Highlights:
C. foetidissima, C. radicans (silvestres), C. moschata, C. argyrosperma and C. pepo were the seeds analyzed.
Triglycerides (TG) 18:2/18:2/18:2 predominates in wild and domesticated seeds.
18:3/18:3/18:3 TG bands are prominent in wild pumpkins.
Reversed-phase HPTLC is a suitable alternative to obtain triglyceride profiles.
Introduction
Pumpkins belong to the genus Cucurbita (Cucurbitaceae) which includes 21 taxa (Barrera et al., 2021) and close 15 species (Lira et al., 2016). In Mexico, 11 wild and domesticated species are mentioned (Ríos, González-Santos, Cadena-Iñiguez, & Mera-Ovando 2018). Wild species include buffalo gourd (Cucurbita foetidissima Kunth) and coyote gourd (C. radicans Naudin). Cucurbita pepo L., C. moschata Duchesne, C. argyrosperma K. Koch and C. ficifolia Bouché are the main domesticated species in Mexico. Domesticated pumpkin seeds consumption has gained interest for being a nutritional source of protein (31 to 51 %), lipids (30 to 51 %), carbohydrates (6 to 37 %), in addition to sterols, tocopherols and minerals (Adepoju & Adebanjo, 2011; Bardaa et al., 2016), resulting in some healing and nutraceutical claims.
Most lipids studies in seeds, including pumpkin seeds, have focused on establishing the fatty acid composition by employing saponification and conversion to methyl esters (FAME) by transesterification and then identification by gas chromatography (Bouazzaoui & Mulengi, 2018; Rezig, Chouaibi, Msaada, & Hamdi, 2012). Although this is a suitable method for routine commercial application, do not disclose information on the composition of the specific triglycerides (TG) present (Eder, 1995). The information on TG composition is, for the moment, used for the characterization of oils in pharmacopoeias and is of interest in the study of the biosynthesis of these compounds. The characterization methodology is basically used in laboratory studies; however, rapid and inexpensive visualization by reversed-phase HPTLC allows rapid separation and differentiation of TG in different tissues, such as wild and domesticated seeds.
TGs represent an efficient form of energy storage in the seed that will be available at the germination and development of the new plant, as they contain high-energy bonds that are easily degraded. The fatty acids esterified to glycerol, can present various chain lengths, generally from C12 to C22, displaying unsaturations as double bonds, all of this, because of their biosynthetic mechanism. The study of TG deserves attention because they represent between 95 to 98 % of the composition of vegetable oils (Contreras et al., 2017). Each oil has a characteristic TG profile, and the physical and chemical properties are established initially by the presence of the various molecular TG species (Ruiz, González, & Cuadros, 2015) which have important physiological effects as components of the human diet (Cherif et al., 2014). Therefore, there is a continuous search for sources of TGs, especially those with components rich in unsaturated or polyunsaturated fatty acids (Zeb, 2012), as they possess specific biological activity and increase the beneficial properties of edible oils (Gao et al., 2017).
Wild pumpkin species TG profile has been established to date (Ali et al., 2017; Azimova, Glushenkova, & Vinogradova, 2011; Neđeral et al., 2012; Zeb & Ahmad, 2017), although there is no comparison of TG composition in domesticated versus wild pumpkin seeds. Some of the methods for the study of TG profile in pumpkins comprise: the use of silica gel plates impregnated with silver nitrate and subsequent evaluation of the fatty acid methyl esters by gas chromatography/mass spectrometry (GC/MS) (Yoshida et al., 2004); the carbon equivalent number (ECN42), International Olive Council (IOC) chromatographic method (Bardaa et al., 2016); the high performance liquid chromatography (HPLC) in its modalities (Ali et al., 2017; Zeb & Ahmad, 2017). and laser-assisted matrix desorption-ionization time-of-flight (MALDI-TOF-MS) (Beneito et al., 2020). There are several mechanisms for isolation and detection of TG, but they are very laborious; therefore, they are not suitable for routine work (Cossignani, Pollini, & Blasi, 2019). These methods have low selectivity for TG that differ only in acyl chain length or position (Cherif et al., 2014) and exhibit poor separation or low resolution (Gao et al., 2017).
High Performance Thin Layer Chromatography (HPTLC), in reverse phase mode, can provide information on triglycerides, polar lipids, sterols, essential oils and multiple organic structures. Currently, at the commercial level, this technique is used for the evaluation of the quality of oils and other components; in addition, it can detect the presence of adulterants (Cañigueral, Frommenwiler, Reich, & Vila, 2018; Pagliuca et al., 2018). For this reason, the use of HPTLC has been adopted by major pharmacopoeias worldwide for the analysis and quality control of herbal preparations (Cañigueral et al., 2018). This technique allows the visualization, quantification and analysis of TG and is based on the separation of the components of a sample according to their differential affinity to a stationary phase with lipophilic residues and the use of a highly polar mobile phase. The implementation of this technique allows easy application of samples in reduced volume using as little as 0.1 µL of the sample in solution. Obtaining good resolution bands helps in the interpretation of retardation factor (Rf) values and phytochemical profile of the compounds in a comparative chromatographic system (Naik & Sellappan, 2020).
This study compared the TG of oils extracted from wild and domesticated pumpkin seeds, using the reversed-phase HPTLC system and making use of a free software commonly used for protein analysis. This program allows the comparison of multiple samples of the displacement of molecules in the form of bands easily, quickly and with results like those obtained with high-priced commercial software systems for thin layer chromatography (TLC). This study will allow to visualize characteristics of taxonomic importance, in addition to contributing with the generation of the first TG profile of wild pumpkin seeds, from which their potential use as non-timber wild natural resources can be identified (Cañigueral et al., 2018) and provide the basis for analyzing the reason for such differences.
Materials and Methods
Mature fruits of two species of the genus Cucurbita were collected in October 2019 at three sites: Cucurbita foetidissima Kunth in the locality of Vaquerías, municipality of Ojuelos, Jalisco (21° 47' 28.39" N, 101° 36' 7.03" W, 2 223 m) and Cucurbita radicans Naudin from San José de Gracia, Jalisco (20° 41' 8.16" N y 102° 34' 11.42” W, 1 947 m) and Cañada de Caracheo, Guanajuato (20° 19' 8.78" N y 100° 57' 2.19" W, 1 772 m). Preliminary identification of wild species was made by reviewing specimens at the herbariums of: UNAM (MEXU); Instituto de Ecología (IE-Bajío); Querétaro (QMEX) and Universidad de Guadalajara (IBUG). The SEINet data portal was also consulted, and the respective floras and specialized bibliographic material (Lira, 2001). The reference specimens were deposited in the Herbarium Luz María Villareal de Puga (IBUG)-CUCBA of the Universidad de Guadalajara.
Regarding domesticated species, C. moschata (calabaza de Castilla or sweet squash, Hortaflor, certified seeds) was acquired in Irapuato, Guanajuato, and C. moschata (calabaza tapatía), C. argyrosperma (silver seed gourd) and C. pepo (courgette) were acquired in the local market in the city of Guadalajara. Sunflower seeds (Helianthus annuus L.) from the local market in the city of Guadalajara, Jalisco, were also used.
Pumpkin fruits were opened in half to separate the seeds and fibrous pulp content. The domesticated seeds testa was removed to work only with the embryonic axis and cotyledons. The process was carried out at the Phytobiochemistry Laboratory of CINVESTAV-IPN, U. Irapuato.
Lipidic components extraction
Around 6 g of seed samples from each collection site, from the domesticated pumpkins and sunflower were weighed on an OHAUS Adventurer™ analytical balance. A six-place Soxhlet extractor (Extraction Unit E-816 SOX-Extraction Solutions, Switzerland) was used; each seeds sample was placed in a 10 x 10 cm filter paper was placed in each Soxhlet container. For the extraction process, 100 mL of light petroleum (Hexanes 4020-Karal, S. A. de C. V.) was used as solvent. The extraction consisted of 10 cycles at 70 °C, a 15 min washing phase and drying for 30 min. Evaporation-condensation time was 1 h 15 min. Excess hexane used as solvent was removed by rotary evaporation. The total lipid content was estimated relative to the density of sunflower oil (0.920 g∙mL-1). Finally, each extract was dissolved in chloroform: methanol (1:1) adjusting to a concentration of 1 mg∙mL-1 and thus stored under refrigeration for subsequent analysis.
Reversed-phase high-performance thin layer chromatography (HPTLC)
The method described by the HPTLC Society, as modified by European Pharmacopoeia 7.0 section 2.3.2 (European Pharmacopoeia Commission, 2011), was used. A total of 20 mg of each lipid extract sample was dissolved in 3 mL of dichloromethane. As reference, triglycerides purchased from Sigma-Aldrich were used: TG 18:3/18:3/18:3, TG 20:1/20:1/20:1, TG 22:1/22:1/22:1 and a mixture of TG 16:1/16:1/16:1 + TG 18:2/18:2/18:2.
For application on the plate samples and references were disposed in vial inserts placed in a vial holder of the TLC 4 autosampler (ATS 4, CAMAG®, Switzerland). Glass HPTLC plates (20 x 10 cm) coated with silica gel 60 (RP-18 F254, MS-grade, Merck, Darmstadt, Germany) were used. The application conditions by microspraying were as follows: 2 µL per band with 8 mm width and 4 mm spacing between each of the 13 tracks, at a distance of 12 mm from the bottom of the plate. Once the samples had been applied, the plate was transferred, for development, to a flat bottom chamber (CAMAG®, Switzerland) previously saturated with a mixture of dichloromethane: acetic acid: acetone (20:40:50 v/v/v). For the development, the solvent ascended by capillary action on the plate, reaching 8 cm above the line of application of the samples. The chamber was kept in cold room at 4 °C prior to full development. The developed plate was removed from the chamber and solvent released by heating at 100 °C for 1 min on a CAMAG® digital hot plate, then brought to room temperature. For visualization, derivatization by immersion and immediately removed in a 20 % phosphomolybdic acid solution dissolved in ethanol. Subsequently, the plate was heated for 3 min at 120 °C. The phosphomolybdic acid reagent produces intense blue-black, uniform zones in the presence of unsaturated TG. This allows digital photographic recording with the CAMAG® Visualizer, illuminated with white light. The bands were analyzed with the VisionCats software (CAMAG®, Switzerland), which is part of the CAMAG HPTLC System, allowing qualitative processing, obtaining the Rf values of each of the bands and the elaboration of densitograms. In the present study the GelAnalyzer software was used as a recommended alternative for the analysis of HPTLC plate images.
GelAnalyzer plate analysis
The developed plates images were analyzed with the freely available software GelAnalyzer 19.1 (Lazar Jr., & Lazar, 2019). This provided component detection, shift bands and intensity in each track, and preliminary assignment from comparison of reference TG. The program also supported the correction of imperfections equivalent to the electrophoretic run by adjusting the application origin and the solvent front to obtain the Rf values. The image was imported into the program in the dark option on illuminated background, rotated from bottom to top and from left to right and the region to be analyzed was defined. The option to select an area was used so that the program would automatically detect the tracks. Each track can also be added manually one at the time. The bands intervals the in the tracks are placed in agreement to the increase of the band values with Rf between the sample application site (value 0.0) and the solvent front at the end of the development (value 1.0). Subsequently, in the calibration mode of bands in linear function, the main reference TG (18:2/18:2/18:2) was observed in most pumpkin seed extracts (Zeb & Ahmad, 2017). The baseline of all leads was defined to display them in the form of a chromatogram. Finally, the Rf values and the areas under the curve, were determined for each band.
Results and Discussion
Figure 1 shows the reversed-phase HPTLC chromatographic plate developed with 13 tracks. Tracks 2 to 10 correspond to TG in seed extracts from domesticated and wild pumpkin seeds. Tracks 1, 11, 12 and 13 indicate the reference TG bands. The dark blue coloration acquired by each band is related to the abundance of certain TG; this occurs due to the application of the derivatizing reagent that improves the visualization of the differences between samples. As a result, a particular pattern of similarity and differentiation was detected among pumpkin seed samples.
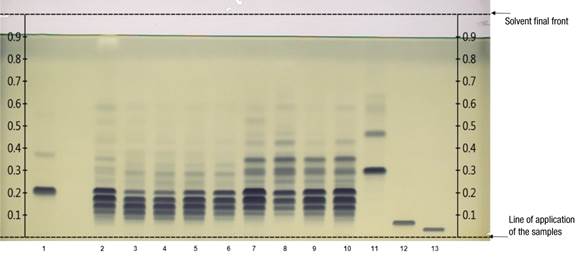
Figure 1 Separation of triglycerides (TG) per track: 1 = reference (16:1/16:1/16:1 + 18:2/18:2/18:2); 2 = sunflower oil; 3 to 6 = seeds from domesticated pumpkins (Cucurbita moschata [calabaza tapatía], C. argyrosperma, C. pepo, C. moschata [calabaza de Castilla]); 7 to 10 = seeds of wild pumpkins (C. foetidissima, C. foetidissima, C. radicans [Cañada de Caracheo], C. radicans [San José de Gracia]); 11 to 13 = references (18:3/18:3/18:3, 20:1/20:1/20:1, 22:1/22:1/22:1). The development solvent was dichloromethane: acetic acid: acetone (20:40:50 v/v/v), phosphomolybdic acid reagent used as derivatizing agent and the plate observed under white light illumination.
Figure 2 shows the integration chromatographic profiling located to the right of tracks 1 and 11 of two TG standards, as well as tracks 3 and 8 corresponding to samples of lipid extracts from C. moschata and C. foetidissima pumpkin, domesticated and wild, respectively. The profile also includes the peak points used as Rf values, which can be compared between samples. The intensity in each band can be tentatively taken as the relative quantitative abundance of the components of each sample.
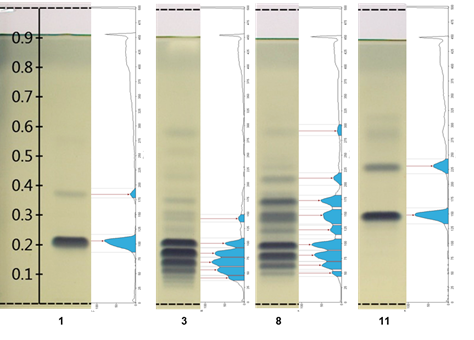
Figure 2 Chromatograms versus chromatographic profiling of the main differences found with the Gel Analyzer software. Track 1 = TG 16:1/16:1/16:1 + TG 18:2/18:2/18:2; track 3 = Cucurbita moschata (calabaza tapatía); track 8 = Cucurbita foetidissima (wild); track 11 = TG 18:3/18:3/18:3.
On the other hand, Figure 3 compares the whole chromatographic profiling of the plate, where the differences regarding the maximum points expressed as Rf of each band can be seen and the area each band occupies among the samples. Obtaining these profiles with their respective Rf values and areas under the curve provides information that can be useful in making quantitative evaluations for a better understanding of the results.

Figure 3 Reversed-phase HPTLC chromatographic profiling for the identification of lipid extracts from seeds of domesticated (tracks 3 to 6: Cucurbita moschata [calabaza tapatía], C. argyrosperma, C. pepo, C. moschata [calbaza de Castilla]) and wild (tracks 7 to 10: C. foetidissima, C. foetidissima, C. radicans [Cañada de Caracheo], C. radicans [San José de Gracia]) pumpkins, as well as sunflower oil (track 2) and reference triglycerides (tracks: 1 = 16:1/16:1/16:1 + 18:2/18:2/18:2; 11 = 18:3/18:3/18:3; 12 = 20:1/20:1/20:1; 13 = 22:1/22:1/22:1)
Báez-Pérez, Quiñones-Galvéz, Santiesteban-Toca, and Molina-Torres (2017) reviewed methods for densitometric analysis of planar chromatographic color images, including the GelAnalyzer software. This software, in addition to being freely available, allows a fast, economic and recommended analysis for HPTLC plates based on the color intensity of the bands, and provides maximum Rf values that can be compared between samples. The use of this image analysis system allows cost savings, as large food and drug companies usually invest in more sophisticated equipment.
According to the analysis, the maximum points or Rf band values of the reference TGs were as follows: track 1: TG (16:1/16:1/16:1) Rf = 0.38 + TG (18:2/18:2/18:2) Rf = 0.22; track 11: TG (18:3/18:3/18:3) Rf = 0.31; track 12: TG (20:1/20:1/20:1) Rf = 0.07; track 13: TG (22:1/22:1/22:1) Rf = 0.04.
When comparing the lipid extracts of wild and domesticated pumpkin seeds, sunflower extract and reference TG, it can be noticed that the component at Rf = 0.22 corresponding to the reference TG 18:2/18:2/18:2 (Figure 1, track 1) is also present in all the samples analyzed. The TG of C. pepo and C. moschata seeds, tracks 5 and 6, confirm as indicated in previous reports on domesticated pumpkins, prevailing studies on C. pepo (Arslan, Gönül, & Yilmaz, 2017; Benalia et al., 2015; Li et al., 2020; Neđeral et al., 2012). The presence of TG 18:2/18:2/18:2 Rf = 0.22 is also an important component in lipid extracts of wild pumpkin seeds (tracks 7 to 10). This TG is prominent in sunflower seeds as indicated by Jakab, Heberger, and Forgacs (2002), who mention that oil from the seeds of domesticated pumpkins has similar characteristics to sunflower oil, although they did not specify the species studied. Salas, Bootello, and Garcés (2015) also mentioned that TG 18:2/18:2/18:2 is one of the most abundant in regular sunflower oil. Hernández et al. (2020) report that C. foetidissima presents a composition of 55.93 % C18:2 fatty acid and 12.65 % C18:1 fatty acid in the triglycerides of its seeds.
For C. radicans, this is the first report for seeds TG. This species presents a TG profile like that of C. foetidissima; TG 18:2/18:2/18:2 and 18:3/18:3/18:3, among others with bands with Rf values higher than 0.31, corresponding to TG 18:3/18:3/18:3, can also be found in these samples. The latter has an additional band with Rf = 0.47. However, being a pure standard, degradation was evidenced after storage time, which is a characteristic of unsaturated TG. It was again possible to confirm that the domesticated pumpkin samples present unsaturated TG in a lower proportion than the wild species. One of the differences between the two wild species is that C. foetidissima had higher Rf values (Figure 3; Rf = 0.53 in track 7 and Rf = 0.59 in track 8) than C. radicans, indicating the presence of more unsaturations in TG of C. foetidissima. Therefore, more replicates and a proper statistical evaluation using the integration values of the GelAnalyzer software are required, as exemplified in Figure 3, which would imply further studies to establish a statistically significant difference between the TG profile of these species.
The reference TG 18:3/18:3/18:3 that stands out in wild pumpkin samples (Figures 2 and 3, track 11) has also been identified in Cucurbita maxima L. (Akintayo, Akintayo, Akinsola, & Ziegler, 2009), while in other domesticated species the presence has not been reported (Montesano, Blasi, Simonetti, Santini, & Cossignani, 2018; Neđeral et al., 2012).
As we have mentioned, there are some bands that stand out due to their importance, because they can help in the discrimination between types of samples or highlight the composition of TG with similar characteristics to commercial oils; for example: C. foetidissima track 8, had a Rf = 0.59 that also is present in the sunflower sample. The value of Rf = 0.09 is present in all the domesticated pumpkin samples, but absent in the wild samples; the band corresponding to that value can serve as a reference to differentiate even in domesticated pumpkin species. They show bands around Rf = 0.12, which is less present in the wild ones. These bands should be characterized in subsequent research. Adewuyi and Oderinde (2012) and Fedko et al. (2020) highlight that the study of seed lipids has shown that the composition varies according to the species, even in pumpkin varieties, which leads to oils with different properties.
The reference TG 16:1/16:1/16:1 (Figure 3, track 1) had an Rf = 0.38, present in the upper bands of wild pumpkins, although it is a light band in the wild samples it is absent in the domesticated ones. On the other hand, both TG 20:1/20:1/20:1 Rf = 0.07 and TG 22:1/22:1/22:1 Rf = 0.04 are not present in pumpkins, which have prevalence of C16 and C18 chain unsaturated TG. It is important to note that the presence of unsaturated TG in wild pumpkin samples is desirable in terms of nutrition and could be considered a substitute for highly polyunsaturated oils such as soybean oil in diets (Nehdi, 2011).
Due to the use of phosphomolybdic acid, which is a specific derivatizing agent to reveal the presence of double bonds, only unsaturated TG, but not saturated ones were observer in this study. Nevertheless, TGs with such characteristics may exist in the analyzed seeds as mentioned by Benalia et al. (2015), who determine that TG 12:0/12:0/12:0 and TG 14:0/14:0/14:0 are present in C. pepo. These can be used as referents to differentiate oils from this species, so it is advisable to use another detection method and such referents to confirm discriminant bands, which is not the purpose of this study.
This research is the first study of the comparative unsaturated TG profile of domesticated (C. moschata, C. argyrosperma and C. pepo) versus wild (C. foetidissima and C. radicans) pumpkins using reversed-phase HPTLC and fast and easy image analysis with GelAnalyzer. Similar results were obtained with this program as when using VisionCats. As could be seen, there are more unsaturated TG bands in the wild samples than in the domesticated samples, coinciding with Facciotti and Knauf (1998), who highlight more diversity in oil compositions in wild species than in domesticated crops.
Band intensities and TG identification analysis evaluation, using techniques such as GC-MS is recommended to be used more detailed information on these results. The European Pharmacopoeia has also considered band intensities as a discrimination criterion. It also highlights the use of hexane as solvent for an adequate extraction of triglycerides. On the other hand, it is advisable to make analysis with fresh samples of mature seeds to avoid degradation of TG containing mainly unsaturated fatty acids.
For species other than Cucurbita, TG comparisons between domesticated and wild species have been carried out. For example, Kamal-Eldin, Yousif, and Appelqvist (1992) mention that, in Sesame spp. seeds, there is similarity in the composition of fatty acids for both types of extracts, although differences are also observed such as the increase of double unsaturated TG in wild species regarding a pure line variety of Sesamum indicum L.
Conclusions
Triglyceride 18:2/18:2/18:2 predominates in the lipid extracts of pumpkin seeds regardless of the origin of the plant, either wild or domesticated. There is a difference in unsaturated triglycerides that are absent in domesticated pumpkins, which confers a higher nutritional value to wild seeds. HPTLC is a suitable tool for chromatographic fingerprinting of lipid extracts that allows the differentiation of lipid content and composition of plant species. By processing the data with the GelAnalyzer it was possible to estimate the delay factors of the triglyceride profile components, which in turn makes it possible to obtain graphs and areas under the curve of each band.











 text in
text in 


