In plants, a decrease in population size or an increased isolation across fragmented populations can lead to significant declines on individual sexual reproductive success and limited population growth (Leimu et al. 2006, Angeloni et al. 2011). This phenomenon is especially important in endemic species because they are usually characterized not only by their restricted geographical distribution but also by dispersed and small populations, thus, this puts them at a higher risk of extinction (Rabinowitz 1981, Kruckeberg & Rabinowitz 1985, Menges 1990, Lavergne et al. 2004, Marrero-Gómez et al. 2007). The decrease in sexual reproductive success of endemic plant species may be partially explained by a limited ability to attract pollinators or the low availability of pairs to mate (Paschke et al. 2002, Busch & Schoen 2008, Albarrán et al. 2017). These conditions result in a strong pollen limitation, resulting in low fruit and seed production (Aguilar et al. 2006). While pollen limitation is a frequent phenomenon in many plant species (Ashman et al. 2004, Knight et al. 2005), there is evidence that endemic species are more limited by pollen than non-endemic (Alonso et al. 2010).
Moreover, the degree of pollen limitation depends largely on the plant mating system being usually higher in self-incompatible species (Fenster & Martén-Rodríguez 2007, Knight et al. 2005, Alonso et al. 2010). Thus, if endemic species face a greater pollen limitation due to small and fragmented populations, self-compatibility could be expected to be a recurrent evolutionary path followed by those species (Kruckeberg & Rabinowitz 1985, Lavergne et al. 2004, Fenster & Martén-Rodríguez 2007, Busch & Schoen 2008). In fact, in a meta-analysis that included 287 plant species, Alonso et al. (2010) found that transitions to endemism were associated with transitions to self-compatibility, which would allow self-compatible endemic species to "scape" from pollen limitation. However, fruit and seed production not only obeys to how much pollen is deposited in the floral stigmas (a quantity limitation), but also of its origin (a quality limitation), i.e., if it comes from genetically related or unrelated individuals (Aizen & Harder 2007, Busch et al. 2010, Alonso et al. 2012).
It is well-stablished also that self-compatibility may incur associated costs derived from inbreeding depression effects (Baker 1955, Charlesworth & Charlesworth 1987, Husband & Schemske 1996, Byers 1998, Dudash & Fenster 2000, Munguía-Rosas et al. 2013). Inbreeding depression, the reduced survival and fertility of offspring of related individuals, is one of the most important factors limiting population growth, since it can occur from early stages of sexual reproduction, such as fruit and seed production (Charlesworth & Charlesworth 1987, Collin et al. 2009, Alonso & García-Sevilla 2013) to later stages of the life cycle (Medrano et al. 2005), thus limiting recruitment in natural populations (Angeloni et al. 2011). In particular, in endemic species, inbreeding depression effects are frequently related to decreased population size, where mating between genetically related individuals increases (e.g., Raijmann et al. 1994, Fischer & Matthies 1998, Paschke et al. 2002).
However, theory also predicts it can be expected that in self-compatible plant species, in which endogamous mating occurs frequently, either by self-pollination or by interbreeding between genetically related individuals, there should be strong selective pressures that would lead to a purge of deleterious alleles, such that eventually these species would not show inbreeding depression (Stebbins 1957, Lande & Schemske 1985, Charlesworth & Charlesworth 1987, Fenster & Martén-Rodríguez 2007, Busch & Schoen 2008). Thus, it is possible that the evolutionary transition to self-compatibility observed in endemic species (Alonso et al. 2010), has been accompanied by a significant decrease of the effects of inbreeding depression (Lande & Schemske 1985, Barrett 2003).
Thus, self-compatible endemic species can be expected to have no or low levels of inbreeding depression. However, some evidences have shown that endemic species experience strong inbreeding depression even in early life-cycle stages (e.g., Alonso & García-Sevilla 2013). Similarly, it is feasible that self-compatible endemic species show pollen limitation, if they are not able to produce fruits and seeds in the absence of pollinators (i.e., inefficient autonomous pollination; Kalisz & Vogler 2003, Becker et al. 2011). Studying the factors that limit the population viability of endemic species is essential to strengthen strategies that contribute to their conservation (Ellstrand & Elam 1993).
The aim of this work was to study some aspects of the reproductive biology of Cienfuegosia yucatanensis Millsp. (Malvaceae), a self-compatible endemic species of the Caribbean region. Populations of this species are distributed mainly in seasonally dry scrublands of the north coast of the peninsula of Yucatán, México (Alonso et al. 2013, Parra-Tabla et al. 2017, GBIF 2020, Tropicos 2020). Specifically, in this work we describe under field conditions the flowering phenology and the pollination success (i.e., pollen loads deposited on stigmas and pollen tubes in the styles) and the post-pollination success (i.e., fruit and seed production), and then we try to answer the following questions, a) Is C. yucatanensis able to autonomous pollination?, b) Are fruit or seed production limited by pollen in C. yucatanensis? and, c) Is there evidence of early inbreeding depression in C. yucatanensis?
Since C. yucatanensis flowers are scarcely visited by pollinators (Téllez 2012, Arceo-Gómez 20016a), and previous observations have shown a low and a highly variable number of pollen tubes on wild populations (Alonso et al. 2013), we predicted that this species will show pollen limitation. Because in the wild populations of this species produce fruits (Téllez 2012), we predicted that C. yucatanensis would autonomous pollinate. Altogether, we expected that C. yucatanensis would show negligible levels of early inbreeding depression.
Materials and methods
Study species. Cienfuegosia yucatanensis Millps. (Malvaceae) is a small perennial herb (10 to 50 cm tall), which is distributed in the Caribbean region, including southern Florida, Cuba and the Bahamas archipelago. However, most known populations are mainly located in the north coast of the Yucatán peninsula (GBIF 2020, Tropicos 2020). In the north coast of Yucatán, C. yucatanensis is distributed in a patchy way, and generally at low densities, in seasonally dry scrublands (Flores & Espejel 1994, Espadas-Manrique et al. 2003, Téllez 2012), which have been extensively disturbed and fragmented mainly by livestock activities (Orellana et al. 2009). C. yucatanensis grows on thin soil atop limestone floodable, with higher rock cover. Plant species richness in these scrublands is low but includes a relatively high level of plant endemism (Espadas-Manrique et al. 2003). The species usually shows solitary open actinomorphic flowers and, although it can be found individuals with 2 to 5 simultaneously open flowers, the total number of flowers produced per individual is low (< 10; Téllez 2012). The flowers are hermaphroditic with yellow corollas and last one day (Figure 1).

Figure 1 Flower of Cienfuegosia yucatanensis visited by the honey bee Apis mellifera in the north coast of the peninsula of Yucatán, México (Photo credit: Luis Salinas-Peba).
The flowers have a solitary style divided apically, in 3-5 stigmas, which are above the anthers (Fryxell 1992). Each flower contains 14-18 ovules (16.4 ± 0.4, Mean ± S.E., Alonso et al. 2013), the fruits are dry and dehiscent, and the seeds pubescent (Fryxell 1992). The flowers produced nectar and are visited by different species of Hymenoptera and Lepidoptera, but at a very low rate (< 0.02 visits/min/flower; Téllez 2012, Arceo-Gómez et al. 2016a). C. yucatanensis is self-compatible (Alonso et al. 2013), but it is not known if it is capable of autonomous pollination. Even though pollen deposition in the stigmas is relatively high (between 100 and 300 grains per stigma), the mean number of pollen tubes per ovule that grow in the styles is usually low but highly variable (0.56 ± 0.75; Mean ± SD; 0- 1.5; Alonso et al. 2013).
Study sites. This study was carried out in two populations of C. yucatanensis located in the north of the state of Yucatán in the rainy season of 2013 and 2014 (from August to October). The first population was located within the municipality of Dzemul (21°18' N; 89°1' W) and the second within the municipality of Chicxulub (21° 08’ N; 89° 30 W). Both sites are separated by ca. 20 km. The climate is warm sub-humid with rains in the summer, an average annual temperature of 26.3 °C and an average annual rainfall of 469 millimeters (Orellana et al. 2009). In the seasonally scrublands in both sites, dominant tree and shrub species such as Bursera simaruba (L.), Acacia collinsii (Saff.) and Gymnopodium floribundum (Rolfe), as well as herbaceous species such as Angelonia angustifolia (Benth.), Tamonea curassavica (L.) and Sida cordifolia (L.), can be found (Flores & Espejel 1994). This scrubland is also characterized by endemic species of Cactaceae such as Nopalea gaumeri (Britton & Rose) and Pilosocereus gaumeri (Britton & Rose) (Espadas-Manrique et al. 2003), and endemic herbaceous such as C. yucatanensis (Millsp.) and Cuphea gaumeri (Koehne) (Alonso et al. 2013).
Flowering phenology, pollination and post-pollination success on field conditions. In 2013, four 20 m transects were established at each site and seven equidistant 2 × 2 m plots were placed in each transect (parallel to each other). The location of the transects was established after a visual inspection through which the presence of C. yucatanensis was detected. Since flowering of most of the herbaceous in the region occur during the rainy season (June to October; Parra-Tabla et al. 2017), weekly censuses were carried out during that period to record the number of open flowers and fruits in a total of 200 individuals (87 at the Dzemul site and 103 at the Chicxulub site). Pollination success was estimated by analyzing the number conspecific pollen grains deposited on the stigmas and the number of pollen tubes growing in the styles. The identification of conspecific pollen was done comparing the samples with a pollen library previously elaborated for endemic species from the study sites (Alonso et al. 2013). Although we were not able to visualize the pollen tubes that penetrated the ovules, the number pollen tubes (and the deposited conspecific pollen grains) are effective variables to describing natural variation in the pollination success at the individual, population, and community levels (Alonso et al. 2012, Arceo-Gómez et al. 2016a, Ashman et al. 2020). To do this, we collected 2 to 3 withered and closed flowers from 20 individuals per site randomly selected during the flowering period. The flowers were placed in 70 % alcohol in individual containers. In the laboratory stigmas and styles were dissected to count the number of pollen grains in the stigma and the growth of pollen tubes with the fluorescence technique (Kearns & Inouye 1993). Styles were softened in 1 N KOH, rinsed with distilled water and stained for 60 min at 68 °C in decolorized aniline blue. Under a fluorescence microscope (Nikon e200©), pollen grains were counted on the stigma and pollen tubes at the base of the style.
Fruit and seed-set (post-pollination success), were evaluated from a subsample of 30 individuals per site, which were marked and followed during all the reproductive season. Fruit-set was estimated as the proportion of the total number of mature fruits respect to the total number of flowers. During each census, we collected 2-3 mature fruits from each individual plant to count the number of seeds (N = 75 and 81 in Dzemul and Chicxulub sites, respectively). Seed-set was calculated as the proportion of the number of seeds respect to the total number of ovules, estimated as the sum of the number of mature seeds and the number of unfertilized ovules (Parra-Tabla et al. 1998).
Pollen limitation and autonomous pollination. To test for pollen limitation, and if C. yucatanensis is capable of autonomous pollination, in the year of 2014 a hand-pollination experiments was carried out under field conditions in 30 individuals at the Chicxulub site. The selected individuals had very similar size (i.e., height and plant cover), and similar floral display size. Given the low number of flowers produced by each individual plant of C. yucatanensis, we were only able to apply the following treatments: (1) Autonomous pollination: individual flowers were bagged before the anthesis to avoid access to pollinators and foreign pollen, (2) Cross-pollination: individual flower buds were emasculated one day before anthesis to avoid self-pollen deposition. At the anthesis we added a pollen mixture of 2 to 3 flowers collected from different and distant individuals (> 10 m); after that the flowers were bagged; and (3) Open pollination: individual flowers with free access to pollinators; when the flowers close these were bagged. All the flowers were individually marked, identifying in each case the treatment to which they were subjected. Because the low number of simultaneously open flowers available, the treatments could not always be applied the same day, and the replication of each treatment was unbalanced. The hand pollinations of the cross-pollination treatment were performed early in the morning (ca. 7-8 AM). We had ca. 90 experimental flowers per treatment (N = 270 total manipulated flowers). The fruits from the experimental flowers were monitored and the mature fruits were collected. Fruit and seed-set were estimated in the same way described above.
Early inbreeding depression. To estimate if C. yucatanensis showed early inbreeding depression, we used as response variables the fruit and seed-set, and the seed weight from the treatments of autonomous pollination (i.e., self-pollination) and cross-pollination of the pollination experiment described above. Mature seeds were individually weighted with an analytical balance (Ohaus® ± 0.001 mg). The magnitude of early inbreeding depression on fruit and seed production and on seed weight, was calculated as δ = 1- (Ws / Wo), where δ is the inbreeding depression index, and Ws and Wo are the mean fitness values of the autonomous self-pollination (Ws) and cross-pollination (Wo) estimated from the pollination experiment described above (Johnston 1992, Ågren & Schemske 1993). These variables are considered good proxies of plant fitness (Endler 1986). In particular, seed weight is closely associated with seedling establishment and future plant survival and reproduction (e.g., Kalisz 1989, Paz et al. 2005).
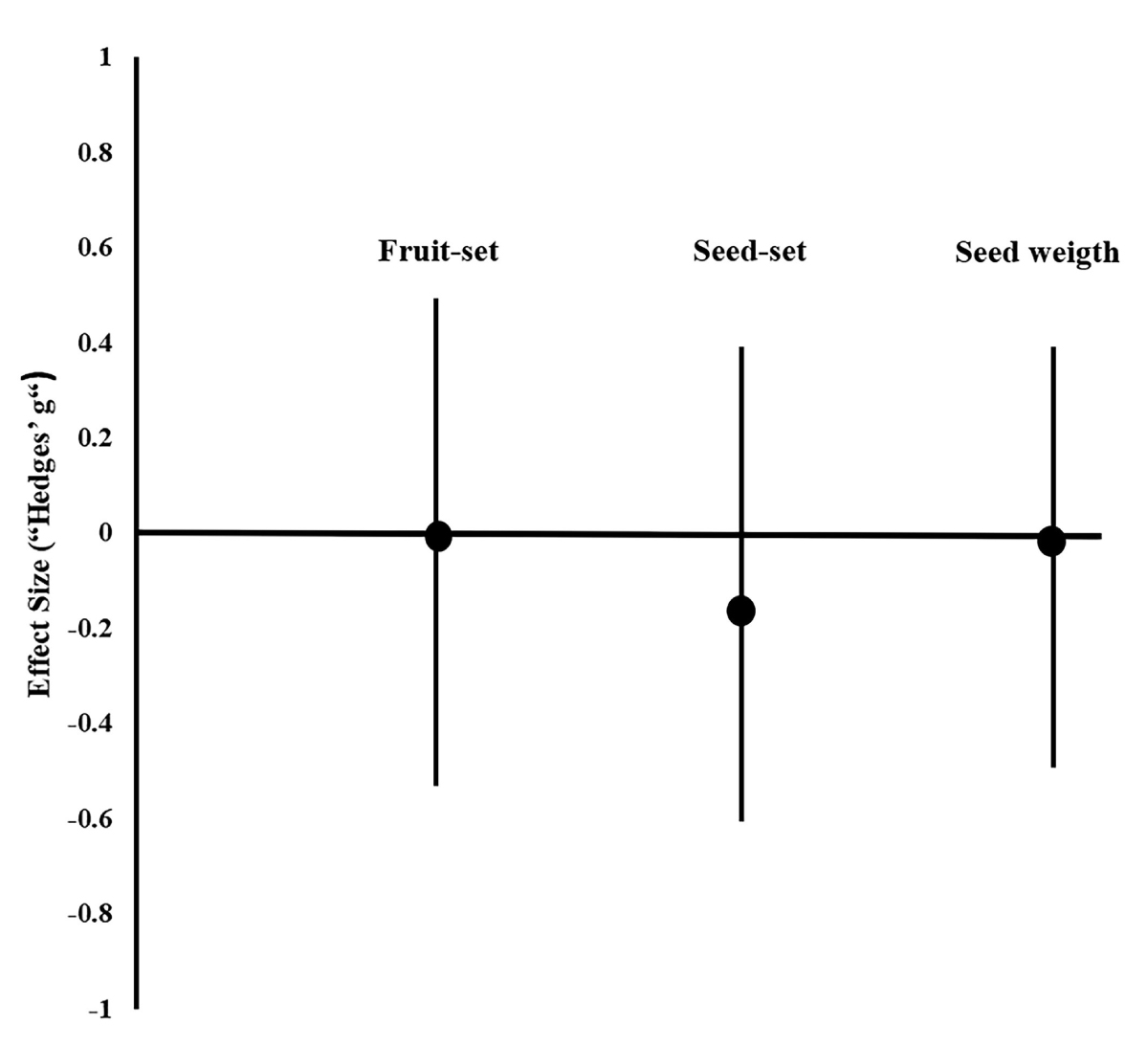
Statistical analyses. Among sites differences in the average of number of flowers, fruits, conspecific pollen on the stigmas, number of pollen tubes on the styles, and fruit and seed production, were evaluated with t-Student tests (Zar 1986). To test differences between the pollination treatments, a mixed generalized linear model was performed, with the pollination treatment as a fixed effect and the individual plants as a random effect. The response variables were fruit and seed-set, in both cases we used a binomial error with log link function (Littel et al. 2006). These analyses were performed with the software SAS ver. 9.1 (SAS 2002). Multiple paired tests among treatments were performed with the pdiff/test procedure (SAS 2002). To assess the effect of early inbreeding depression, the Hedges’ effect size was calculated following Hedges & Olkin (1985), as Hedges’ g = Wo-Ws /SD pooled where Ws and Wo are the mean values of the autonomous self- and cross-pollination, respectively, and SD is the pooled weighted standard deviation. An effect size value of 0 reflects no difference in reproductive success between flowers from the open pollination treatment, and flowers from the autonomous pollination (i.e., no significant inbreeding depression effect). In addition, the mean value of δ for the autonomous pollination and the cross-pollination treatment were calculated (see above). To test if the δ was significantly different from 0, we calculated the mean and the δ ‘s confidence interval (CI) at 95 % (Zar 1986).
Results
Flowering phenology, pollination and post-pollination success under field conditions. The flowering period of C. yucatanensis was short since it only lasted from August to September. The average of total open flowers at the sites was relatively constant throughout flowering, although a higher number of open flowers was observed towards the middle of August. In contrast, a higher number of fruits was observed towards the middle of September (Figure 2). We did not find significant differences between sites for the total number of flowers and fruits per plant (t ≤ 0.8, P > 0.4, in both cases). On the other hand, we did not find significant differences between sites in the fruit-set (Table 1), but we found significant differences between sites for seed-set, in Chicxulub almost twice as many seeds were produced compared to Dzemul (Table 1).

Figure 2 Mean number (± SD) of flowers and fruits of Cienfuegosia yucatanensis in two sites of the north coast of the peninsula of Yucatán, México in the flowering season of 2013 (data from the two study sites combined).
Table 1 Fruit-set and seed-set (Mean ± SD) of the endemic plant Cienfuegosia yucatanensis in two sites of the north of the peninsula of Yucatán. Significance for the comparison among sites (P-values) are shown.
| Dependent variable | Site | Mean ( ± SD) | P |
|---|---|---|---|
| Fruit-set | Chicxulub | 0.61 (0.40) | 0.07 |
| Dzemul | 0.48 (0.41) | ||
| Seed-set | Chicxulub | 8.4 (3.7) | < 0.001 |
| Dzemul | 4.9 (3.4) |
Pollen deposition on floral stigmas was high in both sites (Figure 3A) and the average number of grains per stigma at the Chicxulub site was almost twice as high as the observed at Dzemul (Mean ± S.D. 84.8 ± 56.0 and 43.8 ± 31.5 pollen grains, respectively), this difference was statistically significant (t28 = 7.1, P < 0.01). Regarding the number of pollen tubes, these were much lower compared to the number of pollen grains deposited on the stigmas (Figure 3B), but much higher than the number of pollen tubes per flower in most of the flowering period. The average number of pollen tubes per ovule was three times higher in Chicxulub compared to Dzemul (Mean ± S.D. 2.01 ± 3.9 and 0.56 ± 0.75 pollen tubes, respectively), this difference was statistically significant (t28 = 4.02, P < 0.01).
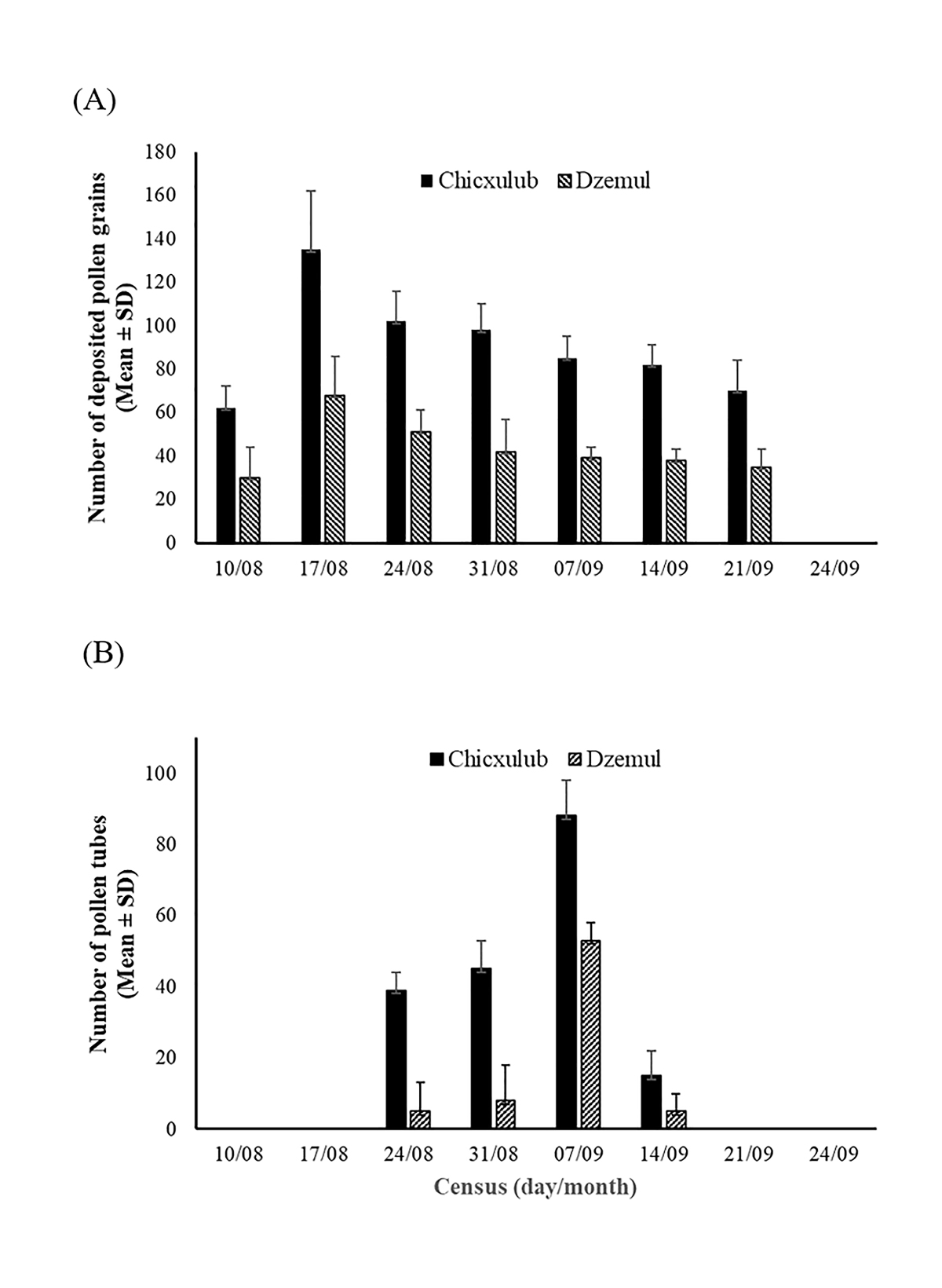
Figure 3 Mean number (± SD) of (A) conspecific pollen deposited on the stigma and (B) pollen tubes growing on the styles of individual flowers of Cienfuegosia yucatanensis along the flowering season of 2013 in two sites of the north coast of the peninsula of Yucatán, México
Pollen limitation and autonomous pollination. The pollination experiment conducted at Chicxulub site showed that the average fruit-set was relatively high for all treatments (ca. 65 %; Table 2). Despite a difference of about 5 % observed in the open pollination treatment, respect to the average of the other two treatments (Table 2), no significant differences were found between treatments due to large variance recorded in all of them (Table 2). On average, seed-set was high in all pollination treatments (> 80 %; Table 2), and the statistical analysis showed significant differences between the cross- and the autonomous pollination treatments compared to the open pollination treatment (P < 0.03 in both cases; Table 2). Open pollination produced almost 10 % more seeds than autonomous and cross-pollination treatments (Table 2, P < 0.05 in both cases), which did not differ from each other (Table 2, P > 0.3). Finally, the seed weight was very similar in all treatments, and no significant differences were found between them (Table 2).
Table 2 Fruit-set, seed-set and seed weight (Mean ± SD) of the endemic plant Cienfuegosia yucatanensis for the pollination treatments. F-values (df = 2, 267) and P-values for comparison among pollination treatments are shown. Different letters denote significant differences between pollination treatments for each dependent variable (P < 0.05). Inbreeding depression index δ (confidence interval at 95 %) for fitness values from cross-pollination offspring and from autonomous self-pollination offspring is shown.
| Dependent variable | Pollination treatments | Mean (± SD) | F | P | δ (CI 95%) |
|---|---|---|---|---|---|
| Fruit-set |
|
|
0.57 | 0.56 | +0.04 (-0.312 to + 0.252) |
| Seed-set |
|
|
4.08 | 0.02 | -0.03 (-0.560 to + 0.200) |
| Seed weight (mg) |
|
|
0.17 | 0.84 | +0.045 (-0.371 to + 0.205) |
Early inbreeding depression. Size effects revealed a minimal and non-significant difference between the cross-pollination (Wo) and autonomous pollination (Ws), for any of the response variables (Figure 4; P > 0.5 in all cases). The average of each variable from both pollination treatments, were very similar to each other (Table 2). Furthermore, the δ ’s confidence interval always overlap zero (Table 2), suggesting also non-significant inbreeding depression effects in this population.
Discussion
The results found in this work were partially consistent with our predictions, since we found that C. yucatanensis is capable of autonomous pollination. In addition, seed but not fruit production, was affected by pollination treatments although in an unexpected way, and we did not find evidence of early inbreeding depression.
Pollen limitation and autonomous pollination. The importance of pollen limitation by both, quantity and quality, depends largely on the plant mating system (Ashman et al. 2004, Knight et al. 2005, Alonso et al. 2010). Since C. yucatanensis is self-compatible, and its flowers are scarcely visited by pollinators (Téllez 2012, Arceo-Gómez 20016a), we predicted that pollen limitation would depend mostly on the autonomy for effective self-pollination and the quality of pollen deposited on the stigma. The results of the pollination experiment done in the Chicxulub site, revealed that C. yucatanensis is capable of effective autonomous pollination in absence of pollen vectors. Furthermore, the flowers of the autonomous pollination treatment showed the same probability to produce fruits as the flowers of hand cross-pollination and those with open pollination, suggesting that fruit production is not limited by pollen in C. yucatanensis. Moreover, the non-significant differences among autonomous and cross-pollination, also suggest that in C. yucatanensis there is not quality pollen limitation of fruit production.
However, the hand-pollination experiment also showed that the open pollination treatment produced ca. 10 % more seeds than both, the autonomous and hand cross-pollination treatments, which would suggest that seed production can be to some extent pollen limited. Unfortunately, our study did not allow us to distinguish if this increase of seed production was due to pollen quantity or pollen quality. On one hand, we did not quantify pollen loads on the stigma of treated flowers so it is possible that, although we used a generous amount of pollen in the cross-pollination treatment, this quantity was less than that received by the open pollinated flowers. Alternatively, it is possible that the supplementary pollen produced a “crowding effect”, inhibiting the ovule fertilization (Young & Young 1992). On the other hand, it is also possible that the pollen they received naturally was of higher quality. In plants with mixed mating systems, differences in the quality of self- vs. cross-pollen have been extensively demonstrated, showing that self-pollen or pollen from individuals genetically related are less effective to ovule fecundation than cross-pollen (e.g.,Kalisz 1989, Dudash 1990, Johnston 1992, Alonso et al. 2012, Abdala-Roberts et al. 2014). Therefore, an experiment that controls both, the pollen source and the amount of pollen deposited by emasculation and hand-pollination with self- and outcross-pollen, is necessary in order to clarify if pollen quantity or pollen quality are more relevant to explain seed production in C. yucatanensis. In addition, emasculation before and after anthesis could further clarify when autonomous pollination occurs (Fenster & Martén-Rodríguez 2007).
Nevertheless, it is important to point out that the pollination experiment of this study was carried out at the Chicxulub site, where the pollen loads on the stigmas was almost twice as high, and the number of pollen tubes was almost three times higher than the Dzemul site. Thus, this result suggests that the extent of pollen limitation, must be variable between populations of C. yucatanensis (e.g., Dart & Eckert 2013).
Unlike other self-compatible endemic species, fruit and seed production in C. yucatanensis was high (ca. 60 and 85 %, respectively) in the absence of pollinators. For instance, Erodium cazorlanum does not produce fruits and seeds autonomously (Alonso & García-Sevilla 2013), and although autonomous fruit production in Echium wildpretii was similar to C. yucatanensis (ca. 55 %), the seed production in this species was 50 % less in autonomous pollinated flowers than in open-pollinated flowers (Sedlacek et al. 2012). These results suggest that self-compatible endemic plants would show different levels of pollen limitation, depending on how efficient autonomous pollination is (see Kalisz & Vogler 2003). Interestingly, the efficiency of autonomous pollination has not been considered in the global analyses of pollen limitation in which self-compatible plants are considered a homogeneous group (Ashman et al. 2004, Knight et al. 2005), and it has been listed among the open relevant questions for pollination studies of XXI century (Mayer et al. 2011). Whether its effect is similar in endemic and non-endemic plants is largely unknown and we suggest that strong selection for reproductive assurance, must drive to an efficient autonomous pollination in self-compatible endemic species (see Fenster & Martén-Rodríguez 2007), particularly when they face great uncertainty in the pollinator’s availability.
Overall, our results suggest that self-compatibility and autonomous pollination are suitable mechanisms of reproductive assurance in C. yucatanensis, but also that the action of natural pollinators contributes to increase seed production. Additionally, for conservation purposes, it is important to consider the spatial variation on pollen limitation effects in endemic species (Arceo-Gómez et al. 2016b), because accumulating evidence supports that ecological disturbances such as habitat fragmentation, decline of pollinators, alien species (Knight et al. 2005, Memmott et al. 2007, Parra-Tabla et al. 2019) or even among-regional differences on plant richness (Alonso et al. 2010), may increase pollen limitation.
Early inbreeding depression. In agreement with our prediction, the results showed no significant differences among autonomous-selfing and experimental hand cross-pollination treatments on fruit and seed production or seed weight, suggesting that in these early stages of the life-cycle of C. yucatanensis, there are no effects of inbreeding depression at least in the Chicxulub site. The effects of inbreeding depression in plants have been widely documented (e.g., Dudash 1990, Holsinger 1991, Byers & Waller 1999). However, theoretical and experimental evidence suggests that species that are subject to repeated events of inbred mating, unmask deleterious alleles, thus decreasing the effects of inbreeding depression (Lande & Schemske 1985). Thus, in addition to the benefit of avoiding pollen limitation, self-compatibility has the associated benefit of purging deleterious alleles (Lande & Schemske 1985, Barrett 2003).
In C. yucatanensis there are two lines of evidence that suggest a highly frequent inbred mating. First, previous observations have shown that the flowers of C. yucatanensis are consistently visited at a very low rate by its insect-pollinators (Téllez 2012, Arceo-Gómez et al. 2016a), which suggest that cross-pollination is not frequent. Second, our results of the pollination experiment revealed that even in the absence of pollinators, C. yucatanensis is capable to produce fruits and seeds via autonomous pollination as efficiently than hand cross-pollination. Interestingly, in other self-compatible endemic species in which autonomous pollination is not efficient, strong effects of inbreeding depression has been observed. For instance, in the endemic self-compatible Erodium cazorlanum, which is not capable of autonomous pollination, it was observed that the number of seeds per fruit and seeds weight, was significantly higher after cross than self-pollinations (Alonso & García-Sevilla 2013). In contrast, in Echium wildpretii, in which the efficiency of autonomous pollination to produced fruits was similar to the observed in C. yucatanensis, weak effects of inbreeding depression were found (Sedlacek et al. 2012). Together, these results suggest that the frequency and efficiency of autonomous pollination is associated with the extent of the inbreeding depression effects (see Fenster & Martén-Rodríguez 2007).
Nevertheless, we cannot rule out the existence of inbreeding depression effects in later stages of the life-cycle of C. yucatanensis, as is common in many self-compatible plant species (e.g., Husband & Schemske 1996, Munguía-Rosas et al. 2013). Furthermore, it is well known that inbreeding depression’s effects occur more frequently when plants are subjected to stressful environments (Armbruster & Reed 2005, Cheptou & Donohue 2011). For instance, in the endemic self-compatible Echium wildpretii, Sedlacek et al. (2012), found that inbreeding depression increased significantly only under hydric stress, reducing the offspring’ survival produced by self-pollination by up to 50 %, respect to offspring produced by cross-pollination. Thus, in order to corroborate that C. yucatanensis does not suffer from inbreeding depression, it is necessary to evaluate differences between self and cross-pollinated progeny in later stages of the life-cycle (e.g., age at first reproduction, survival), as well as evaluating its overall performance under stress conditions.
This latter is reasonable to study in C. yucatanensis for two reasons. First, under wild conditions, C. yucatanensis faces different natural stressful conditions (e.g., recurrent tropical storms, long periods of low water availability and high micro-environmental heterogeneity, such as light exposure and nutrient soil availability). Thus, it is possible that in these temporal and spatial environmental gradients, inbreeding depression may act. Second, the scrublands of the north of Yucatán are being subjected to strong anthropogenic effects, particularly to a high habitat fragmentation, which, in addition to isolating and reducing populations size, could add other important environmental disturbances in the wild populations of C. yucatanensis (see Haddad et al. 2015). For instance, it is well-established that habitat fragmentation can reduce pollinator diversity and abundance, and plant genetic variation (Leimu et al. 2006).
In summary, our results suggest that self-compatibility combined with an efficient autonomous pollination, are suitable mechanisms for the reproductive assurance in C. yucatanensis, with no apparent effects of early inbreeding depression.











 nueva página del texto (beta)
nueva página del texto (beta)