Highlights:
The mushrooms studied: Lactarius indigo, Hypomyces lactifluorum and Ramaria flava.
The effect of heat treatment was evaluated at 50 and 92 °C at 10 to 60 min intervals.
Degradation kinetics of phenolic compounds and antioxidants was first order.
The mushrooms retained more than 50 % of their antioxidant compounds after heat treatment.
L. indigo, H. lactifluorum and R. flava have nutraceutical potential.
Introduction
In Mexico, since pre-Hispanic times, wild edible mushrooms have been a food resource for various ethnic groups in isolated communities (Ruan-Soto, Ordaz-Velázquez, García-Santiago, & Ovando, 2017). According to Garibay-Orijel and Ruan-Soto (2014), there are about 371 taxa of wild edible mushrooms in Mexico. In the Sierra Norte de Puebla, Mexico, some Nahua communities maintain the tradition of identifying and collecting edible mushrooms for self-consumption and commercialization in local markets (Contreras, Vázquez, & Ruan, 2018). Women known as traditional mushroom collectors (hongueras tradicionales) are the main protagonists in collecting, processing and commercialization of wild mushrooms; they are also in charge of sharing ancestral knowledge in order to identify edible and poisonous species and for the recognition of symbiotic associations between mushrooms and trees (Contreras et al., 2018; Garibay-Orijel, Ramírez-Terrazo, & Ordaz-Velázquez, 2012; Jasso-Arriaga, Martínez-Campos, & Dorantes-Coronado, 2019). In several communities surrounding the municipality of Zacapoaxtla, collecting and commercialization of mushrooms in rainy season represent an important household income (Estrada-Martínez, Guzmán, Cibrián-Tovar, & Ortega, 2009).
The mushrooms Lactarius indigo (Schwein.) Fr. (hongo azul), Ramaria flava (Schaeff.) Quél. (changle) e Hypomyces lactifluorum (Schwein.) Tul. & C. Tul. (lobster mushroom), due to their flavor, consistency and color, have high demand and acceptance in the market, although there is the only opportunity to consume them during the rainy season. Hypomyces lactifluorum of the Hypocreaceae family is the result of parasitism by the ascomycete H. lactifluorum (Nectriaceae; Hypocreales) on Russula spp. or Lactarius spp.; the fungus grows on the host's basidiocarp, deforming its cap, stem and gills. This species is highly demanded in popular markets for its color and flavor (Guzmán, 2008; Rochon, Paré, Khasa, & Fortin, 2009). The mushroom L. indigo of the Russulaceae family ha+ been inventoried in the states of Chiapas, Hidalgo, and Querétaro (León-Guzmán, Silva, & López, 1997; López-Vázquez, Prieto-García, Gayosso-Canales, Otazo, & Villagómez, 2017; Ruan-Soto, 2018); the aqueous extract of this species shows antibacterial and cytotoxic activity (Ochoa-Zarzosa, Vázquez-Garcidueñas, Robinson-Fuentes, & Vázquez-Marrufo, 2011). As for R. flava (Gomphaceae family), the ethanolic extract of the fruiting bodies exhibits moderate antibacterial and antifungal activity (Liu, Wang, Zhao, & Wang, 2013).
Mushrooms are characterized by good quality protein, high dietary fiber and low-fat content (Agrahar-Murugkar & Subbulakshmi, 2005; Barros, Venturini, Baptista, Estevinho, & Ferreira, 2008; Wang et al., 2014). Moreover, mushrooms contain primary vitamins such as thiamine, riboflavin, niacin, tocopherol, and vitamin D; however, vitamin D is not found in cultivated mushrooms (Cheung, 2010; Mattila et al., 2001; Sahagún, 2020). Among the bioactive substances present in fungi are phenolic compounds (flavonoids, phenolic and cinnamic acids), tocopherols, ascorbic acid and carotenoids. These substances protect cells from oxidative damage produced by free radicals (Ferreira, Barros, & Abreu, 2009; Sari, Prange, Lelley, & Hambitzer, 2017). Other compounds of interest are β-glucans; these polysaccharides stimulate the immune system, inhibit tumor cells, control blood lipid concentration, and are a source of prebiotics that stimulate the growth of gut microbiota (Liu et al., 2012; Sawangwan, Wansanit, Pattani, & Noysang, 2018; Vaz et al., 2010). Therefore, mushrooms are recognized as nutraceutical foods (Barros et al., 2008).
The processing and preservation of wild edible mushrooms for consumption, either roasted or steamed, most of the time involves heat treatment (Haro-Luna, Ruan-Soto, & Guzmán-Dávalos, 2019), which includes the simultaneous transfer of heat and moisture; therefore, it is important to find a kinetic model to explore its influence on phenolic content. In cultivated mushrooms, such as Pleurotus ostreatus (Jacq.) P. Kumm. and Agaricus bisporus (J. E. Lange) Imbach, Jaworska, Pogòn, Bernas, and Duda-Chodak (2015) indicated that phenolic compounds are degraded during cooking process. The aim of this study was to evaluate nutritional content and heat treatment effect on phenolic compounds and antioxidant capacity of L. indigo, R. flava, and H. lactifluorum. These species were selected based on the traditional mycological knowledge of women collectors and sellers of wild edible mushrooms in the market of Zacapoaxtla, Puebla.
Materials and Methods
Mycological material
To select the study material, a tour was made in the market of the municipality of Zacapoaxtla to find the "traditional mushroom collectors", holders of traditional mycological knowledge. These people provided guidance and accompaniment in the collection (September 2018) of about 20 species of edible mushrooms, from which L. indigo, H. lactifluorum and R. flava were chosen based on their abundance and frequency of consumption (Figure 1).
Collection was done in the community La Loma, municipality of Zacapoaxtla, Puebla, Mexico (19° 49' 38.6” N and -97 36' 10.7” W at 2 140 m) (Figure 2). The vegetation type is temperate pine-oak forest. Mature individuals of Pinus pseudostrobus Brongn. were found in the area; dominance of oaks such as Quercus castanea Née, Q. laurina Bonpl., Q. crassifolia Bonpl. and Q. rugosa Née; individuals of Alnus acuminata Kunth, Buddleja sp. and Juniperus spp. near roads and highways; and presence of herbaceous plants of the families Asteraceae, Lamiaceae and Fabaceae.
Fungal species were determined based on the following literature: Guzmán (1977); Chacón, Guzmán, Montoya-Bello, and Bandala-Muñoz (1995); Montoya-Bello (1987); Mata et al. (2003); and Montoya and Bandala (2005). Descriptions were made from sections of fruiting bodies for observation under stereoscopic and compound microscopy; subsequently, stains were made with potassium hydroxide and lactophenol blue. The scientific names derived from observations were checked against the ethnomycological study of Contreras et al. (2018) and the currency of the scientific name was verified in the Index Fungorum (2021) database. One specimen of each species was deposited in the herbarium collection of the Forest Sciences Division of the Universidad Autónoma Chapingo (UACh).

Figure1. Mushroom species collected in the community La Loma, municipality of Zacapoaxtla, Puebla, Mexico: a) Hypomyces lactifluorum, b) traditional mushroom collectors, c) Ramaria flava, d) Lactarius indigo and e) collection area.
Figure 2 Location of the area where wild edible mushrooms were collected in the municipality of Zacapoaxtla, Puebla, Mexico.
Approximately 2 kg of fruiting bodies were collected from each of the three species of wild edible mushrooms. Agaricus bisporus and P. ostreatus were purchased from a supermarket in Mexico City; these cultivated mushrooms were studied for direct comparison with the wild species. The fruiting bodies were cleaned of excess soil and leaf litter; the basal part of the stipe, when damaged, was removed with a small blade. All specimens were lyophilized and kept frozen at -18 °C until analysis.
Reagents and instrumentation
Folin-Ciocalteu reagents, sodium carbonate, sodium acetate trihydrate 2,4,6-tripyridyl-s triazine (TPTZ), iron(III) chloride hexahydrate, catechin, aluminum chloride, potassium acetate, potassium persulfate, 2,2-azinobis,3-ethylbenzothiazoline-6-sulfonic acid (ABTS) and 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic (trolox) and gallic acids are Sigma Aldrich products (St. Louis, Mo., USA). Absorbance for the quantification of phenols, flavonoids and antioxidant capacity were measured on a Synergy 2 Microplate Reader, Gen5 software (BioTek Instruments Inc., Winooski, VT, USA).
Nutritional properties and nutraceutical potential
The proximate composition of the lyophilized mushrooms was determined in triplicate according to AOAC (2005) methods. Crude protein (N x 6.25) was estimated by the Kjeldahl method, ether extract was obtained by Soxhlet and ash content was determined by ashing at 550 °C.
The nutraceutical composition was evaluated in vitro; for this
purpose, extracts were obtained with 80 % methanol following the method reported
by Hernández-Rodríguez et al. (2016). The
total phenolic content in each extract was quantified by the Folin-Ciocalteu
method adapted to microplates (Hernández-Rodríguez et al., 2016). The calibration curve was
prepared from a stock solution of gallic acid in the linear range of
concentrations from 2.5 to 29.0 µg∙mL-1. Results were expressed as
milligram equivalents of gallic acid per gram of sample on a dry basis (mg EAG∙
Determination of total carotenoids
Carotenoids were determined in H. lactifluorum according to the
procedure of Camargo, Xavier, Alves, Flanch, and
Ruffo (2015) with some modifications. The lyophilized sample (0.2 g)
and protected from light was mixed with 10 mL of a hexane/acetone solution (6:4,
v/v), vortexed for 1 min and incubated for 10 min at 150 rpm. The supernatant
was separated by filtration and the ultraviolet-visible spectrum (UV-VIS) was
obtained in which a maximum was observed at 450 nm, corresponding to
carotenoids. The range of the β-carotene calibration curve was from 0.5 to 4.0
µg∙mL−1. Results were expressed as milligrams of β-carotene per
gram of sample on a dry basis (mg EβC∙
Antioxidant capacity
Antioxidant capacity of the extracts was evaluated by ABTS (Re et al., 1999) and FRAP (ferric reducing ability of
plasma; Benzie & Strain, 1996) assays
adapted to microplates. Trolox calibration curves were prepared in concentration
ranges from 5.0 to 60 μM and from 3.8 to 46.0 μM, for ABTS and FRAP,
respectively. Results were expressed as equivalent micromoles of trolox per gram
of sample on a dry basis (µm ET∙
Heat treatment
The degradation kinetics of total phenolic content and the loss of antioxidant capacity of mushrooms were evaluated at 50 °C (temperature close to the onset of degradation of phenolic compounds; Zhou et al., 2016) and 92 °C (boiling point of water at the experimental site) for 0, 10, 20, 30, 30, 40, 50 and 60 min for each temperature. For each species, a portion (0.5 g) previously lyophilized was transferred to a Pyrex® tube and hydrated with 10 mL of distilled water. Samples were placed in a thermostatic water bath preheated to each of the study temperatures and removed at time intervals of 10 to 60 minutes. Samples were immediately cooled in an ice water bath and lyophilized; total phenolic content and antioxidant capacity were determined in triplicate for each sample.
Data analysis
Phenols, flavonoids, and total carotenoids, as well as antioxidant capacity were determined in triplicate and means, and standard deviation were estimated. Nutrient content was compared between species using a completely randomized design and Tukey's test of means (P ≤ 0.05) was used as the analysis statistic.
The reaction order of the degradation kinetics of phenolic compounds was determined by plotting the concentration of total phenols with respect to time, testing first and second order kinetic models:
where,
k = degradation rate constant (mol∙g-1∙min-1) of antioxidant compounds during heat treatment.
t = treatment time (s)
CA and CA0 = concentrations of phenolic compounds at time t and time 0, respectively.
Results and Discussion
Table 1 shows the results of the proximate analysis of the wild edible mushrooms under study. Moisture content (87 to 90 %) showed no significant differences and was lower (P ≤ 0.05) than that reported for the same species collected in Querétaro, Mexico (Yahia, Gutiérrez-Orozco, & Moreno-Pérez, 2017). Ash content in R. flava was lower (P ≤ 0.05) than in H. lactifluorum and L. indigo; in general, the three species showed higher contents than wild and commercial mushrooms (0.17 to 1.17 %) evaluated by Álvarez-Parrilla, de la Rosa, Martínez, and González (2007) in Chihuahua, Mexico. On the other hand, the dietary fiber content of wild mushrooms (7.58 to 14.64 %) was lower compared to that found by Manzi, Aguzzi, and Pizzoferrato (2001) in A. bisporus and P. ostreatus. With respect to crude protein, H. lactifluorum had the lowest content; however, it was higher than that determined in A. bisporus (13.75 %) and P. ostreatus (8.58 %) by Jaworska, Pogoń, Bernaś, and Duda-Chodak (2015), and that found in R. flava (14.47 %) and L. indigo (13.42 %) by Silva and Lo (1997). These differences could be explained by the edaphoclimatic conditions and the type of substrate where the mushroom grows (Kalač, 2013). According to Cheung (2010), the protein quality of mushrooms is better than vegetable protein, because mushrooms contain all protein amino acids; therefore, such specimens represent an alternative food for vegetarians. The macronutrient profile revealed that all three species are an important source of protein with low fat content. These properties have been described for other wild edible mushrooms (Cheung, 2010).
The nutrient composition of the species studied is very important to maintain a balanced diet and represents an alternative to the lack of resources of local consumers for the acquisition of conventional foods rich in protein.
Table 1. Proximal composition (grams per 100 g dry weight) of wild edible mushrooms collected in Zacapoaxtla, Puebla, Mexico.
| Species | Ash | Crude protein | Total lipids | Crude fiber | Carbohydrates |
|---|---|---|---|---|---|
| Lactarius indigo | 12.93 ± 1.79 a | 23.29 ± 1.30 a | 3.19 ± 0.25 a | 14.64 ± 0.22 a | 45.95 ± 0.00 c |
| Ramaria flava | 7.77 ± 0.46 b | 24.02 ± 0.42 a | 1.20 ± 0.34 b | 7.58 ± 0.09 c | 59.43 ± 0.00 a |
| Hypomyces lactifluorum | 15.06 ± 0.03 a | 20.27 ± 0.78 b | 2.67 ± 0.15 a | 12.08 ± 0.36 b | 49.92 ± 0.00 b |
Different letters in each column indicate significant differences according to Tukey's test (P < 0.05). Values are average ± standard deviation calculated for three replicates per sample.
Nutraceutical composition
Table 2 shows that R.
flava had higher phenolic content (P < 0.05)
than H. lactifluorum and L. indigo; however,
the highest concentration was found in A. bisporus which,
together with P. ostreatus, were studied for direct comparison
with wild species. Total phenol concentrations were lower compared to those
determined by Yahia et al. (2017) for
L. indigo, H. lactifluorum and R.
flava (6.6, 6.33 and 7.26 mg EAG∙
Table 2 Total bioactive compounds and antioxidant capacity (expressed on a dry basis) of edible wild mushrooms collected in Zacapoaxtla, Puebla, and of two commercial species (Agaricus bisporus and Pleurotus ostreatus).
| Total Phenols (mg EAG gdb -1) | Total carotenoids (mg EβC gdb -1) | Total flavonoids (mg EC gdb -1) | ABTS (µmol ET gdb -1) | FRAP (µmol ET gdb -1) | |
|---|---|---|---|---|---|
| Lactarius indigo | 2.92 ± 0.02 c | NC | 0.83 ± 0.05 b | 10.42 ± 0.02 c | 9.03 ± 0.01 c |
| Ramaria flava | 4.40 ± 0.01 b | NC | 2.25 ± 0.04 a | 23.65 ± 0.02 b | 20.17 ± 0.02 a |
| Hypomyces lactifluorum | 2.98 ± 0.04 c | 0.117 | ND | 5.78 ± 0.03 e | 3.75 ± 0.02 d |
| Agaricus bisporus | 6.81 ± 0.03 a | NC | NC | 26.64 ± 0.04 a | 18.79 ± 0.03 b |
| Pleurotus ostreatus | 1.57 ± 0.01 d | NC | NC | 7.47 ± 0.01 d | 2.47 ± 0.01 e |
Assays for antioxidant capacity (µmol ET: micromoles trolox equivalents): ABTS = 2,2-azinobis,3-ethylbenzothiazoline-6-sulfonic acid, FRAP = ferric reducing antioxidant power. mg EAG, mg EβC, mg EC: milligram equivalents of gallic acid, catechin and carotene, respectively. ND = not detected, NC = not quantified. Values with different letters in each column indicate significant differences according to Tukey's test (P < 0.05). Values are average ± standard deviation calculated for three replicates per sample.
On the other hand, as shown in Table 2,
the concentration of total flavonoids in R. flava, with respect
to the total phenolic content, corresponds to 51.1 %, while in H.
lactifluorum, these compounds were not detected, probably due to
the extraction method used. Flavonoid concentrations in L.
indigo and R. flava were lower than those obtained
by Yahia et al. (2017) for the same
species, but in the case of L. indigo (catechin-based, 0.83 mg
EC∙
The UV-VIS spectrum of the hexane/acetone extract of H.
lactifluorum showed a maximum at a wavelength of 450 nm,
characteristic of carotenoids (Camargo et al.,
2015). The carotenoids content in this mushroom was higher than that
found in wild edible mushrooms from Poland and Portugal (Barros, Ferreira, Queirós, Ferreira, & Baptista, 2007;
Robaszkiewichz, Bartosz, Lawrynowicz, &
Soszynski, 2010) and in fruits such as Artocarpus
heterophyllus Lam (0.0018 to 0. 0079 mg∙
Antioxidant capacity
Antioxidant agents reduce free radicals by two mechanisms: by electron transfer or hydrogen atom transfer. The ABTS and FRAP assays are used to measure free radical reduction by electron transfer mechanism (Ozgen, Reese, Tulio, Scheerens, & Miller, 2006); therefore, these two methods were used to corroborate antioxidant capacity in the polar extracts of mushrooms under study. Mushrooms showed significant difference in antioxidant capacity in each of the assays; A. bisporum and R. flava had the highest values, while H. lactifluorum and P. ostreatus showed the lowest (Table 2). According to the results described by Yahia et al. (2017), R. flava also had the highest antioxidant capacity, followed by L. indigo and H. lactifluorum. Since carotenoids are compounds with antioxidant properties it would be advisable to test antioxidant capacity in non-polar extracts.
In general, samples with higher antioxidant capacity also had higher phenolic content (Table 2). According to Cheung, Cheung, and Ooi (2003), antioxidant properties of mushrooms are mainly attributed to low molecular weight compounds such as flavonoids, phenolic and cinnamic acids. In recent years, the demand in the consumption of bioactive compounds has been increasing, therefore, mushrooms are a good alternative.
Phenolic compounds in plant-based foods (Faller & Fialho, 2009; Kalogeropoulos, Grigorakis, Mylona, Falirea, & Andrikopoulos, 2006) and in mushrooms (Jaworska et al., 2015) can be affected by thermal processing. According to Patras, Bruton, Tiwari, and Butler (2011) it is advisable to employ a kinetic model that predicts the influence of food processing on critical quality parameters. The reaction order corresponding to the degradation kinetics of compounds in fungi was determined by plotting the concentration of total phenols against time (0 to 60 min) at 50 and 92 °C, testing first and second order kinetic models, until a linear relationship was found. The kinetic parameters were obtained by applying the equations Ln(CA/CA0) = -kt (first order) and (1/CA -1/CA0) = kt (second order). According to Table 3, both models had acceptable coefficients of determination; however, the first order mathematical model had higher coefficients of determination.
Table 3 Rate constants (k) of degradation of phenolic compounds in wild fungi Hypomyces lactifluorum, Ramaria flava and Lactarius indigo.
| Species | Temperature(ºC) | First order | Second order | ||
|---|---|---|---|---|---|
| k* | R2 | k* | R2 | ||
| H. lactifluorum | 50 | 4 x 10-5 | 0.9930 | 1 x 10-5 | 0.9910 |
| 0.9916 | 0.9883 | ||||
| 0.9895 | 0.9868 | ||||
| 92 | 0.0002 | 0.9828 | 8 x 10-5 | 0.9535 | |
| 0.9798 | 0.9463 | ||||
| 0.9821 | 0.9528 | ||||
| R. flava | 50 | 6 x 10-5 | 0.9926 | 2 x 10-5 | 0.9884 |
| 0.9857 | 0.9805 | ||||
| 0.9868 | 0.9799 | ||||
| 92 | 0.0001 | 0.9885 | 3 x 10-5 | 0.9939 | |
| 0.9908 | 0.9936 | ||||
| 0.9887 | 0.9909 | ||||
| L. indigo | 50 | 7 x 10-5 | 0.9675 | 3 x 10-5 | 0.9589 |
| 0.9713 | 0.9632 | ||||
| 0.9769 | 0.9697 | ||||
| 92 | 0.0001 | 0.9963 | 4 x 10-5 | 0.9982 | |
| 0.9964 | 0.9894 | ||||
| 0.9941 | 0.9900 | ||||
*Values shown are the average of three replicates.
Likewise, degradation kinetics of antioxidant capacity of mushrooms were determined at 50 and 92 °C with intervals of 10 to 60 min. The rate constants of antioxidant capacity loss for each species are shown in Table 4. Application of the first-order kinetic model had higher correlation coefficients.
Table 4 Rate constants (k) of degradation of antioxidant capacity in wild mushrooms Hypomyces lactifluorum, Ramaria flava and Lactarius indigo.
| Species | T (ºC) | Parameter | First order | Second order | ||
|---|---|---|---|---|---|---|
| k* | R2 | k* | R2 | |||
| H. lactifluorum | 50 | ABTS | 4 x 10-5 | 0.9853 | 8 x 10-5 | 0.983 |
| 0.9907 | 0.991 | |||||
| 0.9926 | 0.993 | |||||
| FRAP | 8 x 10-5 | 0.9753 | 2 x 10-5 | 0.9638 | ||
| 0.9659 | 0.952 | |||||
| 0.9796 | 0.9694 | |||||
| 92 | ABTS | 0.0566 | 0.994 | 0.0117 | 0.9903 | |
| 0.9942 | 0.9929 | |||||
| 0.9913 | 0.9921 | |||||
| FRAP | 0.0001 | 0.9921 | 4 x 10-5 | 0.9751 | ||
| 0.9891 | 0.9705 | |||||
| 0.9912 | 0.9742 | |||||
| R. flava | 50 | ABTS | 6 x 10-5 | 0.9803 | 3 x 10-6 | 0.9709 |
| 0.9802 | 0.9708 | |||||
| 0.9833 | 0.9744 | |||||
| FRAP | 8 x 10-5 | 0.9409 | 5 x 10-6 | 0.9275 | ||
| 0.9411 | 0.9281 | |||||
| 0.9371 | 0.9235 | |||||
| 92 | ABTS | 0.0001 | 0.992 | 6 x 10-6 | 0.9921 | |
| 0.9907 | 0.9898 | |||||
| 0.9926 | 0.9911 | |||||
| FRAP | 0.0003 | 0.9423 | 3 x 10-5 | 0.8227 | ||
| 0.943 | 0.8247 | |||||
| 0.942 | 0.8221 | |||||
| L. indigo | 50 | ABTS | 7 x 10-5 | 0.9854 | 7 x 10-6 | 0.9819 |
| 0.9847 | 0.9784 | |||||
| 0.9865 | 0.9818 | |||||
| FRAP | 5 x 10-5 | 0.9753 | 6 x 10-6 | 0.9656 | ||
| 0.982 | 0.9734 | |||||
| 0.9838 | 0.9767 | |||||
| 92 | ABTS | 9 x 10-5 | 0.9778 | 1 x 10-5 | 0.9797 | |
| 0.9793 | 0.9807 | |||||
| 0.9774 | 0.9793 | |||||
| FRAP | 8 x 10-5 | 0.9905 | 1 x 10-5 | 0.9915 | ||
| 0.9854 | 0.9879 | |||||
| 0.99 | 0.9907 | |||||
*Values shown are the average of three replicates. Assays to determine antioxidant capacity: ABTS = 2,2-azinobis,3-ethylbenzothiazoline-6-sulfonic acid, FRAP = ferric reducing antioxidant power.
Figure 3 shows the linear behavior when the first order kinetic model is applied, both of total phenolic content and antioxidant capacity (ABTS and FRAP), in the species under heat treatment of 50 and 92 °C from time 0 to 60 minutes. The same trend was observed in the three variables evaluated, as the cooking time and temperature increased, degradation of phenolic and antioxidant compounds also increased. H. lactifluorum showed greater degradation of total phenols when a temperature of 92 °C was applied for 60 min and R. flava showed a greater decrease in antioxidant capacity at a cooking temperature of 92 °C.
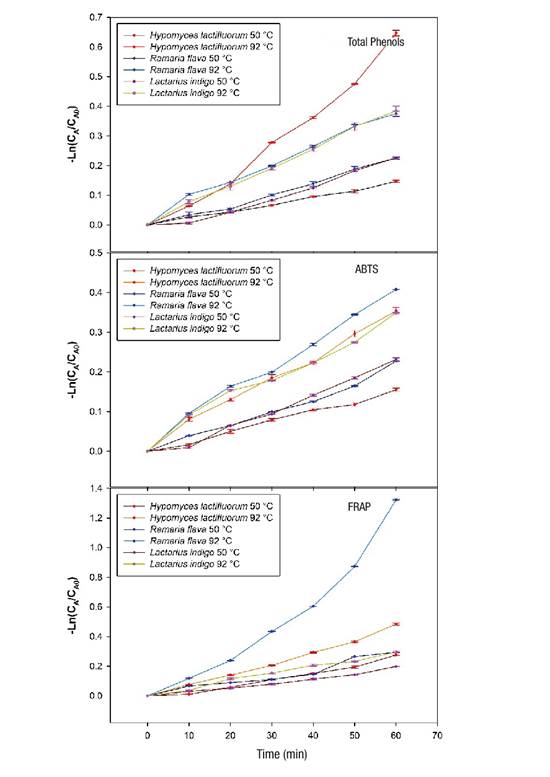
Figure 3 Loss of total phenolic content and antioxidant capacity (ABTS [2,2-azinobis,3-ethylbenzothiazoline-6-sulfonic acid] and FRAP [ferric reducing antioxidant power] assays) in wild edible mushrooms at two temperatures during 60 min of heating. CA and CA0: concentrations of the compounds (phenolics and antioxidants) at time t and time 0, respectively.
According to the results, the losses of total phenolic content were 14 % for H. lactifluorum and 21 % for R. flava under 50 °C conditions for 60 min, but at higher temperature (92 °C, 60 min), the reduction ranged from 31 % for L. indigo to 48 % for H. lactifluorum. These losses are lower than those reported by Jaworska et al. (2015) for A. bisporus (38 %) and P. ostreatus (65 %) cooked in stew. A study by Öztürk, Öztürk, and Duru (2014), shows that before cooking, the methanolic extract of R. flava contained 5.9 mg of phenols per gram of extract and, after cooking, the concentration increased to 9.84 mg of phenols per gram of extract; these results differ from those obtained in the present study. The application of heat treatments to foods rich in antioxidants decreases their content, due to the thermosensitive nature of these compounds. According to Blessington et al. (2010), the loss differences observed could be related to the chemical nature of the phenolic compounds of each species and to the complexity of the matrix in which they are bound to biomolecules such as carbohydrates, fat or proteins.
Concerning antioxidant capacity, in the case of the FRAP assay, the range of loss varied between 26 % for L. indigo and 74 % for R. flava, while for ABTS, the loss was 29 % for L. indigo and 34 % for R. flava. The loss of antioxidant capacity was lower than that reported by Jaworska et al. (2015) for A. bisporus (50.34 % [ABTS] and 53.55 % [FRAP]) and P. ostreatus (53.39 % [ABTS] and 48.52 % [FRAP]).
The kinetic study of the decrease in total phenolic content and antioxidant capacity of the mushrooms evaluated is very useful for the optimization of cooking temperatures and times; for example, according to information provided by people from the municipality of Zacapoaxtla, Puebla, the preparation of the mushrooms collected consists of a boiling heat treatment for 20 minutes. With this information, it is possible to estimate the loss of total phenolic content and antioxidant capacity of each species (Tables 3 and 4); in addition, it could be recommended to lower the cooking temperature for the preservation of bioactive compounds.
Conclusions
Lactarius indigo, Hypomyces lactifluorum and Ramaria flava represent an important source of nutritional and bioactive compounds beneficial to health. The mushrooms had high protein content (20 to 24 %) and low lipid content (1 to 3.2 %). Regarding the content of phenolic compounds (2.9 to 4.4 mg gallic acid equivalents), H. lactifluorum had the highest degradation. On the other hand, R. flava showed the highest antioxidant capacity, which decreased as temperature and cooking time increased; however, all three species retained more than 50 % of their antioxidant properties after thermal processing. To avoid or reduce the degradation of bioactive compounds, it is recommended to reduce the cooking temperature, which is close to or equal to boiling temperature. It is important to generate strategies for the conservation, reproduction, and commercialization of wild mushrooms, and to position them as a functional seasonal food that combines social development, women's empowerment and forest conservation.
Acknowledgments
The authors thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) and the Dirección General de Investigación y Posgrado of the Universidad Autónoma Chapingo for providing funding. The authors also thank the women mushroom collectors who participated in the search for the species studied and thanks to Dr. Emma Estrada Martínez for her guidance in the identification of the species studied.











 texto em
texto em 


