Highlights:
Chemical, mechanical and thermal scarification were evaluated on Vachellia and Prosopis species.
Mechanical treatment (sanding) was the most efficient in breaking physical dormancy.
It required 341 to 669 N of pressure to fracture the seed coat and concluding dormancy.
Dormancy was more intense in Vachellia than in Prosopis.
Introduction
In Mexico, arid regions cover 52 % of the territory. Natural regeneration in these areas is characterized by the scarce and brief rainy season in summer, usually from 100 to 400 mm and 7 to 18 months without rain or more (Rzedowski, 2006), so that moisture is adequate for seeds to germinate (Sánchez et al., 2014).
In some ecosystems, such as Mediterranean ecosystems and savannas, more than 25 % of species show physical dormancy (Hudson, Ayre, & Ooi, 2015). Steinbrecher and Leubner-Metzger (2018) indicate that this phenomenon is originated by water impermeable cell layers found in the seed coat or pericarp. According to Baskin and Baskin (2014), physical dormancy is found in 15 plant families, including legumes. The ecological importance of this family lies in the fact that they are soil improvers, N fixers and food source for wild animals (Odum & Barrett, 2008); in arid and semi-arid places, they serve dual functions as shelter and food for wildlife and as nurse plants in the regeneration of species.
Dormancy can be eliminated by chewing and passage through the digestive tract of animals, by being carried away by surface runoff, or by fire. For example, in the case of legumes, seeds of Prosopis flexuosa DC. and P. chilensis (Molina) Stuntz are physically-biologically (digestive tract) depredated, dispersed and scarified by rodents, other mammals and ants (Velez, 2014). On the other hand, when legume plants are produced in nurseries, the application of scarification practices such as sanding, fracturing, immersing in hot water and immersion in sulfuric acid is necessary (Minchala-Patiño et al., 2014; Miranda, Oliveira, Correia, Almeida, & Pompelli, 2011).
Presence and intensity of dormancy is often taken as a constant in species that have it; however, in the case of legumes such as Prosopis alba Griseb., variability has been observed in the morphological characteristics of the seed, particularly in size (Fontana, Pérez, & Luna, 2015), which possibly influence the intensity of its physical dormancy. Jaganathan (2016) suggest that, during seed development, the environment (relative humidity, temperature and precipitation) of the mother plant influences dormancy intensity.
Genes controlling dormancy and germination show stronger selection than other traits (Penfield, 2017). In this regard, knowledge of the anatomy of seed coat layers allows a better understanding of their role in physical dormancy. Based on the above, it is important to deepen specific information at the time of seed formation. Therefore, the objectives of the present study were to describe the morphology of seed coat layers and to evaluate chemical, mechanical and thermal scarification in three Vachellia and two Prosopis species. The hypothesis was that chemical, mechanical and thermal treatments allow higher germination.
Materials and Methods
Seed morphology and physical dormancy breaking mechanisms of Vachellia schaffneri (S. Watson) Seigler & Eibinger, V. pennatula (S. Watson) Seigler & Eibinger, V. farnesiana, Prosopis laevigata (Humb. & Bonpl. ex Willd.) M. C. Johnst and P. glandulosa were evaluated in this study. These genuses disperse by barocory, anemochory (windblown pods with mature seeds), hydrochory (surface runoff) and endozoochory (Baskin & Baskin, 2014). Huizaches such as Vachellia farnesiana (L.) Wight & Arn. are part of the diet of white-tailed deer and provide cover, shelter, and protection for several wildlife species. Vachellia farnesiana is excellent for poles, the flower is used for perfumery in Europe, and fruits and bark have been used to make ink and obtain tannins (Schiltmeyer & Zouhar, 2020). Mesquite trees, such as Prosopis glandulosa Torr. are used for firewood and charcoal, as well as for furniture manufacture. Fruits serve as food for livestock and a great diversity of wild species (Steinberg, 2001).
For study purposes, seeds were obtained from between five and 10 trees of each species in the vicinity of the community of Juchipila, Zacatecas (1 240 to 1 400 m in semi-dry climate with mean annual temperature of 22.2 °C and mean annual precipitation of 713 mm) in August 2017. Pods were collected and placed in labeled paper bags; seed was extracted, manually cleaned, and stored under cool room conditions. In July 2018, seed was analyzed under ISTA system standards according to Alberta Government (2016), determining purity, weight, moisture content, germination (normal seedlings), and viability (tetrazolium salts test).
Seed morphology and anatomy
Morphological characteristics were evaluated on a random sample consisting of 25 seeds from a selected lot. Weight (Ohaus Scout® scale, precision 0.001 g), length, width and thickness (Truper® digital vernier, precision 0.01 mm) were determined on each seed. Shape was described based on the terminology referred to by Niembro (1988). Microscopic anatomy was described through microphotographs of the cross section of the seed coat. Images were collected from samples prepared with the following techniques: environmental and environmental supplemented with osmium tetroxide, to be viewed under the electron microscope (FEI Quanta 450) with the scanning electron microscope (SEM) technique (Sorrivas, Yañez, & Morales, 2014).
Scarification and breaking resistance
Seeds were subjected to chemical, mechanical and thermal scarification. These treatments emulate natural agents ending physical dormancy (Baskin & Baskin, 2014). We sought to simulate seed passage through digestive tract of wildlife, with chemical scarification; seed dragging on the ground, using sandpaper scarification; chewing and trampling by wildlife, using the breaking resistance test; and fire with thermal scarification.
Chemical scarification consisted of immersing the seed in 37 % (12 N) HCl for 30, 120, 150 and 180 min (treatments T1, T2, T3 and T4, respectively). Thermal scarification was carried out by introducing the seed in an oven at 80, 100, 120 and 140 °C, for 3 min (T5, T6, T7 and T8, respectively). Mechanical scarification (T9) of the seed of the two species of mesquite (Prosopis) was done with 150-gauge, fine-grained wood sandpaper (Truper® brand). For the three species of huizache (Vachellia), Fandeli® brand sandpaper was used for sheeting, 60 X-86 caliber. Sanding was done on one side of the seed until cover deterioration was noticed and cotyledons could be seen. An untreated control (T10) was also used.
The variable resistance by compression was evaluated on samples of 25 seeds per species with the Instron® universal testing machine (Norwood, Massachusetts, USA, model 2285H) and the Instron Bluehill software (Instron, 2006). Displacement velocity was 0.60 mm∙min-1 with a load capacity of 51.521 kN. The test was stopped when a crackle was detected caused by breaking the seed coat, which corresponds with the sudden “drop” of the curve in the graph generated by the computer program.
Sowing and Experimental design
Seeds were sown in plastic boxes (30 x 20 x 15 cm). Seed preparation included disinfection with 98 % alcohol until evaporation, then cream-colored felt cloth was placed as substrate and 80 mL of commercial fungicide Bravo 720® (active ingredient Chlorothalonil: Tetrachloroisoftalonitrile) at 10 % was applied.
The experiment for each species was carried out using a randomized complete block design with 10 treatments (regarding the levels of the factors chemical, mechanical, thermal scarification and a control; compression was considered a separate experiment). A total of 20 seeds (experimental unit) were sown in each germination box (block or repetition), for each of the 10 treatments. Each species involved one experiment with four blocks or repetitions. In total, 20 seeds were sown per experimental unit x 10 treatments x 5 species x 4 repetitions (blocks) = 4 000 seeds.
The boxes were placed in controlled environment chambers (Biotronette®) with a photoperiod (fluorescent light) of 12 h day and 12 h night, and a thermoperiod of 30 °C and 20 °C. The seed was considered germinated when the radicle length equaled the seed length.
Intensity of dormancy
To define the species with the most intense and versatile dormancy to natural agents, the following index was proposed. First, classes were assigned based on germination percentages: 1 = 0 to 67 %, 2 = 68 to 83 %, 3 = 84 to 100 %; for resistance, the intervals and rating value were: 1 = 341 to 450.4 N, 2 = 450.4 to 559.8 N, 3 = 559.8 to 669 N. The figures were summed and divided by the maximum total, equal to 12 (4 factors x the maximum possible rating [3]). The higher the index value (maximum = 1), the more intense the physical latency to agents.
Statistical analysis
Germination and pressure for breaking were analyzed with ANOVA, using the statistical program SAS® version 9.4 (Statistical Analysis System Inc., 2015). Means were compared with Tukey's multiple comparison of means test (α = 0.05). Germination data was transformed with the sine arc function because it is a binomial variable. The statistical model was:
where,
yijk = response value
µ = overall mean
Ti = effect of the i-th treatment
Bj = effect of the j-th block
εijk = independent and identically distributed experimental error with N (0, σ2).
Results
Seed lot quality
According to Table 1, seed lots of the species evaluated had a purity greater than 93 %; impurities were pod residues. The number of seeds per kilogram varied according to size in each species. Seeds of V. schaffneri were relatively large, with 7 400 seeds∙kg-1 (1 000 seeds weighed 135.14 g); the smallest were those of P. laevigata, with 24 313 seeds∙kg-1 (1 000 seeds weighed 41.13 g). Viability of V. farnesiana was 96 % and in the rest of the species reached 100 %. All seeds were orthodox, due to low moisture content (<6 %).
Table 1 Characteristics of seed lots of two legumes collected in Zacatecas.
| Type of analysis | Vachellia schaffneri | Vachellia pennatula | Vachellia farnesiana | Prosopis laevigata | Prosopis glandulosa |
|---|---|---|---|---|---|
| Purity (%) | 95 | 95 | 95 | 94 | 93 |
| No. seeds∙kg-1 | 7 400 | 7 448 | 14 368 | 24 313 | 22 306 |
| Weight of 1 000 seeds (g) | 135.1 | 134.3 | 69.6 | 41.1 | 44.8 |
| Moisture content (%) | 5.7 | 5.9 | 4.2 | 4.2 | 6.4 |
| Viability (%) | 100 | 100 | 96 | 100 | 100 |
Anatomy and morphological characteristics of the seed
The seed coat had cuticle, sclerenchyma and parenchyma. The seed coat was followed by the endosperm and the cotyledonary embryo (Figures 1 and 2). In all five species, the parenchyma showed a wall with linkage-like pores along the endosperm, which is present in different proportions of the seminal cavity, and the pleurogram is well marked with long areoles. However, there is a difference between the genuses studied; in Vachellia, the areola showed fracture lines and in Prosopis they are smooth, as in the rest of the seed coat.

Figure 1 Microphotographs of the seed of the species studied of Vachellia: a) cotyledon, b) endosperm and layers of its seed coat, c) spongy parenchyma, d) sclerenchyma and e) cuticle.
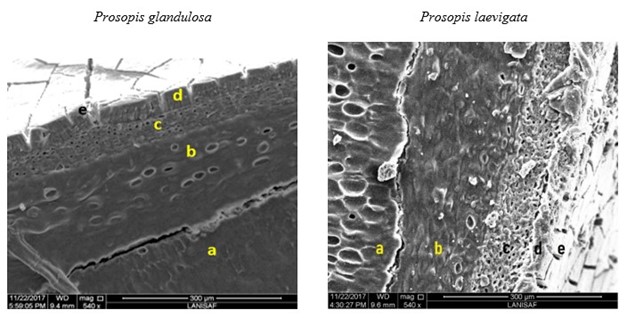
Figure 2 Microphotographs of the seed of the species studied of Prosopis: a) cotyledon, b) endosperm and layers of its seed coat, c) parenchyma, d) sclerenchyma and e) cuticle.
Table 2 indicates that seeds of Vachellia had greater length, width, thickness and weight compared to Prosopis seeds. Vachellia pennatula was the most voluminous and P. laevigata the smallest. With respect to shape, Vachellia seeds are oval, while Prosopis seeds are ovoid and less thick.
Table 2 Morphological characteristics of seeds of two legumes genus collected in Zacatecas.
| Features | Vachellia schaffneri | Vachellia pennatula | Vachellia farnesiana | Prosopis laevigata | Prosopis glandulosa |
|---|---|---|---|---|---|
| Length (mm) | 6.96 ± 0.69 | 7.75 ± 0.90 | 6.12 ± 0.60 | 6.26 ± 0.72 | 6.81 ± 0.89 |
| Width (mm) | 5.94 ± 0.5 | 5.50 ± 0.57 | 4.72 ± 0.39 | 4.62 ± 0.46 | 4.59 ± 1.06 |
| Thickness (mm) | 3.90 ± 0.23 | 3.75 ± 0.33 | 3.17 ± 0.27 | 2.60 ± 0.22 | 2.38 ± 0.20 |
| Weight (g) | 0.14 ± 0.13 | 0.14 ± 0.03 | 0.07 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 |
| Shape | Oblong | Oblong | Oblong | Ovoid | Ovoid |
| Color | Black | Brown | Olive green | Yellow brown | Yellow brown |
| Pleurogram (%) | 90 | 90 | 90 | 90 | 90 |
Seed color was determined subjectively by appreciation. The percentage of the pleurogram is with respect to the 100 % that would represent a complete oval.
Germination
Cumulative germination curve in the two mesquite species was very similar; their germination was fast and uniform, therefore, Figure 3 only shows germination of P. laevigata. In contrast, Figure 4 shows that germination in huizache (Vachellia) seeds was slower and slightly lower (also only one species is shown).

Figure 3 Cumulative germination curves in Prosopis laevigata seeds. Chemical scarification with HCl: T1 = 30 min, T2 = 120 min, T3 = 150 min, and T4 = 180 min; thermal scarification for 3 min: T5 = 80 °C, T6 = 100 °C, T7 = 120 °C and T8 = 140 °C; T9 = sanding; and T10 = control.
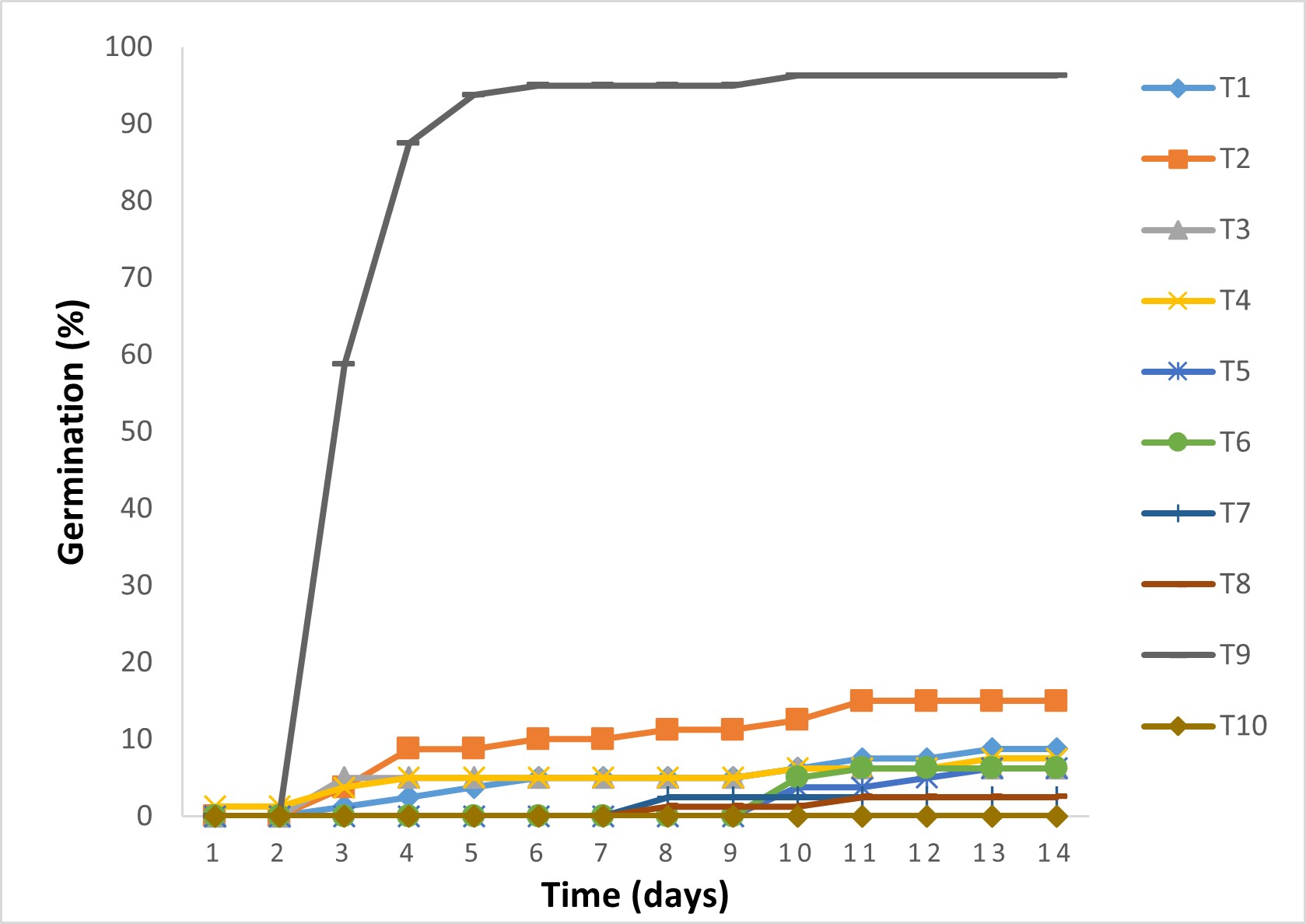
Figure 4 Cumulative germination curves in Vachellia schaffneri seeds. Chemical scarification with HCl: T1 = 30 min, T2 = 120 min, T3 = 150 min, and T4 = 180 min; thermal scarification for 3 min: T5 = 80 °C, T6 = 100 °C, T7 = 120 °C and T8 = 140 °C; T9 = sanding; and T10 = control.
According to Table 3, mechanical scarification (sanding) allowed 100 % of final germination in the two mesquite trees, being statistically higher to the other treatments. In P. laevigata, immersion in HCl for 180 min (72.5 %) was higher to the control (31.3 %) and to the rest of the treatments with chemical and thermal scarification. For Vachellia, the sanding treatment (81.2 to 96.2 % germination) significantly exceeded the rest of the treatments, which showed no differences with respect to the control (27.5 %).
Table 3 Germination with three scarification methods in seed of two legumes genus collected in Zacatecas.
| Method | Treatments | Germination (%) | ||||
|---|---|---|---|---|---|---|
| Vachellia schaffneri | Vachellia farnesiana | Prosopis glandulosa | Vachellia pennatula | Prosopis laevigata | ||
| Chemical (HCl) | 30 min | 7.5 b | 5.0 b | 50.0 b | 21.3 c | 28.8 d |
| 120 min | 15.0 b | 8.7 b | 48.7 b | 31.3 bc | 62.5 bc | |
| 150 min | 6.2 b | 5.0 b | 57.5 b | 33.8 bc | 57.5 bcd | |
| 180 min | 7.5 b | 3.7 b | 53.7 b | 35.0 bc | 72.5 b | |
| Thermal (3 min) | 80 °C | 6.2 b | 3.7 b | 56.2 b | 15.0 c | 30.0 d |
| 100 °C | 1.2 b | 7.5 b | 55.0 b | 31.3 bc | 40.0 cd | |
| 120 °C | 6 2 b | 3.7 b | 66.2 b | 40.0 bc | 58.8 bcd | |
| 140 °C | 3.7 b | 5.0 b | 71.2 b | 58.8 b | 57.5 bcd | |
| Mechanical | Sanding | 96.2 a | 81.2 a | 100.0 a | 92.5 a | 100 a |
| Control | Control | 1.2 b | 5.0 b | 53.7 b | 27.5 bc | 31.3 d |
In each species, germination values with different letters showed statistically significant differences between treatments according to Tukey's test (P ≤ 0.05).
Breaking resistance
Table 4 shows that there are differences in the values of resistance compression until breaking the seed coat. V. schaffneri and V. pennatula had the highest values (669 and 653 N, respectively) and V. farnesiana (482 N) was only higher than P. laevigata (341 N).
Table 4 Compression until breaking the seed coat of five legume species collected in Zacatecas.
| Species | Compression (N) | ||
|---|---|---|---|
| Mean | Minimum | Maximum | |
| Vachellia schaffneri | 669 a | 140 | 1 028 |
| Vachellia pennatula | 653 a | 349 | 1 350 |
| Vachellia farnesiana | 482 b | 215 | 840 |
| Prosopis glandulosa | 473 bc | 134 | 473 |
| Prosopis laevigata | 341 c | 188 | 758 |
Mean values with different letters showed statistically significant differences according to Tukey's test (P ≤ 0.05).
Intensity of dormancy
Based on the proposed index, the species with the most intense dormancy was V. schaffneri, because it showed greater resistance to mechanical breakage and chemical and thermal scarification, while the species with the lowest dormancy was P. glandulosa. The three Vachellia species had more intense physical dormancy compared to the two Prosopis species (Table 5).
Table 5 Intensity of dormancy in seeds of two legumes genus collected in Zacatecas.
| Species | Breaking resistance (Trampling) | Gastric juices (HCl) | Fire (Dry heat) | Dragging (Sanding) | Index |
|---|---|---|---|---|---|
| Vachellia schaffneri | 3 | 3 | 3 | 1 | 0.83 |
| Vachellia pennatula | 3 | 2 | 3 | 1 | 0.75 |
| Vachellia farnesiana | 2 | 2 | 2 | 1 | 0.58 |
| Prosopis laevigata | 1 | 2 | 2 | 1 | 0.5 |
| Prosopis glandulosa | 2 | 1 | 1 | 1 | 0.42 |
Discussion
Seed analysis and anatomy
It seems that the macroscopic anatomy of the seeds of V. farnesiana and V. pennatula has not been published, so their anatomical comparison was made with those of P. juliflora (Sw.) DC. (Niembro, 1988). Capparelli (2008) recognizes for P. chilensis and P. flexuosa DC.: epidermis, subepidermis, spongy parenchyma, palisade cells, albumen and cotyledon. The parts found in the seed, especially the seed coat, are consistent with those of other species of the genuses studied (Capparelli, 2008; Niembro, 1988) and similar to other legumes such as Glycine max L. Merr. (Shao, Meyer, Ma, Peterson, & Bernards, 2007) and Crotalaria juncea L. (Beltramini & Pascualides, 2017). The water impermeability of the seed coat in various legumes is provided by the palisade layer of lignified macrosclereids, which are immersed with phenol and suberin-type impermeable substances (Lazarević et al., 2017; Robles-Díaz, Flores, & Yañez-Espinosa, 2016; Steinbrecher & Leubner-Metzger, 2018). The seed of the five species studied clearly shows this layer of lignified and impermeable macrosclereids. Beltramini and Pascualides (2017) and Shao et al. (2007) report the presence of a layer of cells, the outermost layer of the endosperm neighboring the seed coat, called aleurone. This tissue may be another mechanism that helps regulate water entry to the seed once physical dormancy is overcome, as sudden imbibition causes damage and inhibits germination (Sato, Jitsuyama, Yamada, Liu, & Abe, 2019). This study was not able to clearly distinguish the aleurone layer in the species studied, at least with the technique used, but its possible presence is not ruled out.
Germination
Mesquite germination results coincided with those of Miranda et al. (2011), who also determined a potential germination of 100 % for P. juliflora in seeds without endocarp. Sánchez-Soto et al. (2016) found 98.9 % germination with abrasion, using sandpaper, in Caesalpinia platyloba S. Watson. Data from Ffolliott and Thames (1983) show that germination in species of Prosopis in Latin America is 95 %. Seeds of V. schaffneri from the Mixteca Alta of Oaxaca had 87 % germination by the sanding method (Martínez-Pérez, Orozco-Segovia, & Martorell, 2006). For V. farnesiana,Godínez-Álvarez and Flores-Martínez (2000) reached 86.7 %, and Maldonado-Arciniegas, Ruales, Caviedes, Ramírez, and León-Reyes (2018) had 45 % germination with the sanding of seeds of the South American species Vachellia macracantha (Humb. & Bonpl. ex Willd.) Seigler & Ebinger. These values are lower than those obtained in this research, possibly because the seed used in those experiments may have had lower viability, may not be so new or failed to provide the best environment for germination, such as temperature.
Chemical scarification
In North America, the seeds of P. velutina Wooton are part of the diet of species such as mice (Peromyscus), kangaroo rats (Dipodomis), wood rats (Neotoma), ground squirrels (Sciuridae), rock squirrels (Otospermophilus), cottontail rabbits (Leporidae), skunks (Mephitidae), quail (Cyrtonix montezumae Vigors), pigeons (Columbidae), ravens (Corvidae), hares (Lepus), raccoons (Procyon lotor Linnaeus), coyotes (Canis latrans Say), white-tailed deer (Odocoileus virginianus Zimmermann), mule deer (Odocoileus hemionus Rafinesque), wild turkey (Meleagris gallopavo Linnaeus), among others (Uchytil, 1990). Ecological relevance of endozoochory in seeds with physical dormancy is clear (Jaganathan, Yule, & Liu, 2016). The passage of the seed through the digestive tract of these species with gastric juices that include hydrochloric acid, scarify it, so chemical scarification with acids emulates such a natural process.
Browsing or consumption of mesquite and huizache fruits by wild or domesticated animals, such as ruminants, is an important factor for seed dispersal. The abomasum is the fourth and last compartment of the ruminant's stomach, where gastric juices containing HCl, among other compounds, are concentrated (Rodríguez-Carias & Valencia-Chin, 2007). Kneuper, Scott, and Pinchak (2018) found that the seed of P. glandulosa Torr. var. glandulosa is viably consumed and dispersed by livestock, while sheep and goats consume the fruit and reduce viability.
Germination results using HCl (chemical method) were low (28.8 to 72.5 %) for longer immersion times (up to 180 min), compared with 99 % germination of P. juliflora seeds without endocarp, scarified with H2SO4 for 5 to 40 min (Miranda et al., 2011) and with 100 % germination of P. velutina seeds, subjected to H2SO4 for 10 min (Martínez-Rodríguez, Rivera-Maya, & Santamaría-César, 2000). Also, in Acacia tortilis ssp. tortilis Forsk. (Hayne), from Egypt and Qatar, germination of the control (25 %) increased to 67, 70 and 75 % with chemical scarification (H2SO4 at 98 %) for 20, 25 and 30 min, respectively (El-Azazi, Sourour, Belal, & Khalifa, 2013). In contrast, D'Aubeterre, Principal, and García (2002) had 9 % germination in P. laevigata seeds scarified in H2SO4 for 8 min. The type of acid (sulfuric acid is stronger), immersion time and concentration used can be the difference between a high and low germination value. Adequate immersion time will maximize germination, but a very short immersion, e.g., <120 min for P. laevigata, will not affect the seed coat sufficiently, whereas a very long immersion time may damage the embryo. Ghassali, Salkini, Petersen, Niane, and Louhaichi (2012) tested boiling water, mechanical, and sulfuric acid treatments on 14 Acacia species and found that the best treatment was the chemical treatment because it produced maximum germination in seven species (Acacia farnesiana [L.] Willd., A. victoriae Benth., A. deanei ssp. deanei [R. T. Baker] M. B. Welch et al., A. deanei ssp. paucijuga [F. Muell. ex N. A. Wakef.], A. karroo Hayne, A. pruinocarpa Tindale and A. saligna [Labill.] H. L. Wendl).
Thermal scarification
In a wildfire, fire (≥250 °C) consumes all plant material, but if seeds are little buried, they are protected; however, they receive lower temperatures that can degrade integuments or scarify hard seed coats of species such as legumes (Scott, Bowman, Bond, Pyne, & Alexander, 2014).
Thermal scarification caused no higher germination than the control in the species studied, but neither affected the seeds adversely in this study. It is possible that, at higher temperatures or with higher exposure times, germination of the species studied could be stimulated; for example, in the legume Stylosanthes montevidensis Vogel from a Brazilian grassland, its germination was stimulated at 120 °C for 1 min (Fidelis, Daibes, & Redondo-Martines, 2015). Even Liyanage and Ooi (2015) found in an Australian scrubland that temperatures up to 100 °C stimulated germination of Acacia linifolia (Vent.) Willd., Viminaria juncea (Schard.) Hoffmans., Aotus ericoides (Vent.) Don, Dillwynia floribunda Sm. and Bossiaea heterophylla Vent.; however, there was variability in dormancy intensity and response to heat treatments. Also, seed of the Brazilian legume Mimosa calodendron Mart. had higher germination than the control when subjected to heat treatments (Campos-Dayrell, Goncalves-Alvym, Negreiros, Fernandes, & Oliveira-Silveira, 2015). Prosopis juliflora had higher germination at a constant temperature of 35 °C for 24 h (Ffolliott & Thames, 1983) and P. velutina from Durango germinated 71 % after 10 min of immersion in hot water (85 °C), while germination was 0 % for the control (Martínez et al., 2000).
In another study carried out by Sabiiti and Wein (1987) a 65 % germination rate was achieved in a seed bank of V. siberiana (DC.) Kyal. & Boatwr. collected from the soil of burned areas, compared to 10 % of seed from unburned areas. On the other hand, Zalamea, Sarmiento, Arnold, Davis, and Dalling (2015) indicate that, in tropical forests of Panama, variation in soil temperature was enough to break physical dormancy in the seed of four pioneer tree species. Smoke from forest fires was found in some species of the genuses studied to favor germination. In Australia, this was the case for Acacia hebeclada DC. and in South Africa, for Acacia mearnsii De Wild. (Kulkarni, Sparg, & Van Staden, 2007); however, the possible effect of smoke was not analyzed in this study.
Breaking resistance
During consumption by large herbivores or livestock, the seed can be subjected to strong physical pressure by chewing but can also be trampled. The fracture strength of the seed coat is an indicator of the resistance to compression that the seed offers to these factors. Hence the interest in establishing the breaking resistance of the seed coat. Resistance values measured on the universal testing machine suggest that seeds scarified by livestock, sheep, goats or wild animals trampling should be few, because the live weight of small animals is in the range of 70 to 75 kg, although the conditions for this to happen can occur (on rocks, for example). However, large herbivores and livestock chewing is powerful and can affect the seed coat mechanically. The present study establishes the pressure required to break the seed coat and overcome physical dormancy in the five species studied.
Mechanical scarification is compared with three natural agents: hydrocoral dispersion, surface runoff and trampling or chewing by animals. Low rainfall usually occurs as thunderstorms in arid and semi-arid areas. This causes some seeds to be washed away by surface runoff and to collide and rub against other materials, mainly rocks and soil particles. During this dragging it is possible that seeds suffer light scratches or that small cracks are formed; that is to say, a natural scarification (personal comments provided by the authors). In the natural environment, the combination of breaking by trampling or chewing and scarification may happen in the digestive tract of animals. Seeds may be chewed, consumed and defecated by animals and subsequently trampled by others. These can be benefited or harmed, depending on the intensity of each treatment and the physical dormancy of the species involved.
Conclusions
Mechanical scarification was the most effective for germination of all species. Germination by chemical scarification was highest only at 180 min in Prosopis laevigata. This study proposes an index of intensity and versatility of physical dormancy to environmental factors, but it only works for comparisons between species in the same experiment. There is a direct relationship between seed size and dormancy intensity. The seeds of huizaches (Prosopis) had higher dormancy rates than those of mesquite (Vachellia), so huizache seeds are more adapted to survive to natural scarification agents. This study found that, thanks to their physical dormancy, the seed coat of the seeds of the species studied provides protection against physical, biotic and fire agents.











 text in
text in 


