Arbuscular mycorrhizal fungi (AMF) are mutualistic organisms that are associated with about 80 % of terrestrial plant species with respect to the arbuscular mycorrhiza interaction (Smith & Read 2008). Following Redecker et al. (2013), AMF belong to the Phylum Glomeromycota. Three hundred and forty-six AMF species have been described worldwide, and have been classified into five orders, 16 families, and 48 genera (Oehl et al. 2011b, Błaszkowski et al. 2015, Wijayawardene et al. 2020, Schüβler 2022). However, it is estimated that there are perhaps up to 1,000 AMF species according to modern molecular techniques (Kivlin et al. 2011, Öpik et al. 2013).
AMF are an essential component of rhizosphere and soil in the ecosystems, where they play active roles in the acquisition of water and nutrients (macro- and microelements) for their plant hosts, especially phosphorus, often considered the most limiting nutrient for plants in lowland tropical soils (Hodge & Fitter 2010, Smith & Smith 2011). AMF modulate the populations of several microorganisms, many of which are beneficial for plants (Artursson et al. 2006). AMF supply the plants with up to 80 % of nitrogen and 90 % of the phosphorus required (van der Heijden et al. 1998); AMF also improve survival, growth, reproduction, and resistance to biotic and abiotic stress in plants (Wagg et al. 2014, Van der Heijden et al. 2015).
The first list, to our knowledge, of AMF species tailored for Mexico reported the presence of only 44 species (Varela & Trejo 2001). Years later, in a second approach on AMF species diversity in the country, 95 species were registered (Montaño et al. 2012). The most current studies report the presence of about 160 species (e.g., Chimal-Sánchez et al. 2018, Varela et al. 2019, Polo-Marcial et al. 2021), that is, one half (50 %) of AMF species described throughout the world have been reported in Mexico. In this context, Oaxaca State belongs to the Mesoamerican hotspot of biodiversity of vascular plants (Myers et al. 2000, García-Mendoza et al. 2004), in different ecosystems and agroecosystems including biosphere reserves and natural protected areas for preserving biodiversity and ecosystem services. However, Oaxaca, the most biodiverse state in the country (García-Mendoza et al. 2004) at present lacks an updated checklist of the AMF species it harbors. It is because most of the information generated is scattered and difficult to access (in theses and technical reports), and the vast majority has not been published in scientific journals, hindering access to information.
Considering that Oaxaca State in Mexico is a hotspot zone possessing an important plant species diversity (García-Mendoza et al. 2004), this contribution aimed to investigate the state-of-the-art of AMF species diversity and AMF distribution in the Oaxacan region in order to identify needs and future tasks. In this work, the AMF species richness was considered as the sole index of diversity. This report increases knowledge by providing information regarding trends in geographic and ecological distribution, and the association with ecosystems, crops, or particular plant species. This can allow for the comparison and contrasting of the knowledge concerning this fungal group in other geographical regions and under other conditions, which may provide elements to corroborate or refute different hypotheses. This checklist will continue to be updated when new AMF species registries are added. Additionally, even new species being described from this region will be included, while the information presented herein provides a state-of-the-art panorama of knowledge to date. These data are essential to establish conservation strategies and to better the use of natural resources, through the knowledge of what these species are and where they are located.
Materials and methods
Study area. Oaxaca is located at the southern part of Mexico between 15° 39'-18° 09' N latitude, and 93° 52'-98° 32' W longitude (Figure 1), and covers an area of 95,364 km2, representing 4.8 % of the Mexican territory (García-Mendoza et al. 2004). The topography is exceptionally irregular because of constant tectonic movements. Elevation ranges from sea level along the coastal plains up to 3,000 m asl. The climatic and physiographic variation is reflected in the diversity of soils and vegetation communities that exist. According to economic activities and climate, eight regions are distinguished in Oaxaca: Cañada, Costa, Istmo, Mixteca, Papaloapan, Sierra Norte, Sierra Sur, and Valles Centrales (García-Mendoza et al. 2004). Nearly all vegetation types reported for Mexico are represented in the state, ranging from tropical rainforest to xerophytic vegetation (Rzedowski 1978, García-Mendoza et al. 2004).
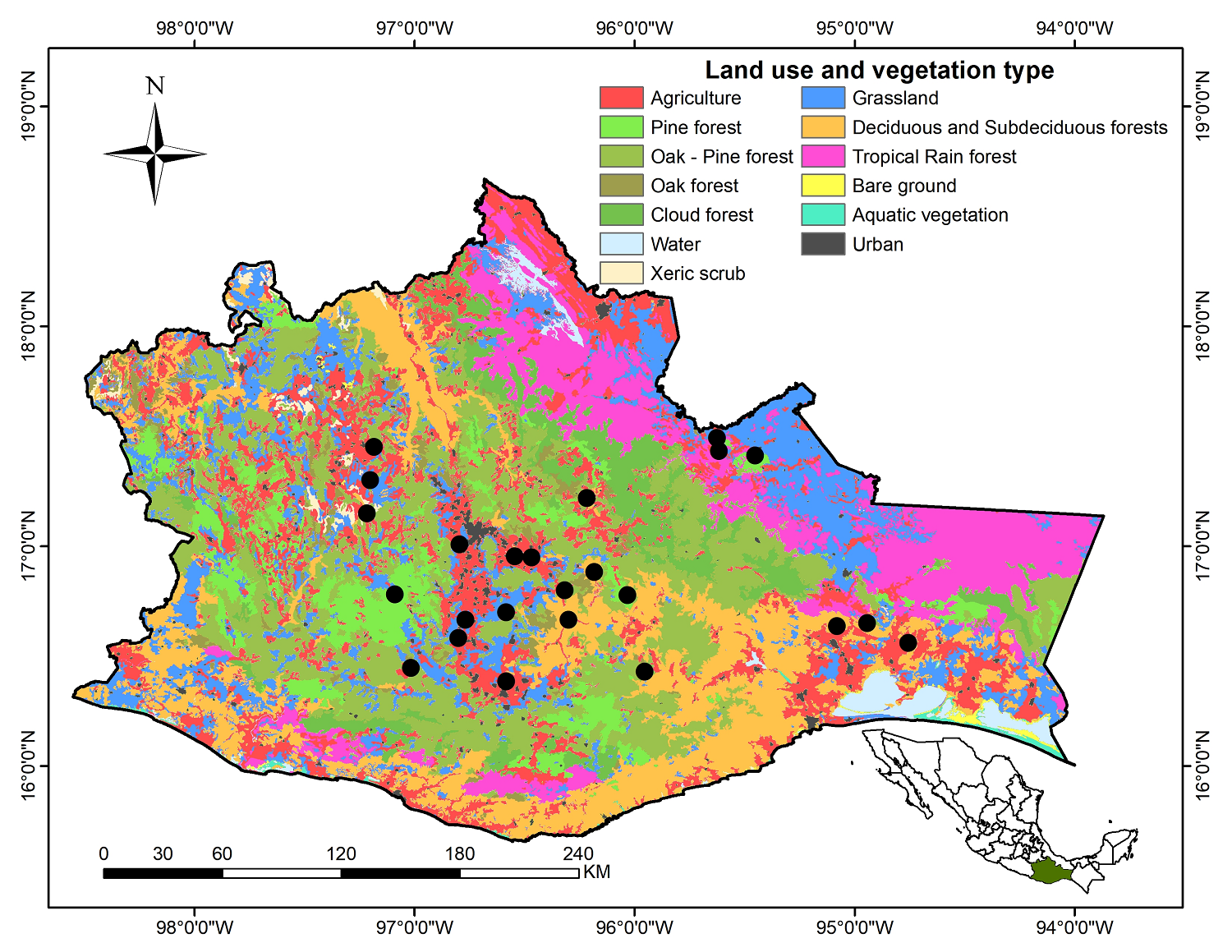
Figure 1 Location of different vegetation types in Oaxaca State, Mexico. The black circles represent the localities where the studies of arbuscular mycorrhizal fungi diversity have been carried out.
Database. We prepared an updated and annotated checklist of the AMF species with frequency (number of times the species has been recorded) of occurrence (%) and the sampling sites where the species had been registered. The AMF species richness was considered as the sole index of diversity. For this checklist, all studies carried out previously (to date, 2022) were considered. Information on AMF taxa was available in scattered form in different publications in physical and electronic media, theses (undergraduate degrees, M.Sc. degrees, and Ph.D. dissertations), projects, technical reports, and scientific papers (Varela & Trejo 2001, López-Guerra 2003, 2006, Guadarrama-Chávez et al. 2007, Carballar-Hernández 2009, Carballar-Hernández et al. 2013, Bautista-Cruz et al. 2014, Robles-Martínez et al. 2015, García-Velasco 2016, Cruz-Luna 2017, Hernández-Morales et al. 2017, Álvarez-Lopeztello et al. 2018, Chimal-Sánchez et al. 2018, Álvarez-Lopeztello et al. 2019a, b, Reyes-Jaramillo et al. 2019, de la Cruz-Ortiz et al. 2020, Chimal-Sánchez et al. 2021, Méndez-Matias et al. 2021). Prior to the elaboration of the checklist from all the sources consulted, only taxa determined at a specific level (with a binomial name) were employed, that is, aff. (affinis) and cf. (conformis) species, genera (i.e., Acaulospora spp.), species with invalid names and morphotypes were deleted. All names of AMF species included in the checklist and the names of the authorities were verified and updated according to the website of AMF Phylogeny of A. Schüßler (www.amf-phylogeny.com; accessed in June 2022). The taxonomic classification of the AMF species in the checklist follows Redecker et al. (2013), updated by A. Schüßler (www.amf-phylogeny.com), who collects all the taxonomical data produced by expert AMF taxonomists, and those available in different scientific journals.
Results
The checklist of AMF registered from Oaxaca includes 78 species, distributed in 23 genera, 10 families, and four orders (Table 1). The best-represented families with respect to their species richness were Glomeraceae (26), followed by Gigasporaceae (20) and Acaulosporaceae (15). The families with highest diversity of genera included Glomeraceae and Gigasporaceae (six, respectively), and Diversisporaceae (three). The taxonomic affinity of Entrophospora infrequens is unclear and it is indicated as incertae sedis (Table 2).
Table 1 Checklist of the arbuscular mycorrhizal fungi species registered from Oaxaca State, Mexico between 2001 and 2022, and the sampling site where the species has been reported (see abbreviations), and its frequency-of-observation between parentheses. Abbreviations: CF = ferns in cloud forest, MT = mine tailings, PAST = pasture, PP = pine plantations, SAV = savanna, STS= semiarid tropical scrub, SV (TDF) = secondary vegetation (tropical dry forest), TC = tomato and chili cultivars’, TDF = tropical dry forest, TRF = tropical rainforest; A. an = Agave angustifolia, A. ka = Agave karwinskii, A. ma = Agave marmorata, A. nu = Agave nussaviorum, A. po = Agave potatorum, and Z. ma = Zea mays. Note: The genus Claroideoglomus in the family Claroideoglomeraceae has recently been described as a new order, Entrophosporales, in the Glomeromycota (Błaszkowski et al. 2022).
| Arbuscular mycorrhizal fungi species |
| Glomerales |
| Claroideoglomeraceae |
| Claroideoglomus claroideum C. Walker & Schuessler; SV (TDF), TDF; A. an, A. ka, A. ma, A. po, Z. ma (44 %) |
| Claroideoglomus drummondii C. Walker & Schuessler; PAST, PP, SAV, TC, TRF (31 %) |
| Claroideoglomus etunicatum C. Walker & Schuessler; MT; A. an, A. ka, A. nu, Z. ma (31 %) |
| Claroideoglomus lamellosum C. Walker & Schuessler; Z. ma (6.3 %) |
| Glomeraceae |
| Funneliformis geosporus C. Walker & Schuessler; CF, MT, PAST, PP, SAV, STS, SV (TDF), TC, TDF, TRF; A. an, A. ka, A. ma, A. nu, A. po, Z. ma (100 %) |
| Funneliformis halonatus Oehl, G.A. Silva & Sieverd.; A. an, A. ka (12.6 %) |
| Funneliformis mosseae C. Walker & Schuessler; CF, MT, STS, TC; A. an, A. ka, A. ma, A. po, Z. ma (56 %) |
| Funneliformis verruculosus C. Walker & Schuessler; SV (TDF); A. an (12.6 %) |
| Glomus ambisporum G.S. Sm. & N.C. Schenck; A. nu (6.3 %) |
| Glomus glomerulatum Sieverd.; PAST, PP, SAV, TC, TRF (31 %) |
| Glomus globiferum Błaszk. & Chwat; A. nu (6.3 %) |
| Glomus macrocarpum Tul. & C. Tul.; A. an, A. ka, A. po (19 %) |
| Glomus microcarpum Tul. & C. Tul.; A. ka, A. ma, A. po (19 %) |
| Glomus spinuliferum Sieverd. & Oehl; CF, MT, STS; A. an, A. ka, A. ma, A. po (44 %) |
| Glomus tenebrosum S.M. Berch; SV (TDF), TDF; Z. ma (19 %) |
| Glomus trufemii B.T. Goto, G.A. Silva & Oehl; PP, SAV, TRF (19 %) |
| Rhizophagus aggregatus C. Walker; SV (TDF); A. an (12.6 %) |
| Rhizophagus clarus C. Walker & Schuessler; CF, MT, STS, TC; A. an, A. po, Z. ma (44 %) |
| Rhizophagus fasciculatus C. Walker & Schuessler; MT, PAST, PP, SAV, TC, TRF; A. an, Z. ma (50 %) |
| Rhizophagus intraradices C. Walker & Schuessler; TC; A. an, A. ka, A. ma, A. nu, A. po, Z. ma (44 %) |
| Rhizoglomus microaggregatum Sieverd., G.A. Silva & Oehl; SV (TDF); A. an, Z. ma (19 %) |
| Sclerocystis liquidambaris C.G. Wu & Z.C. Chen; A. an, A. ka (12.6 %) |
| Sclerocystis clavispora Trappe; SV (TDF), TDF; A. an, A. ka, A. ma, A. po (38 %) |
| Sclerocystis dussi Höhn.; SV (TDF); Z. ma (12.6 %) |
| Sclerocystis rubiformis Gerd. & Trappe; CF, STS; A. an, A. ka, A. nu (31 %) |
| Sclerocystis sinuosa Gerd. & B.K. Bakshi; SV (TDF), TC; A. an, A. ka, Z. ma (31 %) |
| Sclerocystis taiwanensis C.G. Wu & Z.C. Chen; SAV (6.3 %) |
| Septoglomus constrictum Sieverd., G. A. Silva & Oehl; CF, MT, PAST, PP, SAV, TC, TRF; A. an, A. ka, Z. ma (63 %) |
| Septoglomus viscosum C. Walker, D. Redecker, D. Stille & A. Schüßler; TC; A. po, A. nu (19 %) |
| Diversisporales |
| Acaulosporaceae |
| Acaulospora delicata C. Walker, C.M. Pfeiff. & Bloss; SV (TDF), TDF; A. an, Z. ma (25 %) |
| Acaulospora denticulata Sieverd. & S. Toro; CF; A. po (12.6 %) |
| Acaulospora excavata Ingleby & C. Walker; A. an, Z. ma (12.6 %) |
| Acaulospora foveata Trappe & Janos; PAST, PP, SAV, SV (TDF), TRF; A. nu, Z. ma (44 %) |
| Acaulospora kentinensis Kaonongbua, J.B. Morton & Bever; MT, TC; Z. ma (19 %) |
| Acaulospora laevis Gerd. & Trappe; CF, PAST, PP, SAV, SV (TDF), TRF; Z. ma (44 %) |
| Acaulospora mellea Spain & N.C. Schenck; MT, PAST, PP, SAV, SV (TDF), TDF, TRF; A. an, Z. ma (56 %) |
| Acaulospora minuta Oehl, Tchabi, Hountondji, Palenz., I.C. Sánchez & G.A. Silva; A. ka (6.3 %) |
| Acaulospora morrowiae Spain & N.C. Schenck; MT, PAST, PP, SAV, SV (TDF), TC, TRF; A. an, A. ka, A. ma, A. po, Z. ma, (76 %) |
| Acaulospora papillosa C.M.R. Pereira & Oehl; A. an, A. ka (12.6 %) |
| Acaulospora paulinae Błaszk.; A. ka, A. ma, A. po (19 %) |
| Acaulospora reducta Oehl, B.T. Goto & C.M.R. Pereira; A. ka (6.3 %) |
| Acaulospora rehmii Sieverd. & S. Toro; A. an, A. ka, A. po, Z. ma (25 %) |
| Acaulospora scrobiculata Trappe; CF, PAST, PP, SAV, SV (TDF), TC, TDF, TRF; A. an, A. ka, A. ma, A. po, Z. ma (82 %) |
| Acaulospora spinosa C. Walker & Trappe; CF, MT, PAST, PP, SAV, TRF; A. an, A. ka, A. ma, A. po, Z. ma (69 %) |
| Diversisporaceae |
| Diversispora eburnea C. Walker & Schuessler; TC; Z. ma (12.6 %) |
| Diversispora spurca C. Walker & A. Schuessler; PAST, PP, SAV, TRF; A. an, A. ka (38 %) |
| Diversispora trimurales C. Walker & Schuessler; A. ka (6.3 %) |
| Oehlia diaphana Błaszk., Kozłowska & Dalpé; A. nu (6.3 %) |
| Redeckera fulva C. Walker & Schuessler; STS, SV (TDF), TDF; Z. ma (25 %) |
| Gigasporaceae |
| Cetraspora pellucida Oehl, F.A. Souza & Sieverd.; PAST, PP, SAV, STS, SV (TDF), TDF, TRF; A. ka, A. po, Z. ma (63 %) |
| Dentiscutata cerradensis Sieverd., F.A. Souza & Oehl; A. ka (6.3 %) |
| Dentiscutata erythropus C. Walker & D. Redecker; SV (TDF) (6.3 %) |
| Dentiscutata scutata (C. Walker & Dieder.) Sieverd., FA de Souza & Oehl; A. ka (6.3 %) |
| Dentiscutata reticulata Sieverd., F.A. Souza & Oehl; A. nu (6.3 %) |
| Gigaspora albida N.C. Schenck & G.S. Sm.; A. an, A. po (12.6 %) |
| Gigaspora candida Bhattacharjee, Mukerji, J.P. Tewari & Skoropad; A. an, A. ka, Z. ma (19 %) |
| Gigaspora decipiens I.R. Hall & L.K. Abbott; PAST, SAV, SV (TDF), TDF, TRF; A. an, A. ma, A. po, Z. ma (57 %) |
| Gigaspora gigantea Gerd. & Trappe; SV (TDF), TDF; A. ka (19 %) |
| Gigaspora margarita W.N. Becker & I.R. Hall; MT; A. po (12.6 %) |
| Gigaspora ramisporophora Spain, Sieverd. & N.C. Schenck; A. an (6.3 %) |
| Racocetra fulgida Oehl, F.A. Souza & Sieverd; A. an, A. ka, A. ma (19 %) |
| Racocetra gregaria Oehl, F.A. Souza & Sieverd.; PAST, PP, SAV, TRF; A. an (31 %) |
| Racocetra cromosomica Chim.-Sánch., Varela & Montaño; A. ka (6.3 %) |
| Racocetra persica Oehl, F.A. Souza & Sieverd.; PP, SAV, TRF; A. ka (25 %) |
| Racocetra verrucosa Oehl, F.A. Souza & Sieverd.; A. an (6.3 %) |
| Scutellospora calospora C. Walker & F.E. Sanders; STS; A. ka (12.6 %) |
| Scutellospora dipurpurescens J.B. Morton & Koske; PAST, PP, SAV, SV (TDF), TC, TRF; A. ma, A. po, Z. ma (57 %) |
| Scutellospora scutata (C. Walker & Dieder.); A. ka (6.3 %) |
| Sieverdingia tortuosa Błaszk., Niezgoda & B.T. Goto; A. an (6.3 %) |
| Pacisporaceae |
| Pacispora scintillans C. Walker, Vestberg & Schuessler; SV (TDF) (6.3 %) |
| Archaeosporales |
| Ambisporaceae |
| Ambispora appendicula C. Walker; PAST, PP, SAV, TRF; A. an, Z. ma (38 %) |
| Ambispora gerdemannii C. Walker, Vestberg & Schuessler; MT, SV (TDF); Z. ma (19 %) |
| Archaeosporaceae |
| Archaeospora schenckii Walker & Schuessler; PAST, PP, SAV, TRF; A. an (31 %) |
| Archaeospora undulata (Sieverd.) Sieverd., G.A. Silva, B. Goto & Oehl; CF (6.3 %) |
| Paraglomerales |
| Paraglomeraceae |
| Paraglomus bolivianum Oehl & G.A. Silva; A. an, A. ka (12.6 %) |
| Paraglomus occultum J.B. Morton & D. Redecker; Z.ma (6.3 %) |
| Sacculosporaceae |
| Sacculospora baltica; Oehl, Palenz., I.C. Sánchez, B.T. Goto, G.A. Silva & Sieverd.; A. ka (6.3 %) |
| Insertae sedis |
| Entrophospora infrequens R.N. Ames & R.W. Schneid.; CF, MT, STS, SV (TDF), TC, TDF; A. an, A. ka, A. ma, A. po, Z. ma (69 %) |
Table 2 Families, genera, and species of arbuscular mycorrhizal fungi registered in Oaxaca State, Mexico between 2001 and 2022. In parentheses, the proportion of families, genera, and species regarding their total is depicted. Note: The genus Claroideoglomus in the family Claroideoglomeraceae has recently been described as a new order, Entrophosporales, in the Glomeromycota (Błaszkowski et al. 2022).
| Families | Genera (%) | Species (%) |
|---|---|---|
| Claroideoglomeraceae | 1 (4.35) | 4 (5.13) |
| Glomeraceae | 6 (26.09) | 26 (33.33) |
| Acaulosporaceae | 1 (4.35) | 15 (19.23) |
| Diversisporaceae | 3 (13.04) | 5 (6.41) |
| Gigasporaceae | 6 (26.09) | 20 (25.64) |
| Pacisporaceae | 1 (4.35) | 1 (1.28) |
| Ambisporaceae | 1 (4.35) | 2 (2.56) |
| Archaeosporaceae | 1 (4.35) | 2 (2.56) |
| Paraglomeraceae | 1 (4.35) | 2 (2.56) |
| Sacculosporaceae | 1 (4.35) | 1 (1.28) |
| Incertae sedis | 1 (4.35) | 1 (1.28) |
The genera richest in species were Acaulospora, Glomus, Sclerocystis, and Gigaspora (Figure 2A); these four genera concentrate 44 % of the species. The AMF highest in species diversity (in all of the plant communities studied) have been reported under the rhizospheres of Agave angustifolia (39), A. karwinskii (36), and Zea mays (33) (Figure 2B). Funneliformis geosporus was the most frequent species (100 %), followed by Acaulospora scrobiculata (82 %), and A. spinosa and Entrophospora infrequens (69 % each) (see frequency between parentheses in Table 1).

Figure 2 A. Number of arbuscular mycorrhizal fungi species by genera, and B number of arbuscular mycorrhizal fungi species by sampling site in Oaxaca State, Mexico.
Thirty-four species (of the number of total species) were detected in natural ecosystems (without apparent human intervention, i.e., tropical rainforest, cloud-forest ferns, semi-arid tropical scrub, and tropical dry forest) (see Table 1). Ecosystems with highest species diversity were tropical rainforest (20), followed by cloud-forest ferns and tropical dry forest (12 species, respectively); in contrast, semi-arid tropical scrub demonstrated lowest species diversity (8). In these ecosystems, the genera richest in species were Acaulospora (9) and Glomus (4). The genera Dentiscutata, Pacispora, Paraglomus, and Rhizoglomus were absent. Funneliformis geosporus (100 %), A. laevis, A. scrobiculata, and E. infrequens (75 % each, respectively) were the most frequent species (Table 1).
In agroecosystems, 77 species were detected (Table 1). Agroecosystems with the highest species diversity were A. angustifolia (39), A. karwinskii (36), and Z. mays (33); in contrast, A. nussaviorum exhibited lowest species diversity (10). A new AMF species, Racocetra cromosomica, was recently isolated from the rhizosphere of wild A. karwinskii. The genera richest in species were Acaulospora (15), Glomus (8), and Gigaspora (6). Funneliformis geosporus (100 %), A. morrowiae (91.67 %), and A. scrobiculata (83.33 %) were the most frequent species. Archaeospora undulata was absent (see Table 1).
Discussion
The checklist of AMF species reported for Oaxaca State (Mexico) reveals high Glomeromycota species richness (Table 1). Ninety percent of AMF families registered for Mexico by Varela et al. (2019) and Polo-Marcial et al. (2021), and 74 % of families worldwide (Błaszkowski et al. 2015, Wijayawardene et al. 2020) are represented in the region. More than one half of genera reported for the world and for Mexico (> 64 % and > 80 %, respectively), and nearly one quarter of the species described throughout the world (22 %) and one half (49 %) of AMF species registered for Mexico (Montaño et al. 2012, Chimal-Sánchez et al. 2018, Varela et al. 2019, Polo-Marcial et al. 2021, Schüβler 2022) are present in Oaxaca, surpassing the AMF species diversity registered for Chiapas, Tabasco, and Veracruz, which are the Mexican states with the highest fungal diversity reported to date (Varela et al. 2008, Rodríguez-Morelos et al. 2014, Salgado-García et al. 2014, Trejo et al. 2016, Bertolini et al. 2018, Posada et al. 2018). However, this trend may change, as the study and knowledge of AMF species diversity increase in the country, including tropical and subtropical regions and some other states (i.e., the State of Mexico) where AMF species diversity has been poorly explored. This fungal diversity represents increasing efforts for the state of Oaxaca that, up to a few years ago, had been scarcely explored, with only two species recorded (Acaulospora foveata and Sclerocystis clavispora) at the time of the first inventory of AMF species from Mexico (Varela & Trejo 2001).
Nonetheless, in recent years (last 7 years), there has been an increase in the number of studies on AMF taxonomy and diversity at several ecosystems and agroecosystems of Oaxaca (Figure 1). This is mainly due to a search for alternatives to facilitate the nutrition, growth, and development of plants, as well as to improve their tolerance to environmental stress conditions. Despite the increase observed and efforts made in recent years (Figure 3) to acquire knowledge on AMF species diversity in Oaxaca State, it is still far from well-known and possibly hosts a much higher number than is currently known. This Mexican state presents varied topographic, climatic, edaphic conditions, and high vegetational heterogeneity and vast flora diversity with much endemisms (García-Mendoza et al. 2004). The latter could harbor a high and still unknown wealth of AMF species, possibly many of these new species.

Figure 3 Number of taxonomic studies of arbuscular mycorrhizal fungi diversity conducted every 5 years in Oaxaca State, Mexico.
Considering the role of plants as umbrella species, hotspots also function as hotspots for other, less well-studied organisms, such as associated soil fungi (Stork & Habel 2014). In this context, the first AMF was recently described as being from Oaxaca, that is, Racocetra cromosomica (Chimal-Sánchez et al. 2021), isolated from the rhizosphere of Agave karwinskii, a microendemic plant. In addition, Septoglomus mexicanum, another new AMF, was isolated from the Tehuacán-Cuicatlán Biosphere Reserve, a semi-arid ecosystem shared between the states of Puebla and Oaxaca (Chimal-Sánchez et al. 2020). The application of molecular strategies using DNA from the roots or from the rhizospheric soil of selected plants will aid in rapidly increasing knowledge on the AMF community present in Oaxacan systems.
Glomeraceae, Acaulosporaceae, and Gigasporaceae, with highest AMF species richness in Oaxaca State (Table 2), are the very families that also have the highest numbers of species registered for the entire country (Montaño et al. 2012, Varela et al. 2019, Polo-Marcial et al. 2021). According to the richness of AMF recorded in various ecosystems and agroecosystems, Glomeraceae and Acaulosporaceae tend to be the most frequent families (Öpik et al. 2010, Cofré et al. 2019, Varela et al. 2019) and possess a considerable proportion of generalist species, which has been evidenced by their existence under varied and contrasting conditions. In the most recent reviews, to our knowledge, on AMF in Mexico, species from both families prevail (Varela et al. 2019, Polo-Marcial et al. 2021). There is a record of AMF in 19 botanical families, where Cactaceae, Fabaceae and Poaceae have the highest number of analyzed AMF species. No members of Acaulosporaceae, to our knowledge, have been recorded in Asclepiadaceae, Asteraceae, Caricaceae, Chenopodiaceae, Fouqueriaceae, Meliaceae and Selaginaceae. Two reasons can explain these results: the great lack of knowledge on the diversity of AMF associated with particular plant species from various botanical groups, and the lack of Taxonomists studying Glomeromycota. Polo-Marcial et al. (2021) have suggested that one possible explanation for the high species diversity of these families at both the state and national levels is that they are generally more associated with the Neotropical than with the Nearctic region. Acaulospora, Glomus, Sclerocystis, and Gigaspora were the genera with highest specific diversity in Oaxaca State (Figure 2B), as observed in certain other Mexican states (Varela et al. 2008, Rodríguez-Morelos et al. 2014, Salgado-García et al. 2014, Trejo et al. 2016, Bertolini et al. 2018, Posada et al. 2018), and also in Mexico (Montaño et al. 2012, Varela et al. 2019, Polo-Marcial et al. 2021). Acaulospora and Glomus are usually the most frequent and those with greatest AMF diversity in studies on diversity around the world; they are cited as generalist and are capable of existing under environmental and edaphic changeable conditions (Bhardwaj et al. 1997, Carvalho et al. 2003, Escudero & Mendoza 2005). Gigaspora has generally been associated with low-disturbance ecosystems (Álvarez-Lopeztello et al. 2019b) due to its lengthy life cycle and its association with high soil moisture and organic-matter content.
Funneliformis geosporus was the most frequent species and it was present in all vegetation types studied to date (2022), indicating that it could be a generalist species with low environmental specificity, as suggested by Carballar-Hernández et al. (2013) and Guadarrama et al. (2014). The same situation could be true for A. scrobiculata, one of the most cited AMF species in the Mexican territory (Montaño et al. 2012), and the second most frequently registered AMF in Oaxaca. In contrast, other important species, such as Claroideoglomus lamellosum [recently included in the genus Entrophospora (Błaszkowski et al. 2022)], Glomus ambisporum, G. globiferum, G. macrocarpum, Rhizoglomus microaggregatum, Sclerocystis liquidambaris, S. taiwanensis, Acaulospora minuta, A. reducta, Archaeospora undulata, Oehlia diaphana, Dentiscutata reticulata, Gigaspora candida, G. ramisporophora, Racocetra fulgida, R. verrucosa, Scutellospora calospora, Pacispora scintillans, and Paraglomus occultum have been registered only once for Oaxaca State (Table 1). A possible explanation for the low frequency of these species is that no long-term studies (more than 2 years), to our knowledge, have been carried out and only one-half of the studies have considered seasonality (rainy and dry). Therefore, it is recommended that future research consider long-term studies and seasonality. The lack of long-term studies could lead to this AMF species being considered a rare species; for example, A. minuta and A. reducta are known for Mexico as deriving only from Oaxaca (Chimal-Sánchez et al. 2018). If there is no, to our knowledge, further exploration, and number of records, it is valid to consider the species as rare; these are little recorded species, even in the places where they were described (Oehl et al. 2011a, Pereira et al. 2015). These AMF species were found under the rhizosphere of A. karwinskii and A. angustifolia; therefore, this rareness may be the result of the lack of registries; the latter prompts continuing to conduct further AMF taxonomic studies in Oaxaca State, in that there remain many ecosystems that have been little explored, such as tropical rainforest, cloud forest, and natural grasslands (Figure 1). In addition, fewer than 1 % of the rhizospheric soils of wild and cultured plant species in Oaxaca have been explored.
Mirzaei & Moradi (2017) identified a high correlation between plant diversity and AMF diversity in semi-arid forests: to greater diversity of plants, greater diversity of AMF and vice versa. This allowed corroborating that a greater plant diversity favors higher spore density and higher root colonization intensity, which contribute to maintaining the inoculum potential in the soil, although these parameters could depend on the hosts involved and the edaphic conditions. This should undoubtedly be considered to improve conservation strategies by directly influencing the maintenance of the systems. It is difficult to estimate how many AMF species could be expected in Oaxaca based on the number of plant species; only about 346 species of Glomeromycota are known on the Earth and there is abundant evidence of their low specificity. Öpik et al. (2009) pointed out that generalist plant species share the majority of AMF species, including generalists; these authors propose that the relationships are functional rather than specific between plants and AMF.
Mesoamerica is the center-of-origin of maize and agave. Highest AMF-species richness has been found under the rhizosphere of Agave karwinskii, A. angustifolia, and Zea mays and this unequivocally coincides with that these are the most studied species (at the rhizospheric level) and are those entertaining the greatest significant economic and cultural interest in Oaxaca State. However, in many places, these agave species are not cultivated, and the AMF species diversity that they can harbor under natural conditions is unknown. Another important point that could exert a strong negative influence on the AMF diversity associated with these cultivated species is the use of chemical fertilizers and pesticides. It has been demonstrated that chemical pesticides reduce abundance of AMF in agricultural soils (Rivera-Becerril et al. 2017). Nevertheless, the study of the AMF species diversity of these plant species is far from being completed. There remain many sites at which and environmental conditions under which they are cultivated but have not yet been explored.
The isolation and integration of collections of native AMF comprise an important task in order to preserve these bioresources and to explore their potential use for the production of important plants (i.e., maize and agave), or for other purposes such as the restoration of degraded soils and ecosystems. In this regard, some AMF species that have been isolated and propagated include Acaulospora mellea, Acaulospora scrobiculata, Acaulospora spinosa, Claroideoglomus drummondii [recently included in the genus Entrophospora (Błaszkowski et al. 2022)], Diversispora spurca, Funneliformis geosporus, and Septoglomus constrictum. These AMF species have been inoculated in plant species that can become established in early successional stages typical of humid tropical forests (Álvarez-Lopeztello et al. 2021). Agave species, able to adapt to nutrient-deficient soils, high temperatures, and high solar radiation, are candidates for the restoration of arid habitats (Cervera Herrera et al. 2018). Among the AMF reported in this study, species that respond to isolation and propagation could be used in restoration, particularly those that have shown specific benefits to plants. In Mexico, monospecies inocula have been tested to mycorrhize plants for restoration purposes (Hernández-Cuevas et al. 2011, Quiñones-Aguilar et al. 2019) or consortia, including species recorded in Oaxaca (Carballar-Hernández et al. 2018, Quiñones-Aguilar et al. 2019). The recommendation is to work with AMF consortia from environments in ecogeographical regions similar to those to be restored; consortia from stressed environments are particularly attractive.
In conclusion, the findings revealed in this study to date suggest that Oaxaca is the state with the highest AMF species diversity in the country, in that Oaxaca harbors one-half of the species reported for Mexico, reaffirming its place as the most diverse AMF species-rich state. However, this may change, as knowledge and long-term studies on the AMF species diversity increase; different regions have been poorly explored with respect to AMF species diversity. Likewise, our findings also exhibit a lack of studies on AMF diversity; therefore, there is a need to continue exploring the state of Oaxaca, especially in terms of vegetation types such as tropical rainforest, natural grasslands, cloud forest, and temperate forest, plants of economic and cultural interest (maize, tomatoes, and beans), and plants that are endemic that have not been studied (cacti and legumes). The presence of species poorly registered or registered for the first time in Mexico demonstrate the importance of Oaxaca as a reservoir of AMF biodiversity. The number of works on AMF in transformed and non-transformed ecosystems place Oaxaca as one of the best studied states in Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)



