a synonym or basionym, as well as the most recent taxonomic and nomenclatural status. This process involved three steps: (1) the comparison of the scientific names with the Plants of the World (WCVP 2021) and Tropicos (Tropicos 2021), including the Flora Mesoamericana project (legacy.tropicos.org/Project/FM) databases; (2) the comparison with the checklist of the native vascular plants of Mexico (Villaseñor 2016) (see Villaseñor & Meave [2022] for an updated discussion of “of the current status of the Flora of Mexico”); and (3) the names of the species of several groups (e.g., Orchidaceae, Peperomia, Rubiaceae) were reviewed by specialists and updated according to their criteria (see Acknowledgments section). These steps were necessary due to the frequent updates undergone by botanical nomenclature. Furthermore, this process guaranteed that each species’ name and status included in our database was verified (e.g., Grose & Olmstead 2007, Henderson 2011a, b).
Finally, we used various online databases, including Flora Mesoamericana (Tropicos 2021), GBIF (GBIF 2021), Plants of the World (WCVP 2021), and the digital repository of MEXU (IBDATA 2021) to verify distribution status of each species (in order to classify them as native, cultivated or introduced), and determine its distribution within the URB. The outcome of this meticulous review was the final set of records from each source, as follows: 89,317 records from digital collections, 29,173 from the curators, 12,732 from herbarium specimens, 7,222 from the literature review, and 5,897 from vegetation surveys. It must be noted that the database so constructed included some level of redundancy because a given specimen collected by the same person could have fed the database through different sources of information. Hence, the final step consisted in removing 65,530 redundant records from the database, which was carried out with with the dplyr package (Wickham et al. 2019) in R (R Core Team 2018). Accordingly, the final database contains 78,811 records (Figure 2, Jiménez-López et al. 2023). The taxonomic arrangement of the checklist follows Christenhusz et al. (2011b) for ferns and allies, Christenhusz et al. (2011a) for Gymnosperms, and APG IV (2016) for Angiosperms.
Data analysis. To visualize the historical progress of botanical knowledge of the URB, we constructed accumulation curves for species and families, first, collectively for the entire basin, and then for the Mexican and Guatemalan portions separately. This analysis was conducted with the vegan package (Oksanen et al. 2019) in R (R Core Team 2018).
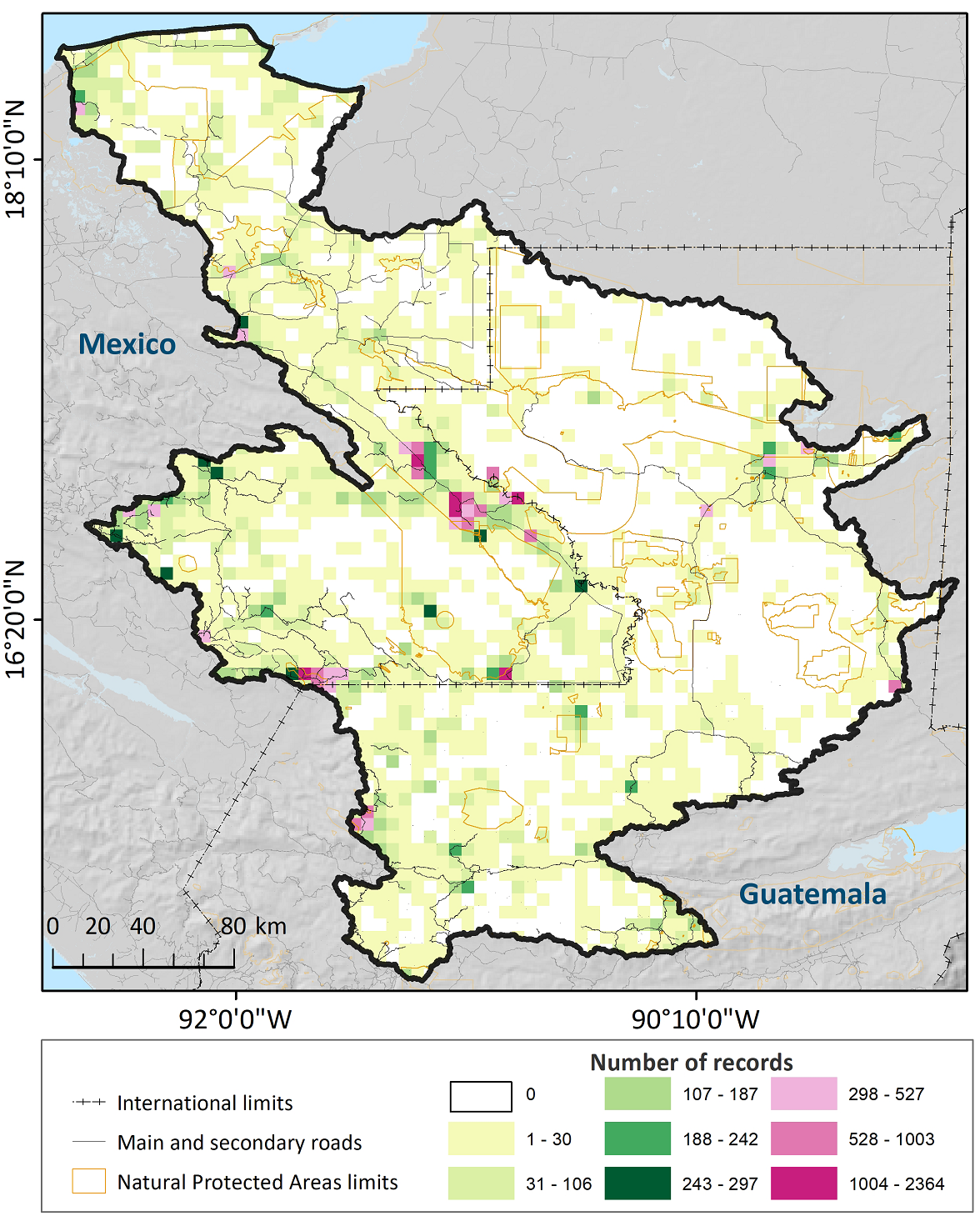
To provide a visual assessment of the geographic coverage of the botanical exploration in the different sectors of the basin, we divided the region into 5 × 5 km cells (2,857 in total) and then calculated the number of records in each cell. All records from the literature review were excluded from this analysis. Geographic layers of protected natural areas and major (primary and secondary) road networks in the region were included to explore the potential relationship between the spatial distribution of the records and these geographical features. The maps and the projection of the numbers of records in the grid cells were produced in the ArcGIS 10.4 ESRI software (ESRI 2016).
Results
Historical growth of the floristic knowledge in the Usumacinta River Basin. The first plant collections from the URB resulted from the work of Belgian botanist Jean J. Linden in 1838. From that year onwards, over 1,420 botanists/explorers have contributed to botanical collections. In general, during the 19th century this activity was moderate and episodic, but in the 20th and 21st centuries, this situation changed drastically as the botanical exploration of the region became much more intense, albeit still irregular (Figure 3). The first significant increase in the number of records occurred in 1905 with 513 records; however, the 1964-2018 period represents the time frame characterized by a constant growth in the number of records, with a mean of 1,165 records per year (minimum = 141 in 1978; maximum = 6,709 in 2002). Particularly fruitful periods in terms of the numbers of plant specimens collected in the URB were 1984-1986 (minimum = 2,218 in 1984; maximum = 3,099 in 1985), followed by important increases in 1998 and 2018 (2,342 and 3,270, respectively), although the highest peak occurred between 2002-2005 (average = 3,275, minimum = 1,185 in 2005, maximum = 6,709 in 2002) (Figure 3). It is important to note that this numerical description of the progress of botanical knowledge in the URB reflects this process in its entire territory, but not necessarily the development made in specific portions of it.
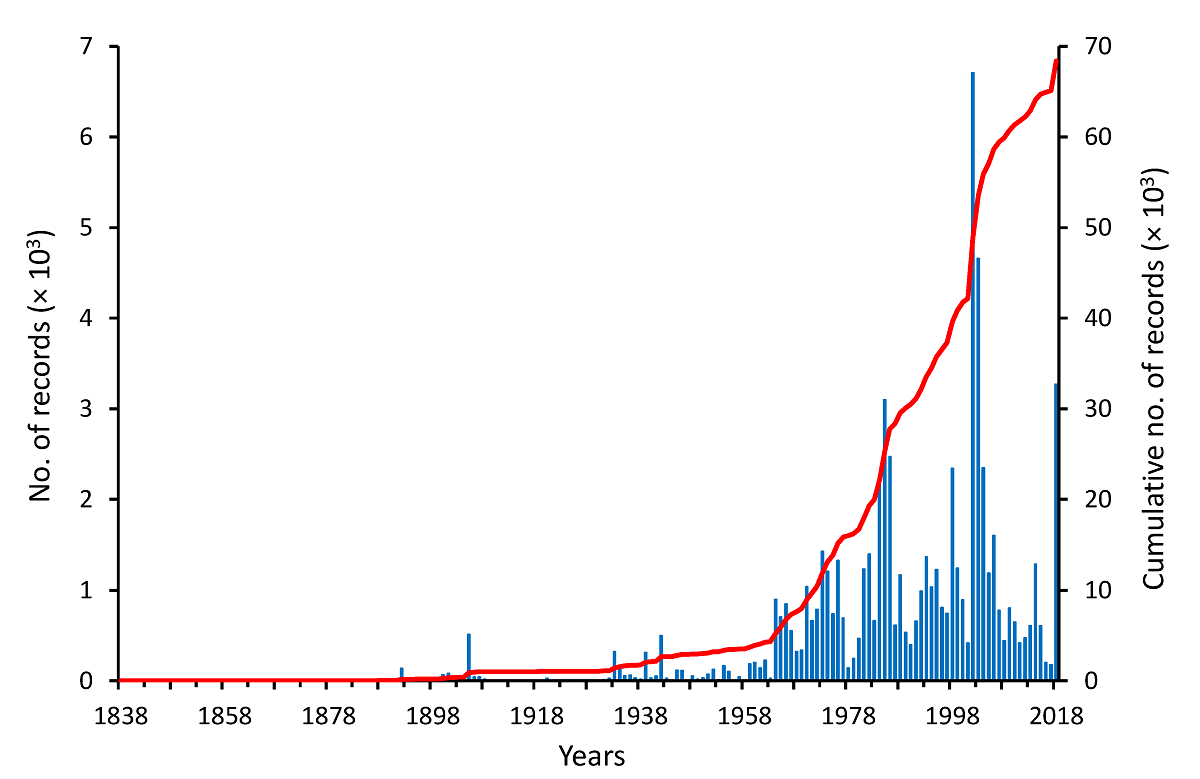
Figure 3 The historical pattern of the accumulation of vascular plant vouchers collected in the Usumacinta River Basin, Guatemala and Mexico, between 1838 and 2018. The red line indicates the accumulation of records over time.
Species richness and composition. The following floristic description for the URB includes all the species regardless of their distribution status (native, introduced, and cultivated). The total list of species per family and their distribution status are given in Table S2 (Supplementary Material). The database of vascular plant species for the entire URB includes 6,977 species, of which the large majority (6,577 species; 94.27 %) are native taxa, with only a small number of introduced or cultivated species (400 species; 5.73 %) (Supplementary Material, Tables S2 and S3). The species in the checklist are distributed among 1,892 genera and 274 families of vascular plants. At the subclass level, the group with the largest number of species was Eudicots with 4,435 species, followed by Monocots with 1,674 species; the group of ferns and its allies was considerably poorer, with 531 species. This pattern was very similar to that observed for genus and family richness in the different subclasses, as Eudicots and Monocots were the groups with the highest richness compared to the other subclasses. By contrast, the basal groups ANA and Gymnosperms had the lowest richness both in terms of genera and families (Table 1).
Table 1 Numerical distribution of family, genus and species richness by subclasses of vascular plants in the Usumacinta River Basin, Guatemala and Mexico. ANA is the angiosperm group with a basal position in recent phylogenetic trees and it represents a paraphyletic group relative to the other angiosperms. Chlorantales are a basal sister group of Magnoliids, but for practical reasons the only species on this list representing this order (Hedyosmum mexicanum C.Cordem., Chloranthaceae) was placed in Magnoliids. Finally, Ceratophyllales is regarded as a basal sister group of Eudicots but their two species reported here (Ceratophyllum muricatum Cham. and Ceratophyllum demersum L., Ceratophyllaceae) were placed in Eudicots.
| Group | Families | Genera | Species |
|---|---|---|---|
| ANA | 2 | 3 | 8 |
| Eudicots | 179 | 1,303 | 4,435 |
| Ferns and allies | 39 | 122 | 531 |
| Gymnosperms | 5 | 11 | 33 |
| Magnoliids | 12 | 38 | 296 |
| Monocots | 37 | 415 | 1,674 |
| Total | 274 | 1,892 | 6,977 |
As is often the case in the Neotropics, there were huge numeric asymmetries in the representation of the different families of this flora. The most speciose family was Orchidaceae (598 species), closely followed by Fabaceae (512) and Asteraceae (476); lastly, Poaceae (403) and Rubiaceae (275), although considerably less rich, were also prominent. These five families together accounted for roughly one-third (32.87 %) of the total species richness within the URB (Figure 4A). Overall, the first 15 families with the largest species richness were also those having the largest numbers of records (mean, 3,275; min, 1,237; max 7,274) (Figure 4A).

Figure 4 The most speciose families and genera in the flora of the Usumacinta River Basin, along with the number of records of specimen collections. (A) Families arranged in decreasing order according to the number of species included in them. (B) Genera arranged in decreasing order according to the number of species included in them. White bars, number of species collected per family or genus; blue and green bars, number of records collected per family or genus, respectively.
At the genus level, Miconia Ruiz & Pav. (Melastomataceae) was the most speciose genus, with 87 species, followed by a notable pair of genera of the same family, namely Piper L. and Peromia Ruiz & Pav. (Piperaceae), with 73 and 64 species, respectively. Next on the list are Epidendrum L. (Orchidaceae) with 60 species, Solanum L. (Solanaceae) with 59 species, and Ipomoea L. (Convolvulaceae), with 53 species. The mean number of records per genus for the 15 most speciose genera was 661.2 (min, 231; max 1,218) (Figure 4B).
Interestingly, the hierarchies displayed by the most speciose families depended upon the sector of the basin considered. For the lower basin, the ranking in decreasing order was Fabaceae (285 species), Poaceae (185), Asteraceae (169), and Orchidaceae (118) (Figure 5A); for the middle basin, the ranking was Orchidaceae (341), Fabaceae (325), Asteraceae (212) and Poaceae (210) (Figure 5B), whereas for the upper basin, the ranking was Orchidaceae (490), Asteraceae (410), Fabaceae (322) and Poaceae (266) (Figure 5C).

Figure 5 The most important families by species richness in the three elevational sectors of the Usumacinta River Basin. (A) lower basin, (B) middle basin, and (C) upper basin.
To analyze the flora in terms of growth form distribution, we classified each species into one of eight groups. Over half of the species present in the URB were herbs (58.72 %), and these were followed by trees (22.03 %), shrubs (13.14 %), and lianas (5.02 %). A considerably smaller proportion of species represented the palm or palm-like growth form, which encompassed all 51 species of the Arecaceae family (0.73 %), the 17 species of tree ferns (0.24 %) and eight species classified as arborescent plants (0.11 %) from Asparagaceae and Zamiaceae.
Collecting effort, contribution by country, and information gaps. The database of vascular plant species for the entire URB encompasses a total of 78,811 entries. This initial effort to systematize such a large number of botanical collections appears adequate given the compilation of records from herbaria with regional (which give priority to information at the state or department level), national (whose efforts aim at achieving knowledge for an entire country) and international scopes, and also due to the inclusion of information drawn from online databases and the direct consultation of vouchers in regional herbaria. Among other things, such a multi-pronged strategy revealed the large assymetry regarding the number of records and species between the two national sectors of the URB. For one, the number of records from the Mexican portion is more than threefold the number of records from Guatemala (58,859 vs. 19,952 records, respectively). Despite this large difference, it was surprising that the number of species recorded for Guatemala was considerably higher in relative terms, i.e., species richness by number of records (Guatemala: 4,445 species, Mexico: 5,746 species) (Table 2).
Table 2 Aereal extent of the Usumacinta River Basin in Guatemala and Mexico, along with the distribution of plant collection records and vascular plant species in the two countries. Numbers in parentheses are the percentages corresponding to these figures. Note that the percentages of numbers of species in the two countries cannot be added.
| Country | Area (km2) | Records | Species |
|---|---|---|---|
| Guatemala | 43,166.7 (55.8 %) | 19,952 (25.32 %) | 4,445 (63.71 %) |
| Mexico | 34,237.4 (44.2 %) | 58,859 (74.68 %) | 5,746 (82.36 %) |
| Total | 77,435.9 (100 %) | 78,811 (100 %) | 6,977 (100 %) |
The species accumulation curves both for the whole URB (Figure 6A) and for each country’s portion separately (Figure 6B) suggest that the catalog of vascular plant species is still incomplete, and that future botanical research would lead to a stronger increase in species richness for each individual sector than for the entire basin. Conversely, cumulative species/time curves for the family level suggest a higher completeness, both for the entire URB (Figure 6C) and for each country separately (Figure 6D).

Figure 6 Historical progress of the floristic knowledge in the Usumacinta River Basin, Guatemala and Mexico, based on the total number of species and families collected each year. (A) Cumulative species richness in the entire basin. (B) Cumulative species richness for the Guatemalan and Mexican portions of the Usumacinta River Basin, separately. (C) Cumulative family richness in the entire basin. (D) Cumulative family richness for the Guatemalan and Mexican portions of the Usumacinta River Basin, separately. In B and D; blue curves, Guatemala; red curves, Mexico.
The database also revealed some aspects of the geographical distribution and potential abundance of some species. The least common species were represented in the database by fewer specimens than species that are more abundant and more widely distributed. For example, the species with the largest number of records in the database (Psychotria costivenia Griseb. [Rubiaceae], Palicourea pubescens (Sw.) Borhidi [Rubiaceae], Trichilia pallida Sw. [Meliaceae], Malvaviscus arboreus Cav. [Malvaceae], Guarea glabra Vahl [Meliaceae] and Aphelandra scabra (Vahl) Sm. [Acanthaceae]; Table 3), are all very common in the vegetation across the URB. By contrast, 1,536 species (22.01 % of the total) have been collected just once (singletons); a smaller number, but no less important (887 species; 12.71 %) have been collected on two occasions only (doubletons). Together, singletons and doubletons accounted for 2,423 (34.73 %) of the species present in the URB. Repeating this analysis for species represented by a less restricted number of specimens (i.e., a species with a maximum of ten records), revealed that these represent a substantial proportion of the flora (4,947 species; 70.90 %).
Table 3 The 15 species with the largest number of records in the Usumacinta River Basin, according to herbaria data.
| Species | Records |
|---|---|
| Psychotria costivenia Griseb. | 307 |
| Palicourea pubescens (Sw.) Borhidi | 284 |
| Trichilia pallida Sw. | 212 |
| Malvaviscus arboreus Cav. | 197 |
| Guarea glabra Vahl | 193 |
| Aphelandra scabra (Vahl) Sm. | 191 |
| Bunchosia lindeniana A.Juss. | 187 |
| Rinorea hummelii Sprague | 183 |
| Dendropanax arboreus (L.) Decne. & Planch. | 170 |
| Palicourea tetragona (Donn.Sm.) C.M.Taylor | 164 |
| Eugenia capuli (Schltdl. & Cham.) Hook. & Arn. | 155 |
| Epidendrum radicans Pav. ex Lindl. | 152 |
| Lasiacis divaricata (L.) Hitchc. | 151 |
| Psychotria limonensis K.Krause | 150 |
| Damburneya salicifolia (Kunth) Trofimov & Rohwer | 146 |
The differences in the quantitative contribution of plant records and species between Guatemala and Mexico for the checklist of the URB suggest a different botanical exploration dynamics for each country. First, it became evident that the effort made towards the growth of botanical knowledge has been larger in the Mexican portion of the URB, in an apparent mismatch with its smaller share of the entire basin. Moreover, we also found a large spatial heterogeneity in terms of collecting effort and the consequent highly irregular floristic knowledge across the URB. Indeed, only for some areas within the URB a more satisfactory level of historical vascular plant collections was appreciated; examples of this situation include the cloud forest regions in the Montebello National Park (Chiapas), and certain parts of the Centla wetlands (Tabasco), both in Mexico. Unfortunately, these outstanding regions in terms of the available botanical knowledge for them appear belittled when compared with the huge sectors of the URB for which records of plant species are scarce or null (Figure 7). Notable examples among the latter are the tropical rain forest core in the Lacandona region, Mexico, as well as the heart of the forest massif in the Petén department and the Sierra de los Cuchumatanes in Guatemala. Lastly, this analysis also revealed a strong concentration of records along the main roads in the region, as well as around or near nature protection areas in both countries.
Discussion
Mesoamerica is one of the most biodiverse regions on Earth (Myers et al. 2000); thus, the quantitative assessment and comprehensive understanding of its biodiversity are long-term challenges. In the case of vascular plants, the Flora Mesoamericana project has invested over three decades of study without approaching completion (Davidse et al. 1994, 2018). Despite its large extent and considerable heterogeneity of its territory and vegetation (Meave et al. 2021), the URB stands out as an area naturally defined by the catchment area of a large and powerful river and its tributaries, although it only represents a small fraction of the Mesoamerican region (< 10 %). Therefore, the estimation provided in this study of the size of the regional flora (ca. 7,000 species), supported by more than 78,000 records, represents an unprecedented figure; it implies that almost one out of every three species in the whole of Mesoamerica (estimated at 24,000 species of vascular plants; Myers et al. 2000) may be found within the URB.
The extremely high floristic richness of the URB can be explained in terms of a complex evolutionary (particularly speciation rates), geologic and ecological history, all of which contribute to its large diversity (Gentry 1982, Jaramillo-Correa et al. 2009, ter Steege et al. 2016). Much of the region is topographically complex, a condition that is mainly linked to the steep elevational gradient and an intricate hydrological network (de la Maza & Carabias 2011). This situation has created different environmental scenarios that have likely promoted the intense diversification of vascular plants. Surprisingly, despite the strong environmental differences existing across the region, the most prominent families by species richness were the same across the three sectors of the URB. These families, namely Orchidaceae, Fabaceae, and Asteraceae, are frequently reported amongst the best-represented high-rank taxa in other floras of the continent (e.g.,ter Steege et al. 2013, Cardoso et al. 2017, Monro et al. 2017, Ulloa Ulloa et al. 2017, Whaley et al. 2019), and are plant groups recognized by their very high diversification rates (Magallon & Sanderson 2001, Schrire et al. 2005, Givnish et al. 2015, Panero & Crozier 2016). We also identified other groups that were particularly speciose in each elevational sector, where the specific habitats may be particularly favorable for their evolution and diversification. In the upper basin this situation is exemplified by Bromeliaceae and Polypodiaceae, both of which are prominent families in this sector. It has been suggested that their high species richness is a consequence of their evolutionary radiation triggered by multiple combinations of geographic, environmental, and historical factors operating in high mountains (Givnish et al. 2014, Kessler et al. 2016). Other noteworthy families in the upper basin represent distinct elements of the plant communities occurring there, although they do not rank high in terms of species richness; for example, species of the Ericaceae dominate non-forest communities in the upper reaches of the basin (Véliz Pérez et al. 2014, Schwery et al. 2016). These families have undergone fast diversification in mountainous areas, where they have benefited from higher humidity and precipitation, and cooler temperatures (Holder 2004, Kessler et al. 2016). In turn, in the lower basin the richness of the Fabaceae and Poaceae families is remarkable; these two families have radiated more intensely in warmer environments, where the presence of tropical rain forests or herbaceous and shrubby communities such as lowland scrub vegetation is common (Punyasena et al. 2008, Canché-Estrada et al. 2018).
The biogeographical relations of the URB flora have their origins in the geological processes that shaped this region, which include the rising of the plateau located in northern Central America during the late Miocene and early Pliocene, and the formation of the volcanic range in the late Pliocene (Williams 1960). Thereafter, the biota began to include mostly Nearctic and Neotropical taxa (Gentry 1982, Graham 2011). The combination of geologic, evolutionary, and ecological events shaped the different phytogeographic ensembles along the environmental gradients, particularly the elevation gradient. For example, in the lower part of the basin, some herbaceous wetlands are dominated by Cyperus L., Eleocharis R.Br., Nymphaea L., Phragmites Adans., and Typha L., all of which have broad (Cosmopolitan) distributions (Qian 2001); by contrast, some forests are dominated by species with Pantropical (e.g., Rhizophora mangle L.), Neotropical or Antillean origins. In the lower and middle basins, tropical forests (0 - 800 m) are dominated by species of Gondwanan and Neotropical origins, exemplified by the genera Dialium L., Terminalia L. and Chamaedorea Willd. In the same sectors of the basin, Laurasian and Pantropical species can also be found, although in lower proportions (Rzedowski 1978, Gentry 1982). In addition, there are some much less common elements of Holarctic and Antillean origins, as well as a share of endemic species (Rzedowski 1978). By contrast, the forests of the upper part of the basin (1,000 - 3,000 m) host a flora with a clear dominance of Holarctic origin (e.g., Abies Mill., Pinus L. and Quercus L.), with notorious Neotropical and Neotropical-Andean elements (Quintana-Ascencio & González-Espinosa 1993, Rzedowski 1991, 2021), as well as taxa of Pantropical or Eastern Asiatic and North American affinities (Quintana-Ascencio & González-Espinosa 1993). In herbaceous and forest communities located in subalpine and alpine areas, the predominant floristic elements have a temperate origin, along with Holartic, Austral-Antarctic and endemic species, albeit in smaller proportions (Islebe & Kappelle 1994). Additional elements of smaller importance include Cosmopolitan and Neotropical species (Islebe & Kappelle 1994). Interestingly, endemic species contribute up to 20 % of the flora in the highest part of the URB (Véliz Pérez 1998, 2014).
Although we did not quantify the number of endemic species in the flora of the URB, some reports have highlighted a considerably high level of endemism for this region, mostly associated with geologically recent mountain chains where allopatric speciation has occurred; this has promoted high levels of localized endemism (Gentry 1982), which is particularly noteworthy in the Sierra de los Cuchumatanes (Véliz Pérez 2008, Véliz Pérez et al. 2014). In this study we documented the presence of endemic or quasi-endemic groups for the URB, many of which represented novel contributions to the flora by the addition of new plant taxa; in fact, some of these groups were originally described based on botanical material collected in the URB. Examples at the family level are Guamatelaceae (Oh & Potter 2006) and Petenaeaceae (Christenhusz et al. 2010), whereas examples at the genus level are Cuchumatanea Seid. & Beaman (Seidenschnur & Beaman 1966), Lacandonia E. Martínez & Ramos (Martínez & Ramos 1989) and Tzeltalia E. Estrada & M. Martínez (Estrada & Martínez 1998).
More than half of the species included in the checklist are herbs, a group mostly comprising orchids, as this family accounts for over 8 % of all species occurring in the URB. This remarkable richness concurs with the suggestion that this is the most diverse plant group in the upper basin (Soto-Arenas 2001, Jiménez-López 2018). Herbs also included numerous Cyperaceae and Poaceae, two broadly distributed families in the extensive wetlands of the lower basin, typically characterized by the abundance and diversity of herbaceous taxa (Méndez-H. et al. 2001, Novelo & Ramos 2005, López-Jiménez et al. 2020). Trees ranked second among growth forms, in agreement with the presence within the URB of one the largest and most important tropical rainforest tracts not only in Mesoamerica but in the world (Miller et al. 2001, Gardner et al. 2009, Meave et al. 2021). Previous studies conducted in the tropical rainforest of the URB all agree that trees are as the best-represented growth form in this community (e.g., Martínez et al. 1994, Meave et al. 2008, Nesheim et al. 2010).
The numerical significance of the vascular plant richness of the URB in the continental context is truly astounding: the region hosts 5.58, 30.38, and 77.18 % of all species, genera, and families, respectively, recorded in the entire territories of North and South America combined (Ulloa Ulloa et al. 2017). A similar appreciation can be made for each of the two countries that share the basin, as the flora in the Mexican portion of the URB represents almost one-fourth of the total species richness estimated for the country (24.64 %; Villaseñor 2016), while its Guatemalan counterpart represents more than half of the estimated flora for Guatemala (55.56 %; Standley & Steyermark 1946). Beyond the implications of such large quantitative contributions to the continental and regional biodiversity wealth, the checklist reported in this study underscores the importance of exploring and quantifying plant biodiversity of natural regions, regardless of political boundaries. In this context, we must highlight the necessity to identify information gaps for which herbaria records are scarce or non-existent, and consequently for which floristic knowledge is completely lacking (Oliveira et al. 2016). Such geographic gaps are mainly visible in those territories where the largest and better-preserved tracts of tropical rainforest are located, such as the core of the Lacandona region (within the Montes Azules Biosphere Reserve; Martínez et al. 1994, de la Maza & Carabias 2011), and a large portion of the Petén department (Nesheim et al. 2010). Future efforts of botanical exploration focused on these areas will document the presence of more species in the URB than those reported here. Ideally, the cataloging of the region’s flora should be a continuous activity; botanical knowledge of the URB would benefit not only from new floristic studies but also from taxonomic and phylogenetic analyses of the plants found in the basin (Villaseñor 2017, Martínez-Camilo et al. 2019, ter Steege et al. 2019). Given this scenario, we highlight the contributions of floristic studies with limited geographical coverage conducted within the URB; in some cases, studies covering as little as a few tens of squared kilometers have documented the occurrence of a considerable number of species. For example, in the lower basin, Nesheim et al. (2010) produced a list of 307 species collected in a 6.5 km2 area in the Maya Biosphere Reserve (Petén); in the middle basin, Meave et al. (2008) recorded 547 species in 22 km2 in the Yaxchilán Natural Monument (Chiapas); and in the upper basin, Véliz Pérez (1998) listed 274 species for the Sierra de los Cuchumatanes (of which 71 species are micro-endemic; Véliz Pérez 2008) in an area smaller than 500 km2.
In this study, we documented the high vascular plant species richness of the URB, which makes of it one of the most biodiverse natural regions, not only in Mesoamerica but also in the world. Other natural regions of the Americas with comparable levels of vascular plant richness are the Balsas River Basin, located in the Mexican states of Guerrero and Michoacán (112,320 km2; 4,442 species; Fernández-Nava et al. 1998); the Río de la Plata grassland region shared by Brazil, Uruguay and Argentina (750,000 km2; 4,864 species; Andrade et al. 2018); and the Chocó region in the Pacific watershed of Colombia (175,000 km2, 4,950 species, Rangel-Ch. 2015). Actually, all these regions lag behind the renowned leaders of the Pan-American biodiversity: Madidi in Bolivia (111,000 km2; 8,244 species; Jørgensen et al. 2012); the eastern tropical Andean region (62,000 km2; > 10,000 species; Barthlott et al. 2005); the entire Amazon River Basin (6 million km2; between 14,003 and 16,000 species; ter Steege et al. 2013, Cardoso et al. 2017); and the Atlantic Forest of Brazil (50,000 km2; 16,000 estimated species; Stehman et al. 2009).
Although we assessed neither the proportion of species in the flora of the URB subjected to legal protection, nor the fraction of species occurring within protected natural areas, where we may foresee better prospects for their conservation, we are deeply concerned about the vulnerability of much of the flora in this region due to forest fragmentation, land use change, and the consequent loss of forest cover, all of which have been repeatedly reported to occur at alarmingly high rates in some parts of the URB (Ortiz-Espejel & Toledo 1998, Fernández-Montes de Oca et al. 2016, Gallardo-Cruz et al. 2019). Wetlands and grasslands in the Mexican portion of the lower basin are being rapidly transformed into other land uses, mainly into oil palm plantations (Gallardo-Cruz et al. 2019). In the Petén region of Guatemala, agriculture has been the main driver of deforestation for decades (Carr 2003). In the middle basin alone, ca. 142,000 ha of forest cover were lost between 2000 and 2012, which translates into the disappearance of some 500 million trees and 32 million Mg of biomass in the Lacandona region, probably the best-preserved tropical rainforest tract in Mesoamerica (Stegen et al. 2009, Fernández-Montes de Oca et al. 2016). Similarly, in the upper basin, deforestation has been related to human migration into areas that were until recently scarcely inhabited (Martínez Velazco 1993); such migration has accelerated forest loss and degradation due to the extraction of firewood and construction timber, and land clearing for agriculture (Islebe 1993).
Our study produced the most complete and updated account of the floristic richness in the URB. Based on this achievement, we can now claim that this natural region hosts the largest plant biodiversity in the entire territories of North and Central America. More than 95 % of the species reported here are associated with information from at least one specimen deposited in one herbarium, which contributes to the high reliability of the checklist.
It must be stressed that assembling this volume of information was only possible thanks to the work of thousands of explorers and plant taxonomists in the past 150 years, as well as owing to the overarching role played by herbaria in the documentation and description of the flora of the URB. These efforts have culminated in this high-level quantification of the floristic richness of the region, similar to what has happened in other areas of the Neotropics, where floristic studies based on enormous amounts of information guarded in scientific collections have led to an updated and accurate assessment of their floras (ter Steege et al. 2013, Cardoso et al. 2017, Ulloa Ulloa et al. 2017, Andrade et al. 2018). It is also clear that the task in the URB is still far from complete, and we strongly urge more researchers to engage in the study of its flora, focusing on its most poorly-known sectors, as well as in the taxonomic analysis of low- and high-rank taxa that are poorly represented in this region.
Supplementary material
Supplemental data for this article can be accessed here: https://doi.org/10.17129/botsci.3253











 nueva página del texto (beta)
nueva página del texto (beta)