Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Botanical Sciences
versión On-line ISSN 2007-4476versión impresa ISSN 2007-4298
Bot. sci vol.93 no.1 México mar. 2015
https://doi.org/10.17129/botsci.230
Botánica estructural
Cytogenetical effects of aluminum on root meristem cells of Helianthus annuus L.
Efectos citogenéticos del aluminio en células del meristemo de la raíz de Helianthus annuus L.
Mei Li1,2, Rong Qin1,3, Wusheng Jiang1 and Donghua Liu1,4
1 Tianjin Key Laboratory of Animal and Plant Resistance, College of Life Sciences, Tianjin Normal University, Tianjin, China.
2 Renhuai No.4 Middle School, Renhuai City, Guizhou Province, China.
3 College of Life Sciences, South China Normal University, Guangzhou, China.
4 Corresponding author: donghua@mail.zlnet.com.cn.
Received: June 17th, 2013.
Accepted: October 12th, 2013.
Abstract
Different aluminum concentrations (5, 50, and 100 μM), as well as different exposure times (24, 48, and 72 h), were applied to investigate cytogenetical alterations in sunflower (Helianthus annuus L.) meristem cells. Nucleolin was also examined under aluminum stress using silver-staining method and indirect immunofluorescent method. Results showed that the mitotic index decreased progressively when either aluminum concentration or exposure time increased. C-mitosis, anaphase bridges, and chromosome stickiness were observed in the aluminum treated root tip cells. Some particulates containing the argyrophilic NOR-associated proteins were distributed in the nucleus of the root-tip cells, and the amount of this particulate material progressively increased with increasing exposure time. Finally, the nucleolar material was extruded from the nucleus into the cytoplasm. The result also indicated the nucleolar protein nucleolin was translocated from nucleolus to nucleoplasm and cytoplasm, that presence of 50 μM of aluminum for 48 h, suggesting that aluminum disturbed rRNA synthesis and induced the abnormal cellular location of nucleolar protein. The strongly toxic effect of aluminum on the nucleolus can inhibit or stop mitosis, and consequently inhibit root growth.
Keywords: aluminum, cytogenetical alterations, mitosis, nucleolin, sunflower.
Resumen
Diferentes concentraciones de aluminio (5, 50 y 100 μM) y diferentes tiempos de exposición (24, 48 y 72 h) se aplicaron a células meristemáticas de girasol (Helianthus annuus L.), para investigar las alteraciones citogenéticas, en particular, dilucidar sobre la nucleolina. La nucleolina fue estudiada bajo el estrés por aluminio, utilizando el método de tinción con plata y el método de inmunofluorescencia indirecta. Los resultados mostraron que el índice mitótico disminuyó progresivamente a medida que aumentó la concentración de aluminio o el tiempo de exposición. Se observaron C-mitosis, puentes en la anafase y rigidez en los cromosomas en todas las células de la punta de la raíz tratadas con aluminio. Algunas partículas contienen argirófilas NOR asociadas a proteínas distribuidas en el núcleo de las células del ápice de la raíz. La cantidad de este material granulado aumentó progresivamente a medida que aumentó el tiempo de exposición. Finalmente, el material nucleolar se expulsó desde el núcleo hacia el citoplasma. El resultado también indica que, en presencia de 50 μM de aluminio por 48 h, la proteína nucleolina se transloca desde el nucleolo al nucleoplasma y citoplasma, lo que sugiere que el aluminio altera la síntesis de ARNr e induce la localización celular anormal de la proteína nucleolar. El fuerte efecto tóxico de aluminio en el nucléolo puede inhibir o detener la mitosis, y por consiguiente, inhibir el crecimiento de la raíz.
Palabras clave: alteraciones citogenéticas, aluminio, girasol, mitosis, nucleolina.
Aluminum (Al), a light metal, represents 7% of the earth's crust and is the third most abundant element after oxygen and silicon (Ma et al., 2001). Aluminum toxicity is a major growth-limiting factor for crop production in acid soils (Liu et al., 2008). Aluminum is a major component of acid toxicity in soils (Foy, 1984).
It is well known that roots are the primary target of aluminum phytotoxic (Doncheva et al., 2005). Clarkson (1965) indicated that cell division as a primary site could induce root growth inhibition under Al stress. Matsumoto et al. (1976) and Morimura et al. (1978) supported the view of Al-induced inhibition of root cell proliferation as a primary target for Al-toxicity. Horst (1995) indicated that apoplastic Al could lead to the inhibition of root cell elongation. Kollmeier et al. (2000) found that Al caused inhibition of basipetal auxin transport in maize roots and inhibited root cell elongation. Limited information is available about the effects of Al on root cell in Helianthus annuus L. For the present investigation, the effects of Al on root growth, cell division and nucleoli in Helianthus annuus were investigated in order to deeply understand the toxic mechanism of Al on root growth.
Materials and methods
Culture condition and aluminum treatment. The seeds of Helianthus annuus were kindly provided by the Centre of Popularizing Agriculture Technique of Tianjin, China, and were used in the present investigation. The healthy and equal-sized seeds were chosen and soaked in aerated distilled water for 36 h before starting the experiments. They were germinated on wet gauze in containers at 25 ºC, producing roots reaching about 1 cm length. After that, they were treated in Petri dishes with different concentrations of Al solutions (5, 50, and 100 μM; aluminum chloride, AlCl3) at pH 4.5 for 24, 48, and 72 h. Controls were grown on distilled water. The test liquids were changed regularly every 24 h. Seedlings were grown in a greenhouse equipped with supplementary 15 h light / 9 h dark at 18-20 ºC diurnal cycle.
Cytological study. Fifteen root tips in each treatment group were washed with tap water and distilled water, and cut at 24, 48 and 72 h, respectively. They were cut and fixed in 3 parts 95% ethanol:2 parts 99.5% acetic acid for 2 h, and hydrolyzed in 5 parts 1 M hydrochloric acid:3 parts 95% ethanol:2 parts 99.8% acetic acid for 9 min at 60 °C. For the observation of changes in nucleolus, ten root tips were cut and squashed in 45% acetic acid, dried, and after 2 days stained with silver nitrate (Li et al., 1990; Liu and Jiang, 1991). For the observation of chromosomal morphology, ten root tips selected from fifteen root tips were squashed in a carbol-fuchsin solution (Li, 1982). Mitotic index was used for the investigation; it means number of dividing cells per 500 observed cells (Fiskesjö, 1985). Data for root length were analyzed with standard statistical software (SigmaPlot 9.0).
Indirect immunofluorescent microscopy. Meristematic zones of root tips from control and seedlings treated with adding 50 μM Al for 48 h were cut and fixed with 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS, pH 7.0) for 2 h in darkness at room temperature and then they were washed with the same buffer. Meristematic cells were digested with a mixture of 2.5% cellulase and 2.5% pectolase at 37 °C, and then washed in PBS for three times. They were squashed on slides and extracted in freshly prepared 1% (v/v) Triton X-100 in PBS when slides dried. After three washings in PBS, the cells were subsequently incubated with mouse primary antibodies respectively against nucleophosmin, nucleolin, or fibrillarin for 1 h at 37 ºC or at 4 ºC overnight in a moist, sealed chamber. After washing (3 × 10 min) in PBS, the cells were incubated with secondary antibodies for detection of the primary antibodies for 45 min in darkness at 37 ºC. After repeated washing in PBS, nuclei were stained with 4', 6-diamidino-2-phenylindole (DAPI, Sigma) at a final concentration of 1 μg per 1 mL for 15 min at room temperature. After washing (3 × 10 min) in PBS, the cells were mounted in antifade mounting medium. The slides were stored at 4 ºC in the dark until viewed. The immunofluorescent specimens were examined under a fluorescence microscope (Nikon, HB-10101AF) with the digital camera Pixera Pro 600CL, using violet (355-425 nm) and blue (450-490 nm) specific filters for proteins and nuclei respectively. Photographs were taken and images were processed with Adobe Photoshop 7.0 software.
Antibodies used in this investigation were as follows, nucleolin/C23, primary antibody: a mouse monoclonal antibody to nucleolin (Santa, SC-8031) at dilution 1:100; secondary antibody: TRITC-conjugated goat anti-mouse IgGs (Sigma, T5393) at dilution 1:50. TRITC was used for the detection of signal.
Statistical analysis. Analysis of variance of the data was done with SigmaPlot 8.0 software. For statistical analysis, one-way analysis of variance (ANOVA) and t-test were used to determine the significance at P < 0.05.
Results
Effects of Al on root growth. The effects of Al on root growth of Helianthus annuus varied with the concentration and treatment time. There is a significant difference between treatment and control groups after 72 h of treatment, demonstrating a dose response (Figure 1). At 5 μM Al there was estimulative effect on root growth (P < 0.05) after 24 to 48 h, whereas 50 μM and 100 μM Al inhibited root growth signifi cantly (P < 0.05) after 24 to 72 h.
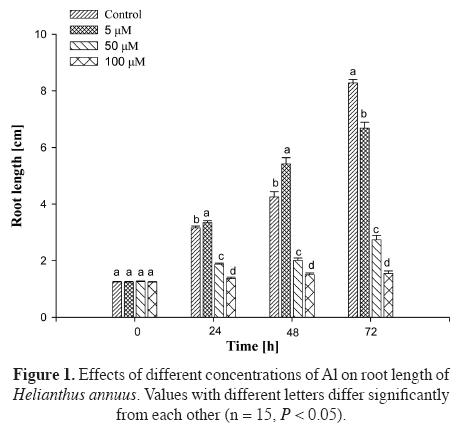
The effects of Al on the morphology of the roots also varied with the different concentrations of aluminum chloride in solution. At 5 μM Al, the morphology of the roots was similar to control during the whole treatment (72 h), but with the increase of the concentration, the color of the root tips became yellow and the color was deeper. At 100 μM Al, the root tips were curved, swollen, cracked, brownish and stubby after 24 h.
Effects of Al on cell division in root tip cells. Observations of roots treated with different levels of Al revealed structural damage that was not observed in the root cells of control plants.
Mitotic index. The mitotic index reflects the frequency of cell division and it is regarded as an important parameter. The mitotic index decreased progressively as a function of increased Al concentration and exposure time (Table 1).
Chromosomal aberrations. The results showed that significant cytotoxic effects emerged after that the root tips of Helianthus annuus were treated with Al. Aluminum ion-induced mitotic aberration types included C-mitosis, chromosome bridges, and chromosome stickiness. C-mitosis was observed in the root tip cells of all treated groups after treatment with Al (Figure 2A). The frequency of cells with C-mitosis increased with increasing Al concentration and with prolonging duration of treatment (Table 1). Anaphase bridges involving one or more chromosomes (Figure 2B-E) were found in the Al treated groups. The frequency of cells with chromosome bridges increased with increasing Al concentration during 48 h of treatment. Al could induce more chromosome stickiness bridges at 100 μM Al treatment group (Figure 2E). Chromosome stickiness refl ects highly toxic effect, probably leading to cell death (Figure 2F). The frequency of cells with chromosome stickiness increased with increasing Al concentration (Table 1).
Effects of Al on nucleolus. Normally, the diploid nucleus of Helianthus annuus contains one or two nucleoli under normal circumstances (Figure 3A). The effects of Al on nucleoli varied with the different concentrations of aluminum chloride used. Three phenomena were observed after these treatments. Firstly, irregular nucleolus was found in the root tips exposed to 50 μM Al for 24 h (Figure 3B). Secondly, some tiny particles containing the argyrophilic NOR-associated (AgNOR) proteins gradually together with the main nucleolus/nucleoli in the nucleus of some root-tip cells increased with increasing of Al concentration and prolonging the duration of treatment (Figure 3C-F). Thirdly, in concentrations of 100 μM Al, the AgNOR protein was extruded from the nucleus into the cytoplasm (Figure 3G-H).
Translocation of nucleolar proteins. The toxic effects of Al on nucleolar protein nucleolin in the root tip cells were observed when compared with control. Using the red fluorescent signal to observe it, the study proved that small immunofluorescence spot of nucleolin was located in nucleolus exclusively in control cells (Figure 4A1-A3). In comparison with control nucleolin in some cells it was located in cytoplasm after the exposure of 50 μM Al for 48 h (Figure 4B3-C3). The nucleolar protein in the cytoplasm increases progressively with longer duration of the treatment (Figure 4E3).
Discussion
Root growth inhibition upon exposure to Al has been used extensively as one of the most distinct and earliest symptoms of Al toxicity (Samuels et al., 1997). Some reports revealed that Al caused the inhibition of root elongation by metal interference with cell division, including inducement of chromosomal aberrations and abnormal mitosis (Zhang, 1995; Qin et al., 2010; Vardar et al., 2011). The results in the present investigation indicated that significant root growth inhibition in Helianthus annuus seedlings exposed to 50 μM and 100 μM Al, which was in agreement with the early findings in H. annuus (Chakravarty and Srivastava, 1992; Kumar and Srivastava, 2006), Vicia faba L. (Zhang, 1995) and Allium cepa L. (Qin et al., 2010).
The silver staining technique has been widely applied in cytological studies aimed at understanding the nucleolar cycle and nucleolar organization in both animals and plants. Nucleolus is well known as the site of transcription of ribosomal genes and further transcript process (Shaw and Jordan, 1995), which contains a set of acidic, nonhistone proteins that bind silver ions and are selectively visualized by silver method. In the present investigation, Al induced irregular nucleolus, fragmentation of particles of argyrophilic proteins scattered in the nuclei, and the material extruded from the nucleus into the cytoplasm. However, the results were, with a few differences, when comparison with the findings of Qin et al. (2010) and Zhang et al. (2009) for the effect of Al on Allium cepa and Vicia faba. For instance, (1) there is not so much nucleolar material released from nuclei into cytoplasm in Helianthus annuus, and (2) Al toxicity on the nucleoli in root tip cells of A. cepa and V. faba is stronger than one in H. annuus.
Amenós et al. (2009) indicated that Al was localized inside the nucleoli of root tip cells of Al sensitive maize. The evidence from the present work shows that once many particles of argyrophilic proteins are scattered in the nuclei, root growth in H. annuus can be inhibited, and once a large amount of nucleolar material is extruded from the nucleus into the cytoplasm, root growth is seriously inhibited or the seedlings died. The strongly toxic effect of Al on the nucleolus often inhibited or stopped mitosis.
Nucleolus is a multifunctional subnuclear territory that plays several important roles in cellular processes (Sirri et al., 2008). Under stress conditions, many nucleolar proteins change their locations and distribute disorderly in and out of nucleolus, furthermore alter their expressions and functions (Mayer et al., 2005; Sun and Yi, 2008). Nucleolus is thought to be a sensor for cellular stress signals (Lewinska et al., 2010).
Data from the present investigation showed that Al had toxic effects on nucleolus and nucleolar proteins. Nucleolin (C23) is a major Ag-NORs protein (Ginisty et al., 1999). C23, the most abundant nucleolar phosphoproteins, is presented in the dense fibrillar component (DFC) and granular component (GC) of the nucleolus and involves in multiple steps of ribosome biogenesis (Chathoth et al., 2009). Data from this work showed that C23 was detected exclusively at the nucleolar territory in control cells and in the treatment group. C23 was observed to be localized to the nucleoplasm and cytoplasm. C23 was demonstrated to be one of the main proteins in nucleolus and oxidative stress could induce the cleavage of it (Wang et al., 2004). van der Aa et al. (2006) found that the nuclear pore complex (NPC) was the most important channel for nuclear material. The phenomenon that the nucleolar material was extruded from the nucleus into the cytoplasm might be explained that the proteins were affected after Al treatment, causing the NPC to lose selectivity. C23 is an important multifunctional protein that has been implicated directly or indirectly in many metabolic processes and is one of the most abundant non-ribosomal proteins of the nucleolus (Bugler et al., 1982). Sripinyowanich et al. (2013) suggest that C23 and ribosomal proteins affect ribosome biogenesis independently or that C23 may interact directly to promote plant growth. It was proved that the retention of nucleolar proteins within the nucleolus was closely related to active rRNA synthesis (Martin et al., 2007; Chen and Jiang, 2004). Rubbi and Milner (2003) reported that inhibition of rRNA synthesis could affect interaction between nucleolar proteins and rRNA, which induced the relocation of nucleolar proteins. Therefore, we supposed that Al disturbed rRNA synthesis and induced the abnormal cellular location of nucleolar protein. The strongly toxic effect of Al on the nucleolus can inhibit or stop mitosis, and consequently inhibit root growth. The results can further confirm our previous observations (Liu and Jiang, 1991), and fi rstly identifies 'Al-structure' named by Fiskesjö (1983, 1990).
The data from these biomarkers can provide valuable information for monitoring and forecasting earlier effects of exposure to Al in real scenarios conditions.
Acknowledgements
This project was supported by the National Natural Science Foundation of China. The authors wish to express their appreciation to the reviewers for this paper.
Literature cited
Amenós M., Corrales I., Poschenrieder C., Illéš P., Baluška F. and Barceló J. 2009. Different effects of aluminum on the actin cytoskeleton and brefeldin A-Sensitive vesicle recycling in root apex cells of two maize varieties differing in root elongation rate and aluminum tolerance. Plant and Cell Physiology 50:528-540. [ Links ]
Bugler B., Caizergues-Ferrer M., Bouche G., Bourbon H. and Amalric F. 1982. Detection and localization of a class of proteins immunologically related to a 100-kDa nucleolar protein. European Journal of Biochemistry 128:475-480. [ Links ]
Chakravarty B. and Srivastava S. 1992. Toxicity of some heavy metals in vivo and in vitro in Helianthus annuus. Mutation Research Letters 283:287-294. [ Links ]
Chathoth K.T., Ganesan G. and Rao M.R.S. 2009. Identification of a novel nucleolin related protein (NRP) gene expressed during rat spermatogenesis. BMC Molecular Biology 10:64. [ Links ]
Chen M. and Jiang P. 2004. Altered subcellular distribution of nucleolar protein fibrillarin by actinomycin D in HEp-2 cells. Acta Pharmacologica Sinica 25:902-906. [ Links ]
Clarkson D.T. 1965. The effect of aluminium and some other trivalent metal cations on cell division in the root apices of Allium cepa. Annals of Botany 29:309-315. [ Links ]
Doncheva S., Amenós M., Poschenrieder C. and Barceló J. 2005. Root cell patterning: a primary target for aluminium toxicity in maize. Journal of Experimental Botany 56:1213-1220. [ Links ]
Fiskesjö G. 1983. Nucleolar dissolution induced by aluminium in root cells of Allium. Physiologia Plantarum 59:508-511. [ Links ]
Fiskesjö G. 1985. The Allium test as standard in environmental monitoring. Hereditas 102:99-112. [ Links ]
Fiskesjö G. 1990. Occurrence and degeneration of 'Al-structures' in root cap cells of Allium cepa L. after Al-treatment. Hereditas 112:193-202. [ Links ]
Ginisty H., Sicard H., Roger B. and Bouvet P. 1999. Structure and functions of nucleolin. Journal of Cell Science 112:761-772. [ Links ]
Foy C.D. 1984. Physiological effects of hydrogen aluminum, and manganese toxicities in acid soil. In: Adams F. Ed. Soil acidity and liming. ASAI Monograph Series12, 2nd ed., pp 57-98, American Society of Agronomy, Inc., Crop Science Society of America, Inc., Soil Science Society of America, Inc., Madison. [ Links ]
Horst W.J. 1995. The role of the apoplast in aluminium toxicity and resistance of higher plants: A review. Zeitschrift für Pflanzenernährung und Bodenkunde 158:419-428. [ Links ]
Kollmeier M., Felle H.H. and Horst W.J. 2000. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminium? Plant Physiology 122:945-956. [ Links ]
Kumar G. and Srivastava S. 2006. Mechanism of aluminum cytotoxicity in meristematic cells of Helianthus annuus L. Journal of Phytological Research 19: 175-178. [ Links ]
Lewinska A., Wnuk M., Grzelak A. and Bartosz G. 2010. Nucleolus as an oxidative stress sensor in the yeast Saccharomyces cerevisiae. Redox Report 15:87-96. [ Links ]
Li M.X. 1982. Introducing a good stain used for nuclei and chromosome. Biological Bulletin 5:53. [ Links ]
Li M.X., Zhang Z.P., Yuan J.S. and Pu X.L. 1990. An improved method for Ag-NOR staining and its application to plant chromosomes. Chinese Bulletin of Botany 7:56-59. [ Links ]
Liu D. and Jiang W. 1991. Effects of Al3+ on the nucleolus in root tip cells of Allium cepa. Hereditas 115:213-219. [ Links ]
Liu Q., Yang J.L., He L.S., Li Y.Y. and Zheng S.J. 2008. Effect of aluminum on cell wall, plasma membrane, antioxidants and root elongation in triticale. Biologia Plantarum 52:87-92. [ Links ]
Ma J.F., Ryan P.R. and Delhaize E. 2001. Aluminum tolerance in plants and the complexing role of organic acids. Trends in Plant Science 6:273-278. [ Links ]
Martin R.M., Tünnemann G., Leonhardt H. and Cardoso M.C. 2007. Nucleolar marker for living cells. Histochemistry and Cell Biology 127:243-251. [ Links ]
Matsumoto H., Hirasawa E., Torikai H. and Takahashi E. 1976. Localization of absorbed aluminium in pea root and its binding to nucleic acids. Plant and Cell Physiology 17:127-137. [ Links ]
Mayer C., Bierhoff H. and Grummt I. 2005. The nucleolus as a stress sensor: JNK2 inactivates the transcription factor TIF-IA and down-regulates rRNA synthesis. Genes and Development 19:933-941. [ Links ]
Morimura S., Takahashi E. and Matsumoto H. 1978. Association of aluminium with nuclei and inhibition of cell division in onion (Allium cepa) roots. Zeitschrift für Pflanzenphysiologie 88:395-401. [ Links ]
Qin R., Jiao Y.Q., Zhang S.S., Jiang W.S. and Liu D.H. 2010. Effects of aluminum on nucleoli in root tip cells and selected physiological and biochemical characters in Allium cepa var. agrogarum L. BMC Plant Biology 10:225. [ Links ]
Rubbi C.P. and Milner J. 2003. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. The EMBO Journal 22:6068-6077. [ Links ]
Samuels T.D., Kucukakyuz K. and Rincon-Zachary M. 1997. Al partitioning patterns and root growth as related to Al sensitivity and Al tolerance in wheat. Plant Physiology 113:527-534. [ Links ]
Shaw P.J. and Jordan E.G. 1995. The nucleolus. Annual Review of Cell and Developmental Biology 11:93-121. [ Links ]
Sirri V., Urcuqui-Inchima S., Roussel P. and Hernandez-Verdun D. 2008. Nucleolus: the fascinating nuclear body. Histochemstry and Cell Biology 129:13-31. [ Links ]
Sripinyowanich S., Chamnanmanoontham N., Udomchalothorn T., Maneeprasopsuk S., Santawee P., Buaboocha T., Qu L.J., Gu H.Y. and Chadchawan S. 2013. Overexpression of a partial fragment of the salt-responsive gene OsNUC1 enhances salt adaptation in transgenic Arabidopsis thaliana and rice (Oryza sativa L.) during salt stress. Plant Science 213: 67-78. [ Links ]
Sun X.X. and Yi J. 2008. Nucleolar proteins: their location, translocation and nucleolar functions. Chinese Journal of Cell Biology 30:569-573. [ Links ]
van der Aa M.A.E.M., Mastrobattista E., Oosting R.S., Hennink W.E., Koning G.A. and Cormmelin D.J.A. 2006. The nuclear pore complex: the gateway to successful nonviral gene delivery. Pharmaceutical Research 23:447-459. [ Links ]
Vardar F., Ismailoglu I., Inan D. and Unal M. 2011. Determination of stress responses induced by aluminum in maize (Zea mays). Acta Biologica Hungarica 62:156-170. [ Links ]
Wang K.K., Jiang L., Liu K., Liu M.D., Wang H., Yi Y.X., Yuan C., E S., Shi Y.Z. and Xiao X.Z. 2004. Effects of heat shock protein 70 on cleavage of nucleolin induced by oxidative stress. Chinese Journal of Arteriosclerosis 12:373-377. [ Links ]
Zhang Y.X. 1995. Effects of aluminum chloride on the nucleus and nucleolus in root tip cells of Hordeum vulgare. Mutation Research/Environmental Mutagenesis and Related Subjects 335:137-142. [ Links ]
Zhang H.M., Zhang S.S., Meng Q.M., Zou J., Jiang W.S. and Liu D.H. 2009. Effects of aluminum on nucleoli in root tip cells, root growth and the antioxidant defense system in Vicia faba L. Acta Biologica Cracoviensia. Series Botanica 51/2:99-106. [ Links ]














