Plant pollination systems form a continuous gradient from highly specialist systems with a sole pollinator to generalist systems with hundreds of pollinator species (Johnson & Steiner 2000). Two of the main factors that promote specialist pollination systems are: 1) asymmetric efficiency in the fecundity of plant species attributed to the different pollinator guilds (Aigner 2001) and 2) pollinator predictability (i.e., low temporal variation and low spatial fluctuation of the most efficient pollinator). On the contrary, a similar efficiency among species and a high unpredictability among pollinator guilds lead to generalist pollination systems (Waser et al. 1996, Johnson & Steiner 2000, Gómez 2002).
Valiente-Banuet et al. (1996, 1997, 2004) suggested that there exists a geographic pollination system pattern in columnar cacti: nectarivorous bat specialized systems prevail closer to the tropics, while generalist systems with a vast variety of pollinators (including birds, bats, and insects) prevail outside the tropics. Such a pattern has been demonstrated on a noteworthy number of studies (see Munguía-Rosas et al. (2009), meta-analysis). This geographic pattern in columnar cactus pollination comes from the predictability of bats on the Leptonycteris Lydekker genus, which are distinctly migratory in the extra tropical deserts and resident throughout the year in central Mexico (between 18° and 20° North latitude according to Valiente-Banuet et al. 1996, Rojas-Martinez et al. 1999, Morales-Garza et al. 2007). Molina-Freaner & Eguiarte (2003) suggested that the same mechanisms that promote specialization and generalization in columnar cacti pollination could be influencing the majority of Agave L. species, because of their overlap in distribution with columnar cacti and the chiropterophily pollination syndrome of their flowers (Gentry 1982); but Munguía-Rosas et al. (2009) did not find this pattern.
Agave horrida Lem. ex Jacobi (subgenus Littaea Tagl; group Marginatae), just like other species from the same genus, is a semelparous plant, with hermaphrodite, protandric flowers whose anthesis lasts several days. Nectar production, anther dehiscence, and stigma receptivity occur at night (A Flores pers. obs.) suggesting that the species is pollinated by nectarivorous bats (Howell 1979, 1981, Schaffer 1977). We studied the pollination of A. horrida to test this hypothesis. For this purpose, we evaluated the floral biology of A. horrida, as well as the composition, frequency and effectiveness of its flower visitors in the seed-set and fruit-set, expecting to find a higher fecundity efficiency due to bat pollinators than to any other guild.
Materials and methods
Study site and species. This study was conducted by the end of February 2005, Northwest of Tepoztlán, on the South slope of the Chichinautzin mountain range, geographically localized at 19° 00’ 59” N and 99° 08’ 55” W, elevation 2,250 m (Figure 1). Climate is temperate, sub-humid with summer rains; mean annual temperature is 14.35 ºC and mean annual precipitation of 1,452 mm. Vegetation is a xerophytic shrubland of Hechtia podantha Mez, Agave horrida, and Yucca L. sp. (Velazquez et al. 2010).
Agave horrida belongs to the subgenus Littaea (group Marginatae), which groups agave species with spike-like inflorescences. It is a solitary, small, compact rosette that only grows on stony landscapes of volcanic origin on mountains of Cuernavaca Morelos, Mexico. See distribution maps and pictures of the species in Gentry (1982) pp. 144-145.
Plant abundance and floral biology. We counted the number of vegetative (without a floral spike) and reproductive (with a floral spike) rosettes in three 500 m2 quadrants in the study site. We also counted the number of open flowers per night on each blooming flower spike. Since Agave horrida is an abundant species in the study site, all values were extrapolated to 1 hectare for better understanding of the text and to allow comparisons with other studies.
To characterize protandry, we selected twelve flowers of Agave horrida (3 flowers from each of 4 different rosettes) and bagged them prior to opening with a nylon mesh netting. The flowers remained bagged at all times except when measures were taken. Starting when the corolla opened (21 h, night zero), we extracted the nectar every 3 h from the same flowers using a graduated one-milliliter syringe. Sugar concentration in nectar was measured with a hand-held refractometer with automatic temperature compensation (American Optical No. 9103). To determine the stigmatic receptivity, style length (from internal base of corolla to the top of the stigma) was measured using a manual caliper every 6 h during the anthesis; the stigmatic opening was also recorded. In addition, we recorded the anthers opening time and pollen release time.
Floral visitors. Early visitation rate (at dusk and dawn, when flower visitors are most active) was calculated from direct observations to an Agave horrida inflorescence five continuous day from 0600 to 0700 h and from 2000 h to 2100 h. Birds visitors were observed from a distance of 10-15 m using binoculars, while bats visitors were observed from the inflorescence base; we counted a “visit” when a visitor touched the inflorescence.
To identify birds and bats visitors, three mist nets (12 m long × 3 m in height or 108 m2 total, Avinet, Dryden, New York, USA) were placed in an area densely covered by Agave horrida individuals, close to their inflorescences. The nets were opened at sunset (2000 h) and closed at noon (1200 h) for five nights/days from February 26 to March 2, 2005 (240 h/netting, 150 nocturnal and 90 diurnal); nets were examined every 30 min.
From each animal caught, the pollen on their body was collected by rubbing a cube of fuchsin-stained jelly over the head (Beattie 1971). The cube was then placed on a microscope slide, melted, and covered with a cover slip for later inspection under the microscope. Pollen presence was considered to be proof of flower visitation. Pollen grains from animal samples were later compared to those obtained from flowers of Agave horrida.
We calculated the relative abundance of effective bird and bat visitors by dividing the total captures of each guild with pollen by the open net hours for that guild. Captured and observed birds were identified using field guide (Howell & Webb 1995) and bats using field guide (Medellín et al. 1997).
Pollination experiments. To determine the type of breeding system and the importance of bird and bat visitors on plant fecundity, we conducted a pollination experiment selecting a total of 553 flowers from 7 different plants of Agave horrida. Different numbers of flowers per plant were marked and emasculated before anther dehiscence; pollinators were excluded by placing a nylon mesh bag around the inflorescence. These flowers were then assigned to one of the following treatments:
Manual cross-pollination (exogamy, n = 113 flowers in 3 plants). Flowers were excluded from pollinators before and after treatment by bagging them with a nylon mesh net. Once the stigmas were receptive, they were pollinated by saturating the stigma with fresh pollen from the recently opened anthers from four different plants. To guarantee maximum pollen transfer, this pollination treatment was conducted before, during and after the night of maximum receptivity.
Manual self-pollination (autogamy, n = 30 flowers in 3 plants). Flowers in this treatment were pollinated by saturating the stigma with fresh pollen from recently opened anthers of other flowers on the same plant, following the same procedure as described before.
Control (n = 85 flowers from 6 plants). Flowers were simply tagged and exposed to natural pollination.
Nocturnal pollination (n = 115 flowers in 4 plants). Flowers were only exposed to nocturnal visitors and excluded from diurnal visitors by bagging them before sunrise, from 0600 h to 2000 h, and removing the bag from 2000 h to 0600 h daily, until the styles wilted.
Diurnal pollination (n = 120 flowers in 4 plants). Flowers were only exposed to diurnal visitors and excluded from nocturnal visitors by bagging them at sunset, from 2000 to 0600 h, and removing the bag at sunrise from 0600 to 2000 h daily, until the styles wilted.
Insect pollination (n = 90 flowers in 3 plants). Flowers in this treatment were isolated from vertebrates with a wire poultry netting (3 cm opening) which allowed entry of only insects (Tegeticula Zeller spp. moths and Apis mellifera L., bees) that were observed visiting flowers of Agave horrida.
The fate of the tagged flowers from each pollination treatment (aborted or developing fruit) was recorded. Fruits were monitored every month and collected when mature. Seeds were quantified directly from the collected fruits and compared with the average number of ovules in order to calculate seed-set.
Statistical analysis. Student’s t-test was used to analyze flower visitation rate variation and relative visitor abundance by guild (diurnal and nocturnal). Our response variables were the success/failure of marked flowers to become fruits, and success/failure of ovules to become seeds (based on an average number of ovules per flower of 320.70 ± 6.3 SE, n = 17 flowers). A logistic regression with a quasi-binomial error (to avoid over dispersion and logit like function) was used to analyze these response variables in function of the pollination treatments (Crawley 2007).
In case of finding statistically significant differences between pollination treatments, a comparison of means was performed using Fisher’s LSD test. The insect-pollination treatment was eliminated from the analysis because no fruits were produced. All statistical analyses were performed using InfoStat v. 2013 statistical package (Di Rienzo et al. 2013).
Results
Plant abundance and floral biology. We found 3,066.60 ± 393.20 vegetative rosettes (mean ± 1 SE) and 253.40 ± 17.6 reproductive rosettes in 1 ha. In the study site, A. horrida offers 55,447.80 ± 3,874.80 open flowers per night in 1 ha.
Agave horrida flowers are protandric and are active for four-night; the staminate phase occurs during the first two nights, followed by the opening of the anthers. When the staminate phase ends, the stamens dry up and the flower begins its pistillate phase. Maximum style elongation and receptivity (total opening and moisture of the stigma) occurs at dawn of the fourth night. The morning following its maximum receptivity, the stigma starts to dry up and decreases in length. Nectar production occurs only during the staminate phase, predominantly at night, starting at 2100 h and ending at 0900 h. The total accumulated nectar produced was 0.1148 ± 0.0044 ml per flower (mean ± 1 SE) and the average sugar concentration 10.47 ± 1.16 % Brix (Figure 2).
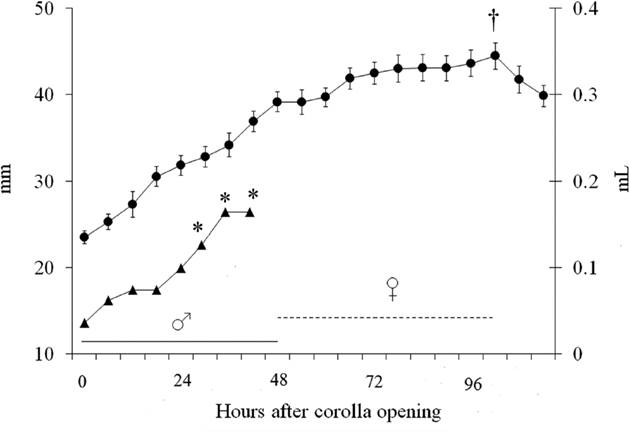
Figure 2 Changes in the flowers of A. horrida in time (n = 12). Black dots represent style growth (mm); black triangles represent accumulated nectar production (mL). The solid line delimits the hours of the staminate-phase (♂), and the dashed line the pistillate-phase (♀). Hours of anther dehiscence (*) and stigma receptivity (†) are also included (defined as total opening and moisture of the stigma). Readings started at 2100 of night zero; all values correspond to the mean ± 1 SE (only for style growth).
Floral visitors. The early visitation rate to Agave horrida inflorescences by nectarivorous bats was greater than by birds (25.10 ± 0.98 visits/h vs. 0.25 ± 0.03 visits/h respectively, t = 4.77, df= 9, P < 0.05. The relative abundance of nectarivorous bats was greater than that of birds (24.6 ± 0.24 h/net vs. 2.22 ± 0.46 respectively, t = 3.41, df= 9, P < 0.05 ). In total, we captured 37 nectarivorous bats and two hummingbirds (one Amazilia violiceps Gould and one Eugenes fulgens Swainson); all bats and hummingbirds had A. horrida pollen on their bodies (Figure 3).
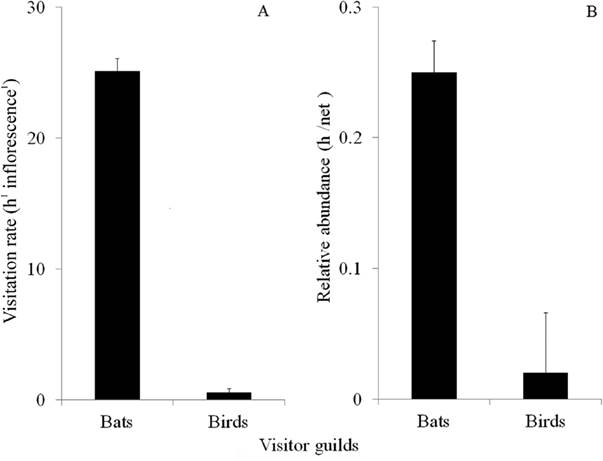
Figure 3 Visitors per hour to an Agave horrida spike (A), and visitor relative abundance per guild (B). All values correspond to the mean ± 1 SE. Five hours of direct observation per guild. 240 h/netting total (150 nocturnal and 90 diurnal). Five days/nights of netting from February 26 to March 2, 2005 using three mist nets (12 m long × 3 m in height). Night hours run from 2000 to 0600 h and diurnal from 0600 to 1200 h.
Pollination experiments. Pollination by insects yielded no fruits. Significant statistical differences in the fruit-set were found due to the pollination treatment (df = 4, χ2 = 87.70, 21 P ≤ 0.05). The manual cross-pollination and control had the largest fruit-set (both with similar production), followed by nocturnal and manual self-pollination (both with similar production), and lastly, the diurnal treatment had the lowest fruit-set.
There were also differences in seed-set among treatments (df = 4, χ2 = 8518.11, P < 0.05). The manual cross-pollination, control and nocturnal pollination had the largest seed-set (no differences were found among them) while manual self-pollination and diurnal treatments (no difference between them) had lowest seed-set (Figure 4).
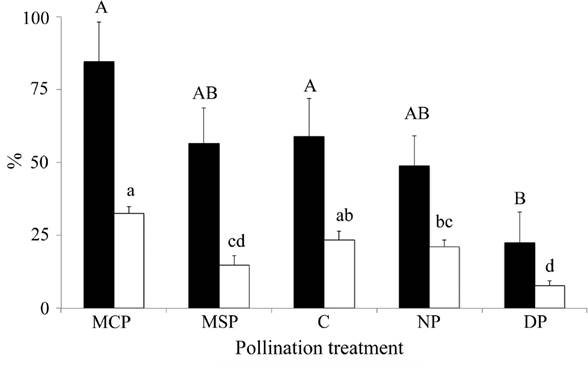
Figure 4 Agave horrida fecundity per pollination treatment. Fruit set (black bars, mean ± 1 SE) and seed-set (white bars, mean ± 1 SE). Means with different letters (fruit-set in uppercase and seed-set in lowercase) are significantly different (Fisher’s LSD p < 0.05). MCP = manual cross-pollination (n = 113 flowers in 3 plants, pollen collected from 4 plants), MSP = manual self-pollination (n = 30 flowers in 3 plants), C = control (n = 85 flowers in 3 plants), NP = nocturnal pollination (n = 115 flowers in 3 plants), DP = diurnal pollination (n = 120 flowers in 4 plants).
Discussion
Our results suggest that, as in other species of Agave in center Mexico, Leptonycteris nivalis Saussure is the most efficient pollinator of Agave horrida and that this is due in part to its predictability in this area and to its visiting behavior to the flowers. A coevolution between this species of agave and nectarivorous bats is suggested by several traits of flower physiology and morphology that correspond in general terms to the chiropterophily syndrome.
Floral biology and visitors. The flowers of Agave horrida are protandrous and clearly chiropterophilous. Like other species of Agave, protandry could be effective in preventing self-pollination within the same flower (Arizaga et al. 2000a) because apparent stigma receptivity occurs 2 nights after the pollen liberation. However, because flowers along the spike are in different phenological stages, geitonogamy might be high in this species. Some degree of self-pollination is common in the Agave genus (Arizaga et al. 2000a, Slauson 2000, Silva-Montellano 2001, Rocha et al. 2005). In A. horrida, maximum fecundity (fruit-set and seed-set) was reached through manual cross-pollination. Our results indicate that self-pollination accounted for at least half of that maximum fecundity. Nevertheless, cases of exclusively exogamous species have been reported (Molina-Freaner & Eguiarte 2003, Rocha et al. 2005, Flores-Torres 2005), in these species, other mechanisms (physiological, genetic or molecular, see Boavida et al. 2005) may be responsible for the strong allogamy, rather than only protandry.
Agave horrida presents floral characteristics related to chiropterophily, such as producing diluted nectar primarily at night (10.47 % Brix), nocturnal anther dehiscence and maximum stigma receptivity at night. These results agree with other works that also report a chiropterophily specialist floral biology (Arizaga et al. 2000a, Slauson 2000, Molina-Freaner & Eguiarte 2003, Rocha et al. 2005). We believe that A. horrida maintains a few hours of diurnal nectar production as a mechanism to attract diurnal visitors, such as birds. Thus, diurnal nectar production could help prevent an extreme specialization and its inherent risks (Howell & Roth 1981, Kearns et al. 1998). It has been suggested that plants with generalist pollination withstand better the complete or temporary absence of its pollinators (Gómez 2002). For example, Agave striata Zucc. (Rocha et al. 2005) and Agave marmorata Roezl (Flores-Torres 2005, Ornelas et al. 2002) are species from central Mexico that have been reported to produce nectar during the day to attract visiting hummingbirds.
Notwithstanding the few hours of Agave horrida diurnal nectar production, the correspondence between its floral biology and the nectar-feeding bats is noteworthy and it reflects the dependency between these species. During the more than two months of its flowering period, A. horrida produces approximately 3.4 liters of nectar per night per ha, enough to maintain approximately 270 nectarivorous bats (see values of energetic flow proposed by Howell, 1979). Agave species are well known for their capacity to offer enough resources to maintain large quantities of pollinators; in central Mexico, their sequential flowering with other chiropterophilous species (agaves and columnar cacti) is likely to be responsible for the presence of resident nectarivorous bats (Rojas-Martínez et al. 1999, Morales et al. 2007). It has also been suggested that the flowering of nine agave species (including A. horrida) forms a corridor that maintains the migration of Leptonycteris nivalis from central Mexico to the South of the U.S. (Gómez-Ruiz & Lacher 2017). This may explain the larger relative abundance and number of visits of nectarivorous bats versus birds (almost tenfold) in A. horrida. Other studies have also reported more visits from bats than from birds in different agave species in central Mexico (Arizaga et al. 2000b, Flores-Torres 2005, Rocha et al. 2005, Trejo-Salazar et al. 2015).
Pollination efficiency of flower visitors. Our experiments show that insects behave as nectar robbers because they cannot pollinate A. horrida flowers. Even when the visitation rate was not evaluated, A. horrida flowers are visited by two type of insects; their visit behavior seem to be responsible for their inability to produce fruits: at night, moths from the Tegeticula genus occasionally visit both pistillate and staminate flowers randomly but without making contact with the stigma; during the day, the non-native bees Apis mellifera visit frequently only the staminate flowers, but at that time the stigma is not receptive. Both insect species have been reported as flower visitants in other agave species (Estrella-Ruiz 2005, Flores-Torres 2005).
Although traditionally it has been proposed that agaves of the subgenus Littaea are pollinated mainly by insects (Schaffer & Schaffer 1977, Howell 1979, Howell & Roth 1981), there are at least 5 other species that are mainly pollinated by nectarivorous bats (Rocha et al. 2005, Trejo-Salazar et al. 2015).
Our results suggest that A. horrida does not have strong pollination limitation (i.e., control fruit-set and seed-set values are similar to those of the manual cross-pollination treatment). This pattern is consistent with the majority of agave pollination experiments in central Mexico, which suggest that in general there is no visitor limitation in Agave genus pollination in this area (Arizaga et al. 2000a, Rocha et al. 2005, Flores-Torres 2005).
Nectarivorous bats in Agave horrida seem to be responsible for the majority of fruits and seeds obtained by natural pollination (no difference in fruit-set and seed-set was found between the nocturnal treatment and control). Therefore, we propose that nectarivorous bats have a greater impact in this species fecundity than birds. Similar results have been found for other agave species in central Mexico (Arizaga et al. 2000, b, Rocha et al. 2005, Flores-Torres 2005).
We can conclude that Agave horrida has a pollination system with a tendency to specialize to nectarivorous bats (Waser et al. 1996, Johnson & Steiner 2000, Gómez 2002). Consequently, this agave depends largely (but not exclusively) on these bats for its reproductive success. Our study corroborates the role of this visitor guild as the most efficient pollinator for agaves in central Mexico in accordance with Molina-Freaner & Eguiarte (2003) suggestion, and contrasts with the tendency to a generalist pollination system in Agave plants found outside central Mexico (Slauson 2000, Molina-Freaner & Eguiarte 2003, Silva-Montellano & Eguiarte 2003).











 text new page (beta)
text new page (beta)



