Allelopathy can be studied from an ecological or applied approach. Allelopathy is considered a significant ecological factor in determining the structure, composition and dynamics of plant communities (Muller 1966, Ballester & Vieitez 1978, Rice 1984, Wardle et al. 1998, Inderjit & Duke 2003). One of the applied uses of allelopathy focuses on the interactions of cultivated species with another crop or weed, and the search of new bioactive compounds with herbicidal potential or as plant growth regulators (Reigosa et al. 2013, da Silva et al. 2017). There is great interest in the biological control of pests through the application of allelochemical compounds (Narwal 2010, Cheng & Cheng 2015, Jabran et al. 2015, Macías et al. 2019), because allelopathic compounds are considered safe and beneficial for the environment and human health (El-Kenany & El-Darier 2013). In addition, the selective pressure generated by the massive use of herbicides has led to the development of resistance in a large number of weeds (Cheng & Cheng 2015, Al-Samarai et al. 2018). Thus, there is a need to constantly search for new herbicidal compounds to avoid the resistance mechanisms of weeds (Gaines et al. 2020).
The family Poaceae has a significant number of species with allelopathic properties (Chou & Young 1975, Rietveld 1977, Bokhari 1978, Hussain et al. 1982, Li et al. 2005), with Cymbopogon Spreng. and Bothriochloa Kuntze (Poaceae: Andropogoneae) being the main aromatic genera known to date (Hussain et al. 1982, Kaul & Vats 1998, Scrivanti et al. 2009, 2011, Scrivanti 2010, Scrivanti & Anton 2019). The genus Imperata Cirillo (Poaceae: Andropogoneae) includes nine species distributed in tropical, subtropical, and temperate regions of both hemispheres (Gabel 1982). Imperata cylindrica has been widely studied for its invasive behavior and was reported as one of most damaging weeds, reducing biodiversity and causing losses in production of various crops globally (Jose et al. 2002, MacDonald 2004, Collins et al. 2007, Global Invasive Species Database 2021). Early in vitro and field studies demonstrated that aqueous extract of I. cylindrica inhibits the growth of some plants in vitro and on soil through leaching from rainfall and irrigation in field experiments. (Abdul-Wahab & Al Naib 1972, Eussen & Wirjahardja 1973, Eussen 1979, Eussen & Niemann 1981, Inderjit & Dakshini 1991). More recent studies have confirmed the presence of allelochemicals in rhizomes and aerial parts of I. cylindrica, and some authors have suggested their possible contribution to its invasion success (Inderjit & Dakshini 1991, Koger et al. 2004, Xuan et al. 2009, Cerdeira et al. 2012, Hagan et al. 2013, Suzuki et al. 2018). The production of allelochemicals by I. cylindrica has also been investigated in search of an alternative strategy to traditional herbicides for weed control. Aqueous extracts of I. cylindrica have shown an inhibitory effect on germination and development in different weed species, such as Cynodon dactylon (L.) Pers. and Lolium multiflorum Lam. (Koger et al. 2004), Parthenium hysterophorus L. (Anjum et al. 2016), and Amaranthus spinosus L. (Erida et al. 2019). Of the rest of Imperata species (I. brasiliensis Trin., I. brevifolia Vasey., I. condensata Steud., I. conferta (J. Presl) Ohwi, I. cheesemanii Hack., I. contracta (Humb. Bonpl. & Kunth) Hitchc., I. minutiflora Hack. and I. tenuis Hack.) allelopathic activity has only been demonstrated on I. brasiliensis Trin. as the aqueous extract of the whole plant inhibited germination and stem growth of Oryza sativa L. (Casini et al. 1998).
Most allelopathic or allelochemical compounds are intermediate molecules or products of secondary metabolism (Lotina-Hennsen et al. 2006). Evidence exists that ancestral members of a clade evolved the biosynthetic capacity to produce a similar secondary metabolite and bioactive properties (Wink 2003, Chon & Nelson 2010, Imatomi et al. 2013, Liu et al. 2017).
Considering the allelochemical biosynthesis pathway can be shared by all or some species of a plant genus, the species of Imperata may synthesize compounds with allelopathic activity like I. cylindrica.
Hence, this paper aims to carry out a first approach to the presence of allelopathic compounds in the South American species of Imperata. For this purpose, we tested the effect of leaf aqueous extract of I. brasiliensis, I. condensata, I. minutiflora, and I. tenuis on seed germination and early seedling growth of Lactuca sativa, Zea mays, and Solanum Lycopersicum. with rapid germination and high sensitivity.
Material and Methods
Plant material. Four Imperata species were studied: I. brasiliensis, I. condensata, I. minutiflora, and I. tenuis. Plants were collected from Argentina and Brazil (Table 1). Voucher specimens are deposited in the Museo Botánico de Córdoba (CORD) herbarium. Plants were cultivated using pots in a greenhouse; fresh mature expanded leaves were collected to obtain aqueous extract. Seeds of three tested species ‒lettuce (Lactuca sativa), maize (Zea mays), and tomato (Solanum lycopersicum) were used for germination and seedling growth assays. Tested species selected are used as evaluation models due to their rapid germination and high sensitivity. Lactuca sativa is the most widely used in allelopathy bioassays (Carvalho et al. 2019, Tigre et al. 2012, Wang et al. 2016). The inhibitory or stimulatory effect on shoot and root growth and germination is considered an indirect measure of how allelochemicals affect other internal physiological processes in the plant (Macías et al. 2019).
Table 1 Imperata specimens collected from field and used in bioassays.
| Imperata species | Collection number (CORD) | Location | Coordinates |
|---|---|---|---|
| I. brasiliensis | Scrivanti 564 | Formosa, Clorinda, Argentina | 25° 36’ 6.761” S 57° 54’ 26.3” W |
| I. condensata | Scrivanti 566 | San Luis, Villa Mercedes, Argentina | 33° 36’ 43.1” S 65° 34’ 46.5” W |
| I. minutiflora | Scrivanti 568 | Jujuy, San Salvador de Jujuy, Argentina | 24° 9’ 43.72” S 65° 23’ 16.77” W |
| I. tenuis | Scrivanti 561 | RGS, Dom Pedrito, Brazil | 30° 52’ 45” S 54° 53’ 26” W |
Aqueous extracts. In order to obtain the aqueous extracts, we used a method adapted from the one described by Scrivanti (Scrivanti 2010, Scrivanti et al. 2011, Scrivanti & Anton 2019, 2021). Five grams of fresh leaf blades from each Imperata plant were crushed with a blender. Each sample was placed in a tube containing 50 mL of deionized water. The mixture was kept in a refrigerator for 24 h; then it was stirred in a rotary shaker for 1 h and centrifuged at 1500 revolutions min-1 for 15 min. The supernatant was recovered and filtered using filter paper. This process was performed in duplicate to remove as much of the undesirable residue from the extract as possible. The supernatant was recovered and stored in a refrigerator until being used as a crude water-soluble extract (100%). Other test solutions (75, 50 and 25% v/v) were prepared by diluting stock solution with sterile distilled water. The extracts were stored at 4ºC.
Bioassay. To evaluate the allelopathic effects of aqueous leaf extracts, seeds of lettuce, maize, and tomato (25 seeds each) were placed in respective Petri dishes containing two layers of filter paper moistened with 5 mL of aqueous extracts. The control Petri dishes received 5 mL deionized water. The Petri dishes were kept in the greenhouse under homogeneous conditions of light (12 h) and mean temperature of 25°C during seed germination and seedling growth for three (Lettuce), four (Maize), and five (Tomato) days. The treatments were replicated five times in a completely randomized design, so we used fifty seeds per treatment. Tests were previously conducted to establish incubation times using seeds of the tested plants and distilled water; this procedure allowed us to determine the germination time and seedling growth period of each crop. After incubation, germinated seeds were counted and length of shoot and root was measured using ruler; measurement was taken in 10 randomly selected seedlings per treatment.
Percentage of inhibition of germination was calculated as follows (Scrivanti 2010, Scrivanti et al. 2011, Scrivanti & Anton 2019):
Were, I: percentage of inhibition; G: average germination percentage in the treatment; C: average germination percentage in the control.
Germination percentages were analyzed using a Chi-square (χ2) test to determine the existence of differences between treatments and control at a probability level of 0.05, using the R software (R Core Team 2022).
Percentage of shoot and root growth reduction was calculated as follows (Scrivanti 2010, Scrivanti et al. 2011, Scrivanti & Anton 2019):
Where, I: percentage of inhibition; T: mean root or shoot length (mm) in the treatment; C: mean root or shoot length (mm) in the control.
Data from inhibition (%) of root and shoot length growth were subjected to an analysis of variance (ANOVA) followed by a posteriori test of multiple comparisons based on the false discovery rate (Benjamini & Hochberg 1995) to determine the existence of differences of the treatments with respect to the control and between treatments, at a probability level of 0.05, using the R software (R Core Team 2022).
Results
Seed germination. Aqueous leaf extract of I. brasiliensis and I. tenuis reduced germination of tomato at all tested concentrations (25, 50, 75, and 100 %) as compared to the control (Figure 1 and Table 2). Aqueous leaf extract of I. minutiflora reduced germination of lettuce at the highest (100 %) concentration (Figure 1 and Table 2). Aqueous leaf extract of I. brasiliensis, I. condensata, I. minutiflora, and I. tenuis reduced germination of lettuce at the highest (100 %) concentration as compared to the control (Figure 1 and Table 2). None of the aqueous extracts tested reduced germination of maize with respect to control.
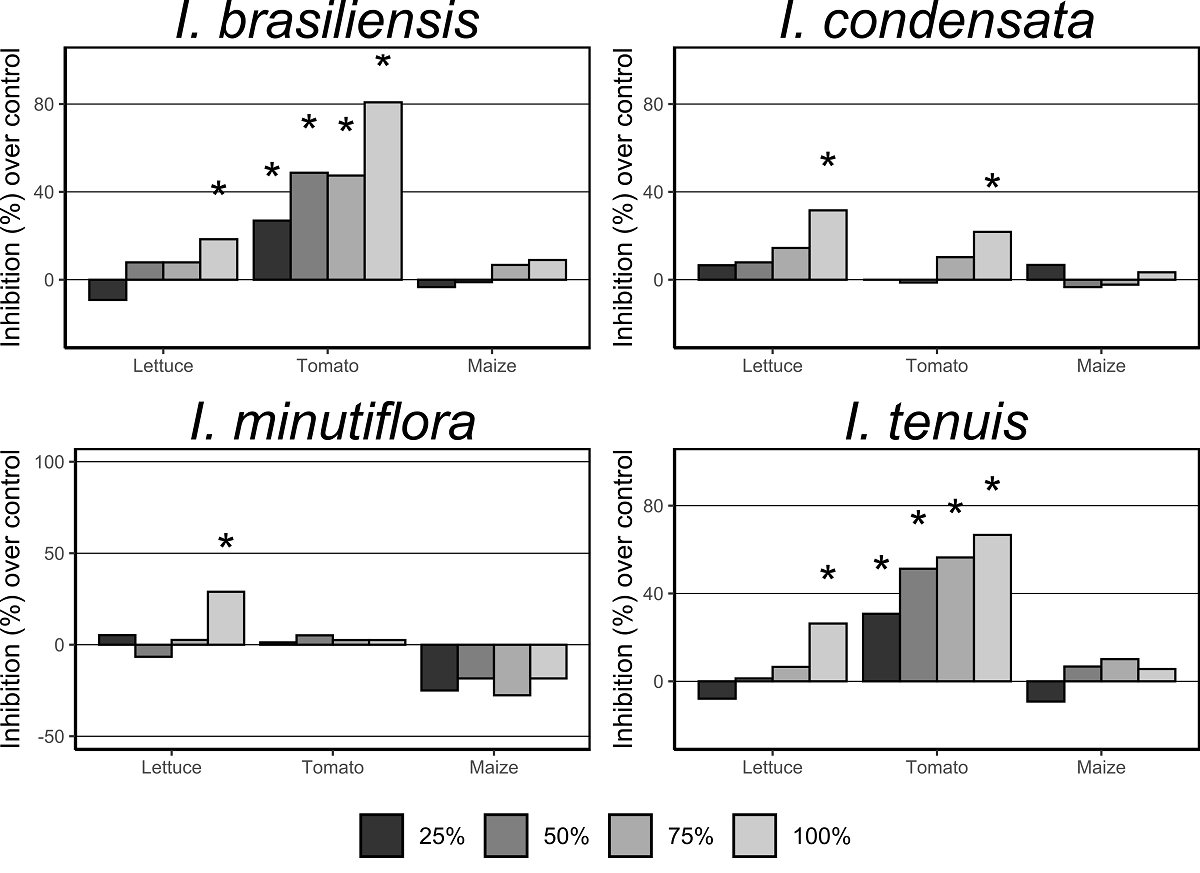
Figure 1 Mean inhibitory effects of aqueous leaf extracts of Imperata species on germination of lettuce, tomato, and maize. Symbol above the bars indicates values significantly less than the respective control (P ≤ 0.05).
Table 2 Mean inhibitory effects of aqueous leaf extracts of Imperata species on germination of lettuce, tomato, and maize. A statistically significant value denotes inhibition over control (* P ≤ 0.05).
| Imperata species | Aqueous extract concentration |
Test species | ||
|---|---|---|---|---|
| Inhibition (%) over control | ||||
| Lettuce | Tomato | Maize | ||
| I. brasiliensis | 25 % | -9.2 | 26.9* | -3.3 |
| 50 % | 7.8 | 48.7* | -1.1 | |
| 75 % | 7.8 | 47.4* | 6.7 | |
| 100 % | 18.4* | 80.7* | 8.9 | |
| I. condensata | 25 % | 6.5 | 0 | 6.7 |
| 50 % | 7.8 | -1.2 | -3.3 | |
| 75 % | 14.4 | 10.2 | -2.2 | |
| 100 % | 31.5* | 21.7* | 3.3 | |
| I. minutiflora | 25 % | 5.2 | 1.2 | -25 |
| 50 % | -6.5 | 5.1 | -18.4 | |
| 75 % | 2.6 | 2.5 | -27.6 | |
| 100 % | 28.9* | 2.5 | -18.4 | |
| I. tenuis | 25 % | -7.8 | 30.7* | -9.2 |
| 50 % | 1.3 | 51.2* | 6.7 | |
| 75 % | 6.5 | 56.4* | 10.1 | |
| 100 % | 26.3* | 66.6* | 5.6 | |
Root and Shoot growth. Aqueous leaf extract of I. brasiliensis inhibited the growth of lettuce and tomato root at 50, 75, and 100 %, and at all concentrations, respectively, as compared to the control (Figure 2 and Table 3). The inhibition percentage was statistically different between 50 and 100 % concentrations for lettuce and between 25 % and the remaining concentrations for tomato (Figure 2 and Table 3). The aqueous leaf extract of I. brasiliensis did not produce a statistically significant reduction in the growth of the maize root as compared to the control at any concentration (Figure 2 and Table 3). Regarding shoot growth, the aqueous extract of I. brasiliensis inhibited the growth of all the test plants as compared to the control only at the highest concentration (100 %) (Figure 2 and Table 3).

Figure 2 Mean inhibitory effects of aqueous leaf extracts of Imperata species on root and shoot growth of lettuce, tomato, and maize. Symbol above the bars indicates values significantly less than the respective control (P ≤ 0.05). Different letters indicate significant differences among treatments within each test plant (P ≤ 0.05).
Table 3 Mean inhibitory effects of aqueous leaf extracts of Imperata species on root and shoot growth of lettuce, tomato, and maize. A statistically significant value denotes inhibition over control (* P ≤ 0.05). Different letters indicate significant differences among treatments within each test plant (P ≤ 0.05).
| Imperata species | Aqueous extract concentration |
Test species | |||||
|---|---|---|---|---|---|---|---|
| Inhibition (%) over control | |||||||
| Root lenght | Shoot lenght | ||||||
| Lettuce | Tomato | Maize | Lettuce | Tomato | Maize | ||
| I. brasiliensis | 25% | 3.8a | 22.8a* | 6.8a | -7.8a | -21.2a | 9a |
| 50% | 16b* | 43.2b* | 5.2a | -3.5ab | 1.2ab | 6.3a | |
| 75% | 22.6bc* | 38.2b* | 7.3a | 7.1bc | 7.2bc | 10.7a | |
| 100% | 28.2c* | 58.4b* | 14.6a | 19.1c* | 40.5c* | 22.3a* | |
| I. condensata | 25% | 22.5a* | 39.1a* | 0.6a | -3.9a | -7.3a | 14.1a* |
| 50% | 26.4b* | 32.5a* | 2.3a | -2.3ab | 3.6ab | 11.5a | |
| 75% | 34.4c* | 41.7a* | 8.8a | 7.1b | 11.9ab | 21.2ab* | |
| 100% | 33bc* | 54.4b* | 26.4b* | 2.9a | 19.2b | 32.4b* | |
| I. minutiflora | 25% | 6.9a | -5.1a | 12.4a | -9.6ab | -66.5a* | 18.1a* |
| 50% | 7a | -9.7a | 13.3a | -13.8b | -80.4a* | 19.4a* | |
| 75% | 21.1b* | 8.5ab | 8.4a | 0.4ac | -55a* | 18.6a* | |
| 100% | 22.1b* | 14.1b* | 0.2a | 5.4c | -49.6a* | 18.9a* | |
| I. tenuis | 25% | 19.1a* | 43.6a* | 19.8a* | 8.5a | 10.4a | 20.2a* |
| 50% | 19.1a* | 54.6ab* | 12.3a | -0.8b | 27.3ab* | 5.4a | |
| 75% | 37.7b* | 63.8b* | 9.4a | 17.6c* | 35.8b* | 9.4a | |
| 100% | 41.6b* | 66.6b* | 23.3a* | 20c* | 47.9b* | 18.4a* | |
Aqueous leaf extract of I. condensata inhibited the growth of lettuce and tomato root at all concentrations with respect to control, and maize root only at the highest concentration (Figure 2 and Table 3). The inhibition percentage was statistically different between 25 % and the remaining concentrations for lettuce, and between 100 % and the remaining concentrations for tomato (Figure 2 and Table 3). Regarding shoot growth, the aqueous extract of I. condensata inhibited maize growth at all concentrations compared to the control, with inhibition percentage being statistically different between 25-50 % and 100 % (Figure 2 and Table 3). The aqueous extract of I. condensata did not inhibit shoot growth of either lettuce or tomato (Figure 2 and Table 3).
Aqueous extract of I. minutiflora inhibited root growth of lettuce (at 75 and 100 %) and tomato (at 100 %) as compared to the control (Figure 2 and Table 3). The inhibition percentage was not statistically different between 75 and 100 % concentrations for lettuce (Figure 2 and Table 3). Regarding shoot growth, the aqueous extract of I. minutiflora inhibited the growth of maize at all concentrations as compared to the control, with no statistical differences among concentrations (Figure 2 and Table 3). Aqueous extract of I. minutiflora stimulated the growth of tomato shoot at all concentrations as compared to the control, with no statistical differences in inhibition percentage among concentrations (Figure 2 and Table 3).
The aqueous leaf extract of I. tenuis inhibited root growth of lettuce and tomato as compared to the control at all concentrations, and of maize at 25 and 100 % (Figure 2 and Table 3). The inhibition percentage was statistically different between 25-50 and 75-100 % concentrations for lettuce, and between 25 and 75-100 % concentrations for tomato (Figure 2 and Table 3). The inhibition percentage was not statistically different between the concentrations tested for maize (Figure 2 and Table 3). Regarding shoot growth, the aqueous extract of I. tenuis inhibited the growth of lettuce at 75 and 100 % concentrations, of tomato at 50, 75, and 100 %, and of maize at 25 and 100 %, with no statistical differences in inhibition percentage among concentrations (Figure 2 and Table 3).
Discussion
Bioassays are an initial and essential stage to determine allelopathic activity in plants. In this sense, in this work, the aqueous leaf extracts of South American Imperata taxa showed allelopathic effects on seed germination and seedling growth of maize, lettuce, and tomato. An interesting future approach would be the use of non-allelopathic plants as controls to verify that the procedures and methodologies are not creating artifacts. In general, the inhibitory effects of aqueous extracts of Imperata species were greater on root and shoot growth than on germination of the tested plants. The results obtained with aqueous extract of I. brasiliensis reinforce previous findings by Casini et al. (1998). The aqueous extract of I. tenuis exhibited the highest inhibitory activity on the tested plants, followed by the aqueous extracts of I. brasiliensis, I. condensata, and I. minutiflora. The concentration of the crude extract (100 %) is probably not found in nature, however using the crude extract allows us to make sure that if these plants produce allelopathic compounds we could detect some effect and also test a possible application as a herbicide. An interesting result is the growth stimulation of the shoot of tomato by the aqueous extract of I. minutiflora. The fact that allelopathic substances can produce inhibitory and stimulatory effects has already been reported and is currently being investigated for their agronomic use (Abbas et al. 2017). In general, the effects of the different concentrations did not differ in each treatment. However, when there were differences between the effects at the different concentrations, the degree of inhibition increased with the increasing extract concentration.
Studies on the production and release of allelopathic compounds by I. cylindrica have covered both the ecological and the agronomic aspects. Phenolic compounds and aldehydes were identified in the aqueous leaf extracts of I. cylindrical as responsible for the allelopathic activity, such as p- and o-coumaric acid, gentisic acid, vanillic acid, p-hydroxybenzoic acid, scopolin, scopoletin, chlorogenic, and isochlorogenic acid, vanillin and p-hydroxy-benzaldehyde (Abdul-Wahab & Al Naib 1972, Eussen & Wirjahardja 1973, Eussen 1979, Eussen & Niemann 1981). Inderjit & Dakshini (1991) found that the phenolic fraction of the aqueous extracts of I. cylindrica was responsible for the phytotoxic activity and that the release of the compounds to the ground could be selective. Hussain & Abidi (1991) identified caffeic, p-coumaric, p-hydroxybenzoic, syringic, chlorogenic, and vanillic acids as responsible for the allelopathic effects generated by aqueous extracts and rain leachates. In addition, a great variety of chemical compounds with allelopathic activity have been found in aqueous root and rhizome extracts of I. cylindrical, including gallic acid, iso-Eugenol, ferulic acid, caffeic acid, 4-acetyl-2-methoxy phenol, 5-methoxy flavone, and 5,2'-dimethoxyflavone (Xuan et al. 2009, Hagan et al. 2013, Suzuki et al. 2018). The mixture of phenolic acids is the factor that contributes to the allelopathic effect, since each acid separately has a weak effect (Blum et al. 1985). Phenolic compounds can cause this inhibition through different mechanisms that affect vital physiological processes for plants such as nutrient absorption, cell elongation, photosynthesis, and respiration (Li et al. 2010). The biosynthetic pathways of phenolic compounds have been traced by natural selection throughout evolution between specific plant lineages, especially when these compounds perform specific advantageous functions (Li et al. 2010). Although the chemical composition of the aqueous extracts of South American taxa has not been studied, the phytotoxic activity observed in this work is likely due to the phenolic fraction, as has been demonstrated in I. cylindrica. Furthermore, the results showed differences in the effects on germination and seedling growth. Allelochemical composition and allelopathic effect are influenced by the historical biogeography of the species (Irimia et al. 2019). Considering that South American species of Imperata and I. cylindrica have different evolutionary histories (Cordobés et al. 2021), the observed differences in the effect of aqueous extracts could reflect the production of different compounds by each taxon. The results also show that the compounds produced by these plants may potentially be used as natural herbicides.
The results obtained are promising and provide the basis for future studies on the role of the production of allelopathic compounds in the South American Imperata taxa analyzed. The results also show that the compounds produced by these plants may potentially be used as natural herbicides. The characterization of the allelochemicals and their release and movement under field conditions are important guidelines for future research in the ecology of Imperata and potential agroecological uses.











 text new page (beta)
text new page (beta)



