Cactaceae family have evolved various adaptations in response to stressful conditions associated with severe aridity. These include the presence of specialized defense mechanisms, such as spinescence, and sclerophilia (Hanley et al. 2007), which are important because under extreme conditions originated by different factors (e.g., excessive heat, frost, natural fire, herbivore damage) (Rhoades 1979, Lundberg & Palo 1993), it becomes more difficult to regenerate damaged tissue. A variety of herbivore-response mechanisms are divided into two main categories: resistance and tolerance. Previous studies to understand the causes of both mechanisms, and their effects on fitness and genetic diversity, have been developed in several gymnosperms and angiosperms (Abreu et al. 2012), but few are found in Cactaceae. Resistance, defined as the ability of plants to avoid damage, has been documented in multiple species with leaves, and most frequently relates to resistance to herbivores (Rasmann et al. 2011, Mithöfer & Boland 2012). Tolerance, defined as the ability of plants to produce new branches, and reallocate resources, among other responses, as well as fitness maintenance in the presence of damage (Rasmann et al. 2011), has been largely unexplored in plant lineages (Juenger & Lennartsson 2000) and only one study has been documented for cacti (Medel 2001).
Mexico is the main area of diversification of Cactaceae, especially the columnar cacti, which are represented by some 80 species. In addition to their taxonomic and ecological importance (Godinez-Alvarez et al. 2003), many species (about 45) have been exploited since pre-Hispanic times (Callen 1967, Casas 2002, Luna-Morales 2004). A current serious problem in this botanical family is the presence of damage in stems and branches, which has been observed in populations of multiple species. Field observations of different columnar cacti growing in semiarid region of Central Mexico showed various types and extents of damage, ranging from apical cuts by ants, to total decay of complete individuals due to rot damage (Bravo-Avilez 2017). Previous reports have documented many herbivores (see Mann 1969) and vectors of damage, but specific aspects related with defense mechanisms are practically nonexistent.
Based on this, we considered necessary to summarize knowledge about damage in cacti in the Americas, as well as previous documentation of defense mechanisms in this family. The aims of the present review are: 1) to summarize literature focused on damage in cacti and defense mechanisms in the Americas, 2) to elucidate possible related patterns of types of damage, factors causing damage, and distribution of damaged cacti species, and 3) to show new evidence from field observations about rot damage in some species of columnar cacti from Central Mexico.
Materials and methods
The following electronic databases were consulted: ISI Web of Knowledge, Cabdirect, and Google Scholar. We included the keywords: damage, cacti, insect, herbivores, pest, and disease (English and Spanish words). A database of presence - absence was elaborated by: subfamily, tribe, and cacti species, considering its distribution, and kind of damage: biotic (including different interactions, like herbivory, parasitism, and commensalism): nine nematodes’ taxa, 23 insects, nine mammals, one taxon of birds (corresponding to four species), one parasitic plant, seven yeast, and two bacteria; and abiotic (including in one category heat, frost, fire, wind, human damage by tools, and barking, which was included here because it is not consequence of any biotic interaction). The final database consisted of 51 kinds of damage on 58 taxa of Cactaceae. Looking for a possible pattern between these factors, as well as the geographical distribution of cacti, a Cluster Analysis using the Ward´s method on the Euclidian distance matrix was applied, using the statistical program XLSTAT (2016).
Evidence from field surveys consisted of direct observations of individuals from different species presenting rot damage in Central Mexico. Extensive field surveys were carried out through three years (2012 to 2014), accounting for damage in cacti in different locations at the Mixteca Baja (in Puebla and Oaxaca), and Tehuacán Valley, Puebla. Damaged individuals were photographed.
Results
State of knowledge of damage in Cactaceae. Literature referent to damage in cacti comes from the late 1970’s. Despite the existence of about 1,500 species of cacti (Anderson 2001), only 58 species have been analyzed in aspects related to damage. They correspond to 29 out of about 100 recognized genera; that is, most include one or two species per each genus studied. Different kinds of damage are reported in cacti distributed throughout the Americas, from the northern part of the United States to Chile, as well as in the Caribbean region. Damaged cacti studied differ in terms of the kind of damage (biotic or abiotic), the species causing the damage in the former case, and its taxonomic distribution, inside each subfamily and tribe.
Subfamily Cactoideae. Studies are focused on columnar species of the tribe Echinocereeae in Mexico and North America, and species belonging to tribes Cereeae, Trichocereeae, and Browningieae, most of them columnar cacti of South America.
Regarding the tribe Echinocereeae, studies are focused on six genera distributed in the deserts of Sonora, and Baja California, in the semiarid region of Central Mexico, particularly in the Tehuacán Valley (Puebla), Mixteca Baja (Oaxaca, Puebla and Guerrero), and Cuba. The genera analyzed were: Carnegiea, Dendrocereus, Myrtillocactus, Cephalocereus (= Neobuxbaumia), Pachycereus and Stenocereus. Damage by herbivores is caused by larvae and adults of different insects: Scyphophorus, Cactophagus, Nasutitermes, Neotermes, and Hymenoptera (Anderson 1948, Vila-Marín et al. 2004, Villalobos et al. 2007, Maya et al. 2011, Bravo-Avilez et al. 2014). Damage is caused by ants of the Atta genus (Pimienta et al. 1999) that trim the apical zone and flowers, by the foraging of branches by mammals (e.g., goats, mouse), or by birds such as Melanerpes and Colaptes (Villalobos et al. 2007, Danzer & Drezner 2014). "Fish eye" and "gray crust" diseases are caused by yeasts like Fusarium, Cladosporium, Colletotrichum, Phoma, and Molinia, in association with some Isoptera (Vila-Marín et al. 2004, Monreal-Vargas et al. 2014). Damage by abiotic factors, commonly superficial, is usually expressed as a dark surface on the epidermis, and is caused by UV-B radiation, freezing of branches, and cutlass used by peasants, among other factors (Nobel 1980, Evans et al. 1992, Holguin et al. 1993, Bashan et al. 1995, Evans et al. 2001, Flores & Yeaton 2003, Evans 2005, Villalobos et al. 2007). In some cases, the origin of damage is unknown (Flores & Yeaton 2003, Evans 2005).
For the tribe Cereeae, five genera were analyzed, mostly columnar species of Cereus, Cipocereus, Pilosocereus, Praecereus, and the genus Melocactus, a globose cactus.
Damage by herbivores is caused mainly by insects like Hypogeococcus festerianus (mealybug), Cactophagus spinolae, and different species of Cerambycidae (Vaurie 1967, Pérez Sandi y Cuen et al. 2006, Abreu et al. 2012). Some nematodes, like Meloidogyne incognita, and bacteria like Erwinia (Ortega & Fernández 1989), are also reported. Few cacti exhibit superficial damage by herbivores (Evans & Macri 2008).
The tribe Trichocereeae has been analyzed on four genera of columnar cacti: Cleistocactus, Harrisia, Echinopsis and Trichocereus, and on the globose Gymnocalycium. Damage by biotic factors is caused by the cactus borer Moneilema sp., the nematode Meloidogyne incognita, and the bacteria Erwinia sp., and Hypogeococcus festerianus (Ortega & Fernández 1989, Pérez Sandi y Cuen et al. 2006), as well as cattle (Peco et al. 2011, Malo et al. 2011). There are also reports of damage by the parasitic plant Tristerix aphyllus (Silva & Martínez del Río 1996, Medel et al. 2010). Also, superficial lesions on branches by accumulation of epicuticular waxes has been reported for Echinopsis, as a consequence of different kinds of biotic damage. Damage by abiotic factors, such as human tools, is significant because they cut branches for handicrafts such as the "rain stick", which is made from wood (Evans et al. 1994, Montenegro et al. 1999, Ginocchio-Cea & Montenegro-Rizzardini 2000).
Only four genera of the Cacteae tribe distributed in Mexico have been assessed: Astrophytum, Mammillaria, Ferocactus and Echinocactus, all globose taxa (Appendix 1). Damage caused by biotic factors correspond to different genera of Cerambicidae, Cactophagus and Narnia, mammalian herbivores like squirrels (Spermophilus mexicanus), rodents (Mus sp.), rabbits (Sylvilagus sp.), and donkeys (Equus asinus) (Vaurie 1967, Blom & Clarck 1980, Martínez-Ávalos et al. 2007, Jiménez-Sierra & Eguiarte 2010). Other kinds of damage by biotic factors include fungi (Phytophthora infestans), bacteria (Erwinia sp.), and nematodes (Meloidogyne incognita) (Ortega & Fernández 1989, Martínez-Ávalos et al. 2007). Damage resulting from chewing, necrotic flesh, and apex destruction is also reported, but causes are unknown (McIntosh et al. 2011).
Tribe Browningieae has been assessed from three genera: Armatocereus, Neoraimondia, and Jasminocereus in some countries of South America, where herbivory by Hymenoptera (Camponotus sp.) has been reported by (Novoa et al. 2005), besides abiotic damage in branches by solar radiation (Evans & Macri 2008).
Two genus of the tribe Hylocereeae has been studied for southern Mexico and Brazil, Selenicereus (= Hylocereus) and Acanthocereus. Herbivory by insects (larvae and adults) of Cactophagus, Ozamia, Narnia, Euphoria, nematodes like Helicotylenchus, Meloidogyne, Dorylaimus, Tylenchus, Aphelenchus and Pratylenchus, and unidentified bacteria that promote soft rot in stems has been reported (Valencia-Botín et al. 2003, Ramírez-Delgadillo 2011, Ramírez-Delgadillo et al. 2011, Guzmán-Piedrahita et al. 2012, López-Martínez et al. 2016). There is a report damage by the nematodes: Meloidogyne, Helicotylenchus, Tylenchorhynchus, Trichodorus, and Hemicycliophora (Rincon et al. 1989).
Tribe Notocacteae has been analyzed only from the genus Eulychnia, distributed in South America. Damage is produced solely by the parasitic plant Tristerix aphyllus (Medel et al. 2010).
Finally, we did not find any records of damage for the tribes Calymmantheae and Rhipsalideae.
Subfamily Opuntioideae. Damage in branches, and flowers has been reported in different species of Opuntia (tribe Opuntieae), which has been widely studied because its economic importance in México, and South America: Opuntia ficus-indica, O. ondulata, O. cochenillifera, O. humifusa, O. stricta, O. macrocentra, and Opuntia spp. Damage is caused by herbivorous insects such as Cactophagus, Dactylopius, Metamasius, Cylindrocopturus, Cactoblastis, Platynota, Hypogeococcus as well as mammals such as rabbits, and hares (Vaurie 1967, Hoffman et al. 1993, Zimmermann et al. 2005, Rodríguez-Fuentes et al. 2009, da Silva et al. 2010, Zimmermann & Pérez Sandi y Cuen 2010, Falcão et al. 2012, Jezorek & Stilling 2012, Bautista-Martínez et al. 2014), damage due to abiotic factors, is caused mainly by frost in others Opuntia (Bobich et al. 2014).
Damage in branches by the herbivore Cactophagus spinolae (Vaurie 1967) has also been reported in the tribe Cylindropuntieae, on different species (Cylindropuntia spp.); damage due to abiotic factors, is caused mainly by frost in C. ganderi (Bobich et al. 2014).
Subfamily Pereskioideae. Reports of damage in this subfamily are scarce. Studies have been made only for Pereskia aculeata. Various insects from different orders cause herbivore damage: Catorhintha schaffneri, Acanthodoxus machacalis, Maracayia chlorisalis, Cryptorhynchus sp., and Asphondylia sp. (Paterson et al. 2014).
Defense mechanisms in Cactaceae. Growth and branching patterns in columnar cacti determine their adult form. Growth is related with the prevailing type of dominance (apical or lateral), the increase in photosynthetic surface area, as well as the capacity of water storage. Particularly in columnar cacti, growth under natural conditions may be associated with the branching pattern of each species. Few species are monopodic with apex dominance bearing only one stem without branching: Cephalocereus columna-trajani and C. mezcalaensis (Zavala-Hurtado & Díaz-Solís 1995). Others produce a branched stem with presence of apical and lateral dominance: Carnegiea gigantea, Cephalocereus tetetzo, and C. macrocephalus. A third group grows highly branched, so lateral dominance contributes more to growth through the production of new branches (most of branched columnar cacti, such as: Stenocereus spp., Lophocereus schottii, Myrtillocactus, Escontria, Pachycereus weberi, Isolatocereus dumortieri, and others).
Growth and branching patterns can be modified under stress conditions by wind, frost, human damage by tools, or herbivores, depending on the architecture model of each species. The implications of natural damage have been observed mainly in changes in branching, growth rate and reproduction, according to the height of the plant before and after the damage occurs in the giant Cephalocereus columna-trajani (Zavala-Hurtado & Díaz-Solís 1995). However, this study did not address the analysis of defense mechanisms.
Defense mechanisms in cacti have been poorly analyzed. In the present review, few studies with this scope were found. Of the two mechanisms described in literature, resistance and tolerance, the former has been analyzed by physical structures, such as thorns in Echinopsis chiloensis (Medel 2001), and cuticle in some species of Opuntia (da Silva et al. 2010, Falcão et al. 2012). They demonstrated that thorns confer resistance against the parasitic plant Tristerix aphyllus, and cuticle against various insect herbivores. In the case of tolerance, the only study that involves the analysis of this mechanism and confirmed the existence of a compensatory response after damage, measured as an increase in branching, is that of Medel (2001), with E. chiloensis.
Cluster analysis between hosts and kinds of damage. The cluster analysis grouped cacti species in four groups, according to the kind of damage reported in literature (Figure 1): Group I: the most numerous, included climbing or epiphytic, as well as globose cacti, belonging to different genera, various species of Opuntia, and some columnar cacti. This group is characterized by the presence of only biotic damage produced by small Mammals (squirrels, rabbits and mice) and invertebrates, insects of diverse families: Cerambycidae, Curculionidae, Formicidae, Pseudococcidae, also, nematodes and a parasitic plant Tristerix aphyllus. A subgroup I-B (Figure 1) was formed with species damaged mainly by C. espinolae, and other insects; Group II, includes species of the tribe Echinocereeae (Stenocereus from Mexico and Colombia: S. pruinosus, S. stellatus, and S. griseus), and a globose from Mexico, belonging to the tribe Cacteae (Echinocactus platyacanthus), all of them subject to human management; and two South American species from tribe Trichocereeae (Trichocereus terscheckii and Echinopsis leucantha). All of them exhibit similar kinds of biotic damage due to foraging of domesticated mammals (donkeys, cows, goats), and birds, as well as abiotic damage, caused by human tools; Group III, corresponds to columnar and arborescent species from northern Mexico and South America, subject to damage by abiotic factors, including wind, extreme heath, and frost. These species belong to the tribes Echinocereeae, Cereeae, Trichocereeae, Browningieae, Opuntieae and Cylindropuntieae. Finally, the group IV included species of the genus Selenicereus distributed in Central Mexico, where Cactophagus spinolae, along with Coleoptera, Lepidoptera, as well as unidentified bacteria, are the main herbivores that cause damage.
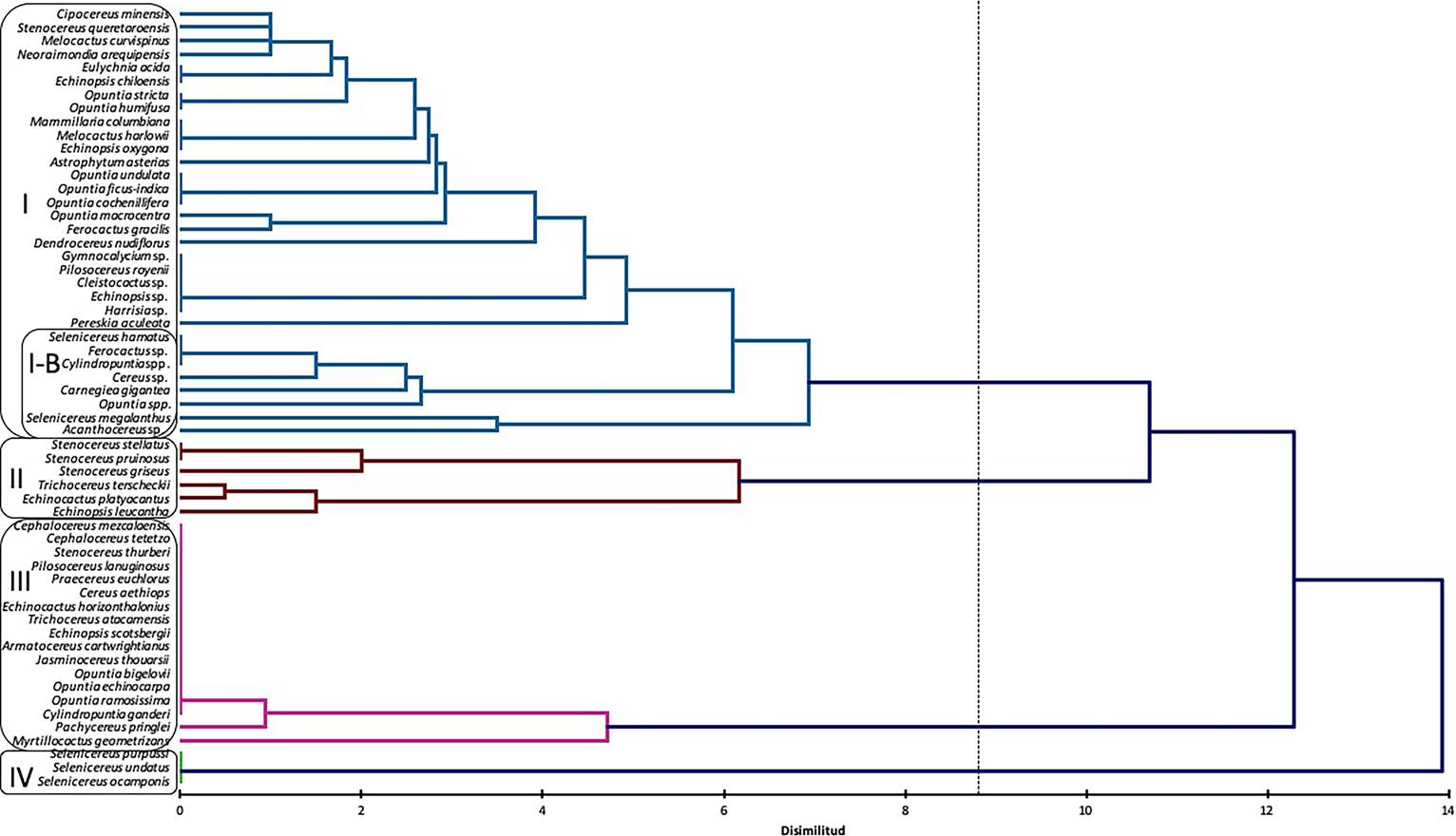
Figure 1 Dendrogram based on 58 taxa of cacti with presence of damage. Four groups were defined, based on kind of damage.
Geographic distribution of damage among Cactaceae. There is a clear geographic distribution pattern of the recognized kinds of damage among Cactaceae in the Americas (Figure 2). Most of the damages are distributed in desert areas of North latitude. Nevertheless, within this region, there is a subregional distribution of some kinds of biotic damage. Cactophagus spinolae (Coleoptera) are distributed along Mexico and southern United States. Hypogeococcus festerianus (Hemiptera) and Cactoblastis cactorum (Lepidoptera), are more frequent in the Caribbean region and southern United States. Damage by small mammals is frequent in globose cacti of Northern Mexico, Different species of Melanerpes birds are distributed in Northern and Central Mexico, as well as in Colombia. The nematode Meloidogyne is frequent in Cactaceae distributed in tropical areas of Caribbean region and Ecuador. In the case of South latitude, few agents causing damage have been reported. Cerambycidae (Coleoptera) has an interesting pattern because is reported in Northern Mexico and South America (Brazil); damage by the parasitic plant T. aphyllus, is only reported in Cactaceae from Chile. Damage by large mammals, mainly cattle (donkey, cow, goat), is reported mainly in managed populations of different cacti of Northern México, and Central and South America. These biotic damages, as well as abiotic damage caused by humans, is generalized in the Americas. Although damage due to abiotic factors is common throughout the continent, it is more frequent in extreme latitudes, where extreme weather, as well as wind and radiation have direct effects on cacti.
Cacti species under human management and presence of damage. Some columnar cacti reported with presence of damage are subject to different forms of human management: M. geometrizans (edible fruits called “garambullos”), Cephalocereus spp. (edible buds called “tetechas”), Stenocereus spp. (edible fruits called “pitayas”), E. platyacanthus, (edible stem called “acitrón”), Ferocactus sp., (edible fruits), Selenicereus spp., (edible fruits called “pitahayas”), and Opuntia spp., (edible cladodes, called “nopales”, and edible fruits called “tunas”). These species show different kinds of biotic damage, one of them caused by Cactophagus spinolae, which has been reported more recently in cultivated species, and can be related with some kind of disturbance. Damage by goats, cows, and donkeys, is also frequent in this group of cacti. Finally, damage by birds (Melanerpes sp.) that nest in the branches, is very common in managed plants, especially in species of Stenocereus, although rot effect associated with this damage has not been reported.
Field evidence of damage in Cactaceae of Central Mexico. Different kinds of damage have been observed in columnar cacti in Central Mexico. Among them, the most worrying is rotting of apparently healthy branches, probably related to the herbivory of larvae of C. spinolae, because it causes the death of the branches, or even of the whole plant (Ramírez-Delgadillo et al. 2011, Maya et al. 2011, Bravo-Avilez et al. 2014). It has been observed in the States of Puebla and Oaxaca: Tehuacán Valley (Santiago Miahuatlán, Ajalpan, Zapotitlán Salinas, and Coxcatlán); the Mixteca Baja Poblana (Acatlán de Osorio, and Xayacatlán de Bravo); and the Mixteca Baja Oaxaqueña (Cosoltepec, and San Pedro and San Pablo Tequixtepec), (Figure 3). The damage is expressed as a rot on the branches (or the main stem on the unbranched species) of the standing plants. This damage is probably caused by bacteria, fungi and viruses, which inhabit in the mouthpieces of the adults of C. spinolae and are inoculated when Coleoptera forage these plants and lay eggs (Solís-Aguilar et al. 2001). Also, these microorganisms enter by themselves when stems are damaged. Then, larvae of C. spinolae, continue feeding into the stems. This pattern has been reported in the association Scyphophorus acupunctactus - Agave (Solís-Aguilar et al. 2001).
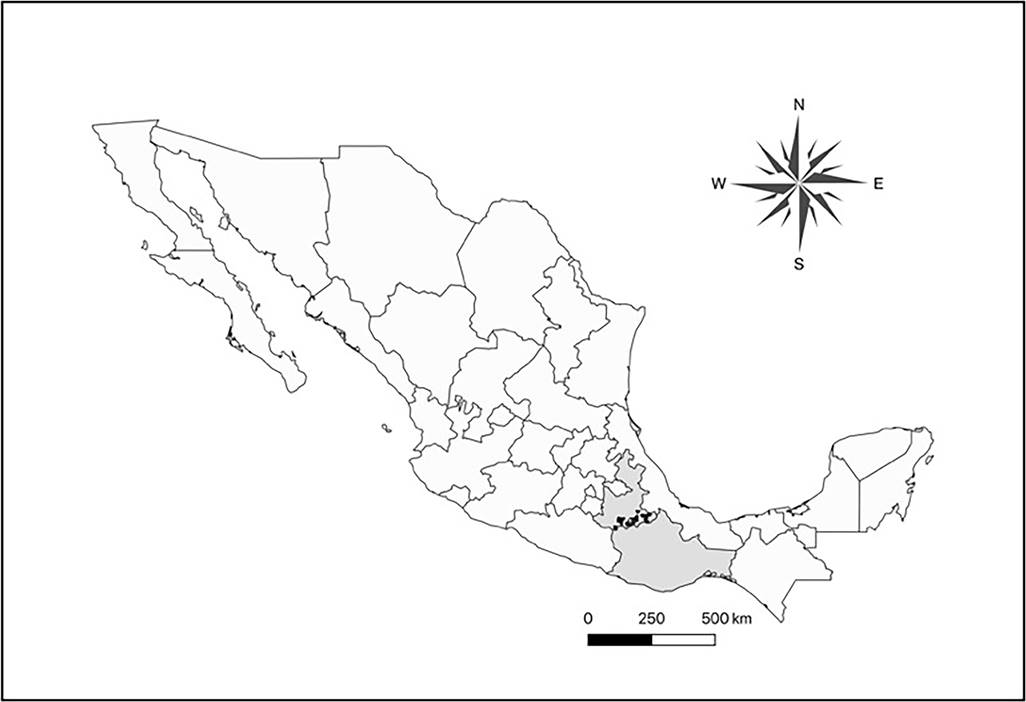
Figure 3 Municipalities of Oaxaca and Puebla states, where the 14 species of columnar cacti with presence of damage by rotting in the center of Mexico were observed (in black).
In the early stages of rotting, there is a brown spot on the surface tissue (cuticle and parenchyma); later, a brownish viscous liquid is produced inside. Days later, the branch deforms, swells and emits an unpleasant odor; in some cases, the viscous liquid runs through the branches. In advanced stages, the branch falls down because it is weak, but the whole plant recovers after the damage, and new branches and buds are produced. In some cases, the rot spreads to other branches of the same plant, including the main stem, due to the movement of the larvae, killing the plant; so, species with monopodic architecture, such as C. mezcalaensis, are killed. Rotting seems to be contagious, because individuals growing near the damage one, also exhibit the presence of damage (Bravo-Avilez, field obs.). This type of damage causes serious problems in populations of wild species, but also in populations of cultivated species that have been used and managed by humans since prehispanic times (Smith 1967, Casas 2002, Luna-Morales 2004), that currently have economic importance in this region. Managed cacti that exhibit rot damage include: "pitayas”, Stenocereus pruinosus (Otto ex Pfeiff.) Buxb., and S. stellatus (Pfeiff.) Riccob; "jiotilla", Escontria chiotilla (F.A.C. Weber) Rose; wild species which are gathered by their edible reproductive structures (fruits or flowers): Myrtillocactus geometrizans (Mart. Ex Pfeiff.) Console, Cephalocereus tetetzo (F.A.C. Weber ex J.M. Coult.) Diguet, C. mezcalaensis Bravo, Pachycereus weberi (J.M. Coult.) Backeb., Pilosocereus chrysacanthus (F.A.C. Weber ex Schum.) Byles & G.D. Rowley; wild species with diverse uses: Marginatocereus marginatus (DC.) Backeb., Lemaireocereus hollianus (F.A.C. Weber) Britton & Rose, Isolatocereus dumortieri (Scheidw.) Backeb., Pachycereus grandis Rose (Figure 4); and wild species with no human uses: Cephalocereus columna-trajani (Karw. ex Pfeiff.) K. Schum. and C. macrocephalus F.A.C. Weber ex K. Schum.
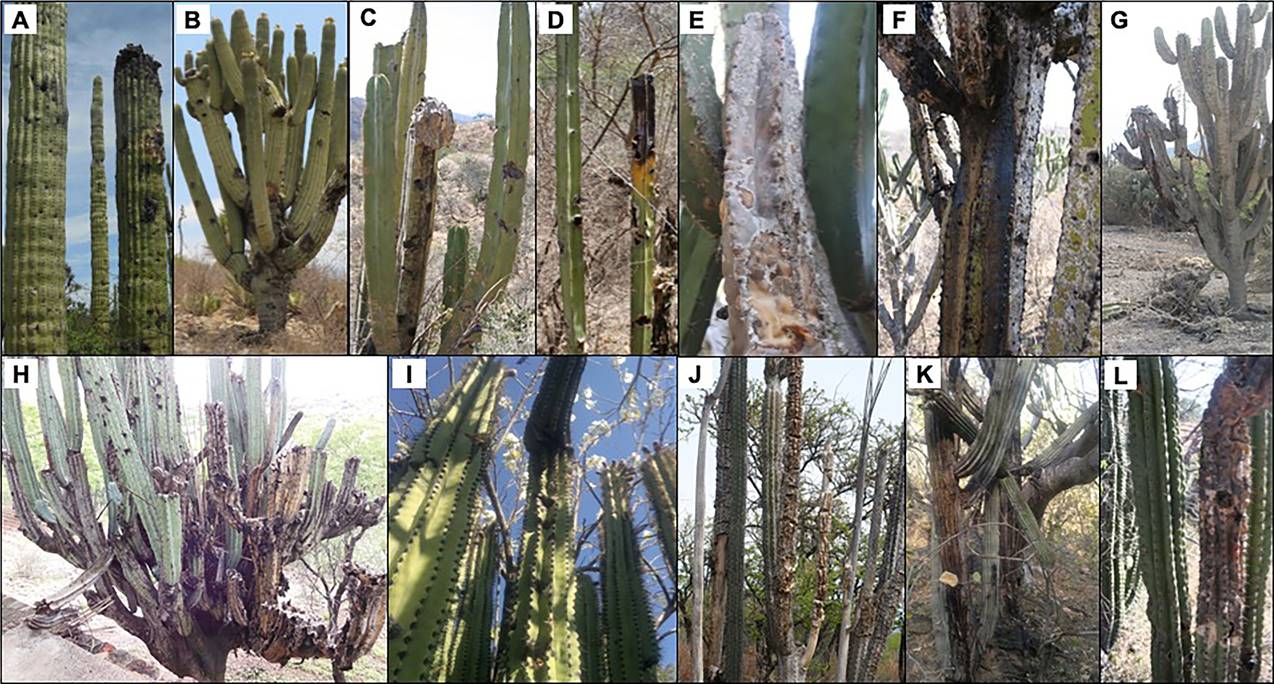
Figure 4 Species with rot damage observed in Central Mexico. A. Cephalocereus mezcalaensis, recovering from damage; B. Cephalocereus tetetzo, with several damaged branches; C. Isolatocereus dumortieri, with several damaged branches; D. Marginatocereus marginatus, main branch with damage; E. Stenocereus pruinosus, fallen branch by rot; F. Escontria chiotilla, it shows the brown liquid, which drains from the rot; G. Myrtillocactus geometrizans, individual with branches damaged by rotting, the liquid that runs off is observed; H. Pachycereus weberi, individual with many lost branches, due the damage; I. Pachycereus grandis, a branch near the area damaged; J. Lemaireocereus hollianus, severely damaged individuals, K. Pilosocereus chrysacanthus, with liquid draining from damage; L. Stenocereus stellatus, individual with severely damaged branch.
Discussion
Studies that quantify, analyze and even describe damage in cacti are scarce and are concentrated in certain taxa; even fewer are those that analyze mechanisms of defense and the effect on fitness in this group of plants. Literature focuses on studies of damage by herbivory in leafy annual plants, and little attention has been focused on perennials (Rasmann et al. 2011). However, for cacti there is a long list of organisms associated with damage: mammals, birds, insects, nematodes, bacteria, yeasts, parasitic plants and several abiotic factors. Nevertheless, most aspects of which defense mechanisms intervene and how they are activated in this group of plants are unknown.
A clear separation in four groups of cacti where obtained, based on kind of damage: abiotic and biotic, and inside this by the kind of organisms recorded. This means that some abiotic factors are promoting that some animals, bacteria, and nematodes, modify their foraging and dispersion patterns, becoming herbivores or pests of wild cacti. It is evident that human activities (directly or indirectly promoted) have induced these changes. Direct effects are evident for group II, for example, where human and domesticated mammals produce the most important damage, which is linked to domesticated cacti.
Also, group III indicate that abiotic factors have increased their effects in populations of many cacti species in the southern latitudes. Recent evidence suggests that a latitudinal pattern could be related with abundance and richness of herbivore species, and generalist herbivores are more common in lower latitudes (Salazar & Marquis 2012). The present review does not exhibit this pattern. Most of the damage reported due to biotic factors is located in latitude north and near Equator. A possible explanation is that research studies have been developed in this region, which is supported by the number of papers published. Even when the desert region of South America is also considered an area of diversification of Cactaceae, few studies with this scope have been developed.
Damage due to biotic factors was characterized by the presence of many herbivores, which can become aggressive pests, like Hypogeococcus festerianus which has been reported feeding on many ornamental cacti, and it is possible that it turns into an aggressive pest through the Americas (Zimmermann & Pérez Sandi y Cuen 2010). Cactoblastis cactorum "palomilla del nopal", is another species with potential effect as a destructive pest. This hemipteran, used as a biological control in Africa against some species of Opuntia, became a dangerous pest of wild cacti in the Caribbean region (Zimmermann et al. 2005); at the present, it has also been reported as a pest of different wild and cultivated Opuntia species in the southeastern United States, and Mexico (Zimmermann et al. 2005). Cactophagus spinolae, which had been originally reported only in Opuntia species in the central region of Mexico, now appears in new host plants (Ramírez-Delgadillo et al. 2011, Bravo-Avilez et al. 2014, López-Martínez et al. 2016) and its populations could increase by changes in environmental conditions due to human disturbance (habitat loss and conversion to intensive agriculture and urbanization, use of agrochemicals, deforestation) in the same region, as it has been annotated by Sánchez-Bayo & Wyckhuys (2019). It can be expected that this herbivore would be present in the rest of the columnar cacti that exhibit the same type of damage by rotting.
The damage by rot, in some cases is documented as a secondary damage, which occurs after primary damage by different herbivores (Vaurie 1967, Vila-Marin et al. 2004, Maya et al. 2011), but in other cases, only rot damage is reported without clarifying whether it is a primary or secondary damage (Valencia-Botín et al. 2003).
We consider that it is difficult for rot damage to occur without apparent causes. Authors like Evans, in the 1960s conclude that the cacti had bacterial rot. Subsequent researches found bacteria to be secondary invaders, and not the primary cause of damage (pers. com., anonymous reviewer). In the present review many species present damage such as the surface lesions on branch, epidermal browning and barking (Evans et al. 1992, 1994, 2001, 2005, Ginocchio-Cea & Montenegro-Rizzardini 2000, Evans & Macri 2008). In some cases, the cause is unknown, in others it is attributed to UV radiation. However, it seems that after this damage, the plant is more susceptible to the entry of herbivores, or directly to pathogens that cause rot. In the case of our evidence, we have found similar damage in the Stenocereus species (Bravo-Avilez 2017). We do not rule out the possibility that excess solar radiation causes bark damage making these species susceptible to invasion by herbivores and pathogens that cause their rot. This damage is becoming a major concern and needs special attention because it shows that the columnar cacti seem to be the main "target" of agents that cause it. As it was described before, if it extends through the branches, could kill the whole individual. Furthermore, tissue decay can be propagated to other individuals via biological vectors, affecting local plant populations.
An interesting damage is that caused by insectivorous bird species belonging to the genus Melanerpes (Ramírez-Albores & Ramírez-Cedillo 2002). Although they cause "damage" to the columnar cacti when they construct their nests, it is evident that they do cause rot in the branches; even when the holes are deep, branches still have the photosynthetic capacity to survive, and even to produce reproductive structures. This suggests a commensal relationship between these birds and cacti. It would be important to elucidate the role of these birds as natural predators of the larvae of C. spinolae, and Lepidoptera insects, that cause damage to the columnar cacti, because citizen science data suggests that birds eat these insects. It would be interesting to deepen insights on these interactions.
Studies focused on damage in columnar cacti are mostly descriptive, despite the nutritional, economic and cultural importance of cacti, and the implications of being damaged. It is important to highlight the fact that there are no ecological studies that compare the variation in damage in populations distributed in different geographic areas, or even between populations subject to different forms of management (wild, tolerated, cultivated). Recent field evidence indicates differences between populations damaged by C. spinolae, located in the Tehuacán Valley and the Mixteca Baja (belonging to the Mexican states of Puebla and Oaxaca), as well as populations subject to different forms of management (Bravo-Avilez et al. 2014, Bravo-Avilez 2017).
Models developed to explain the evolution, and ecology of defense mechanisms in plants are based on comparisons between wild species, and there are not studies that put into perspective the effect of management within a single species (Endara & Coley 2011). Likewise, the few studies that exist in domesticated plants have been focused on the study of worldwide commercially important species, most of them annual plants (cotton, soybean, corn) and have addressed the problem from a molecular perspective (All et al. 1989, Brooks et al. 2007). Comparisons are made between domesticated species and wild relatives, but not in the context of the complex dynamics of the forms of actual management, in particular under traditional management (Chaudhary 2013 and citations within).
Damage caused by human tools and domesticated mammals is widely distributed in the Americas, and it is possible that a synergic effect with other kinds of damage is happening. So, it is necessary to understand these interactions in order to delimitate with more accuracy natural areas with less affectation of domesticated mammals, and of human activity.
There is evidence of rot damage in 14 species of columnar cacti from field observations. Of them, eight species have not been reported previously in the literature and all have different degrees of endemism. Some are widely distributed only in Mexico as Stenocereus pruinosus, Marginatocereus marginatus, Myrtillocactus geometrizans, and Isolatocereus dumortieri. Others are endemic to the central region of Mexico, in the states of Puebla, Guerrero and Oaxaca: Stenocereus stellatus, Pachycereus grandis, Escontria chiotilla, Pachycereus weberi and Pilosocereus chrysacanthus. Finally, there are species with a restricted range of distribution, like Lemaireocereus hollianus, Cephalocereus columna-trajani, C. tetetzo, and C. macrocephalus, which only grow in the Tehuacán-Cuicatlán Valley, in Puebla and Oaxaca states (Esparza-Olguín et al. 2005), and C. macrocephalus, which habitat is restricted to the Tehuacán Valley, Puebla (Bravo-Hollis & Sanchez-Mejorada 1991, Esparza-Olguin et al. 2002). Pachycereus weberi, and P. grandis are considered species with “decreasing populations”, and the last one is also classified as vulnerable species in the IUCN Red List of Threatened Species (IUCN 2019). The other 12 species are cataloged as least concern species. The status of conservation of many columnar cacti adds to the necessity to identify biotic and abiotic factors that cause damage, in order to take appropriate measures to enhance the protection of this group of plants in Central Mexico.
Future directions. Key research can be developed in the next years: The dynamics of dispersion of rot damage where various types of organisms such as bacteria, yeast, flies and beetles, among others, are involved and take advantage of changes in the conditions in the plant tissue from the production of a wound for their establishment. Also, the sequential scheme of spread between branches within and between individuals, which can affect entire populations, could be addressed from an epidemiological approach seeking to create predictive models that reveal the dynamics of spread and eventual damage control. These studies need to incorporate socioeconomic components such as agriculture, livestock, and even the same urbanization processes, that might be favoring population growth of some of these herbivores, fungi, viruses and bacteria involved in damage.
Ecological interactions among different organisms that are part of the damage process in cacti must be understood. At the present, most of the papers analyze damage in a descriptive way: vectors, herbivores, and parasites are described separately. It is necessary to deep on dynamic interactions of different actors from a community level, in order to understand their role, and to assess the consequences of decreasing or increasing their abundances. This is necessary because several herbivores tend to become generalists and look for new hosts. Thus, an approach from a community level would help to understand which factors (biotic, or abiotic, e.g., climate change, desertification, aridization, changes in land use) could be responsible of the movement and enlargement of niches and borders of herbivores that could be considered a pest. This approach also would help to find a real control of potential pests, without the introduction of foreign organisms (biological control), chemical control (pesticides), or destructive methods (removal of damaged hosts) (Dobson & Crawley 1994).
Furthermore, it is necessary to develop studies to estimate the change in the defense mechanisms in populations with different forms of management due to active domestication processes, in a similar way as morphological, genetic, and ecological traits (Casas et al. 1997, Casas et al. 1999, Rojas-Aréchiga et al. 2001, Guillén et al. 2009), and trade-offs between defense mechanisms, fitness, and domestication have been analyzed. Finally, from an applied perspective, studies involved in the agronomic assortment of selected resistant phenotypes and/or tolerant individuals to organisms that produce damage, particularly in cacti species of economic value, as already have been developed in Opuntia (Falcão et al. 2012) must be carried on.











 text new page (beta)
text new page (beta)




