Highlights:
Pinus pseudostrobus was in contact with Gloeophyllum trabeum for nine months.
The wood was resistant to degradation for G. trabeum, considering only weight loss.
G. trabeum affects the chemical and mechanical properties in wood.
The static bending and perpendicular compression to the grain suffer the greatest mechanical losses.
The durability of the wood should not be classified only by its weight loss.
Introduction
Fungi are eukaryotic organisms with unicellular and multicellular aggregation; they are heterotrophs with diverse structure and functionality. Their more than 1.5 million species impact natural and artificial ecosystems in many ways. Within the wide fungal variety, xylophages use the major structural components of wood and metabolize them (Hiscox, O'Leary, & Boddy, 2018; Hunt et al., 2018). Two main groups of xylophagous fungi have been recognized. Those included in the Basidiomycota division and those belonging to the Ascomycota group. The organisms that can cause deterioration are white-rot fungi, soft-rot fungi, and brown-rot fungi (Krah et al., 2018). The latter being the most aggressive, caused by basidiomycete organisms e.g., G. trabeum, Laetiporus portentosus (Berk.), and Fomitopsis lilacinogilva (Berk.), which degrade cellulose and hemicellulose, without producing severe lignin changes (Aguiar, Gavioli, & Ferraz, 2013).
Wood is susceptible to fungal biodegradation, especially, when used in a high ambient humidity environment (Ramage et al., 2017), resulting in decay (Johnston, Boddy, & Weightman, 2016). Wood rot is a common problem in urban environments, causing human hazards by a progressive deterioration of the cell walls that reduces the durability of the material (Daniel, 2016; Terho, Hantula, & Hallaksela, 2007). Damage is caused by enzymes and low molecular weight metabolites that act as precursors and coagents of enzymatic degradation, decreasing mechanical properties (Daniel, 2016). Enzymes that degrade cellulose and hemicellulose in the cell wall belong predominantly to hydrolases. Cellulose is hydrolyzed by cellulases, cellulose 1,4-β-cellobiosidase and β-glucosidase. The xylan and mannan of hemicellulose are degraded by endo-1,4-β-xylanase and endo-1,4-β-monosidase, respectively, followed by xylan 1,4-β-xylosidase and β-monosidase and other enzymes acting on the side chains. Lignin is degraded by oxidoreductases, lignin peroxidase and manganese peroxidase (Álvarez, Reyes‐Sosa, & Díez, 2016; Dashtban, Schraft, Syed, & Qin, 2010; Witomski, Olek, & Bonarski, 2016).
The global use of wood as a construction material is estimated based on the world production of sawn wood, which was 468 million m3 in 2016 (Mantau et al., 2019; Proskurina, Junginger, Heinimö, Tekinel, & Vakkilainen, 2019). During the last decades, softwood species, such as pine, have been the most widely used in the industrial sector. It has been used in interiors, moldings, coatings, architectural carpentry and other applications (Ramage et al., 2017). For estimating the durability and resistance of wood, the tests used are based on weight loss of the material (Arantes & Goodell, 2014). However, the interaction between wood and xylophage organisms has not been assessed in relation to mechanical damage or chemical degradation occurring in the lignocellulosic material. The damage caused to the structural components of wood could be detrimental and be significantly reflected in mechanical properties, compromising its integrity (Ortiz, Jamet, Herrera, Vindigni, & Pereira, 2011).
For this reason, the purpose of this study was to determine how weight loss affects chemical and mechanical properties of Pinus pseudostrobus Lindl. wood exposed to brown-rot fungi G. trabeum.
Materials and methods
Material preparation
Sawn timber from P. pseudostrobus was collected from a natural forest with forest management in San Miguel del Monte, Morelia, Michoacán, Mexico (Longitude: -101.134 167, Latitude: 19.620 278, altitude: 2 140 m).
According to the International Standardization Organization (ISO, 1975), samples for wood-fungus interaction tests were 135 wood pieces (P. pseudostrobus) with the following dimensions: 45 pieces 20 mm × 20 mm × 60 mm (parallel compression [PC]; ISO 3345), 45 pieces 20 mm × 20 mm × 50 mm (compression perpendicular to the grain [CPG]; ISO 3132) and 45 pieces 20 mm × 20 mm × 300 mm (static bending [SB]; ISO 3133).
Inoculum preparation
The fungi G. trabeum (donated by the Department of Wood, Cellulose and Paper of the University of Guadalajara) was grown on Potato Dextrose Agar (PDA) medium in Petri dishes and incubated in the dark at 32 °C for seven days (QUINCY LAB, Mod QL-12-140E). At the end of the incubation period, the total mycelium biomass was recovered by adding 10 mL of sterile distilled water and subsequently adjusted to a final volume of 1 L with 106 CFU∙mL-1 (read at 530 nm in a spectrophotometer, Thermo Scientific, model Multiskan GO).
Wood-fungus interaction test
The durability test of wood pieces (wood probes) with G. trabeum was carried out with a variation of the system known as the soil-block test (Green, Jones, Nicholas, Schimleck, & Shmulsky, 2011). For this, 20 L containers were used, which contained a substrate (50 % pine-oak forest soil and 50 % horticultural grade vermiculite ACCIMINTM), previously sterilized in autoclaved (AESA Mod CV-300) at 15 lb pressure and 120 °C for 30 min.
The sterilized wood probes were separated according to the measurements required for the PC and Fe tests, and covered with the substrate. Previously, a 50 mL aliquot of G. trabeum (106 CFU∙mL-1 [530 nm]) was inoculated into each of the wood probes (emptying the content onto the pieces). The control treatment was free of G. trabeum. The bioassay was maintained for up to nine months in a dark room at a temperature of 20 ± 2 °C (average) and 70 ± 5 % substrate humidity.
Wood probes were studied at zero, three, six and nine months. The material was reconditioned for mechanical, chemical, and microscopic tests. The pieces were carefully cleaned and placed at room temperature (18 ± 2 °C) until constant weight was achieved (HONGZUANMT equipment, model HZ-2003), with measurements taken every 24 h. The data were reported as percentage loss in the sample. The data were reported as percentage of weight loss, according to the standard of American Society for Testing and Materials (ASTM, 2005).
Mechanical test
Mechanical tests were performed at each of the established periods (zero, three, six and nine months). The PC and the CPG were evaluated using a 10-t capacity SHIMADZU SEISAKUSHO (Japan, model 70133) universal mechanical testing machine, where a constant force was applied at a compression rate of 3 mm∙min-1 until failure of the specimen was achieved. The PC was calculated through the ratio between the maximum applied load and the cross-sectional area of the part with the formula of ISO 3345 (ISO, 1975):
where,
σ = parallel compression
Pmax = maximum load capacity (N)
b and h = breadth and height, respectively (mm).
The CPG was performed according to ISO 3132 (ISO, 1975), where the proportionality limit (R) in perpendicular compression was calculated as the quotient between the maximum applied load and the longitudinal area of the wood piece.
where,
R = proportionality limit
Pmax = maximum load capacity (N)
b and l = breadth and length, respectively (mm).
The static bending (SB) test was performed according to ISO 3133 (ISO, 1975), where a constant load was applied in the center by placing the wood probe on two supports, leaving a space of 25 cm between them. The calculation was made using the following formula:
where,
MOR = modulus of rupture
Pmax = maximum load capacity (N)
L = length between the centers of the support (mm)
b and h = breadth and height, respectively (mm)
where,
MOE = modulus of elasticity
ΔF = force difference in elastic field
Δd = deformation in the interval F.
Chemical analysis of wood exposed to Gloeophyllum trabeum
The probes used in the mechanical tests (CP, SB and CPG) were also used to calculate the percentage of the main structural components. At each of the established periods, 15 g of anhydrous grinded wood were analyzed for successive reflux extraction using Soxhlet equipment (KIMAXR, model 24027 45/50) with 150 mL of the following solvents: cyclohexane, acetone, methanol and hot water. The extraction lasted six hours for each solvent. The solvents were recovered in a rotary evaporator (BÜCHI, R-200) under vacuum. The extract obtained was placed in a desiccator (PHARMA GLASS, Mod 3120-250) until constant weight was achieved. The extract yield was calculated according to Mejía-Díaz and Rutiaga-Quiñones (2007). Samples free of extractable compounds were used for holocellulose, cellulose and lignin derivatives.
Holocellulose determination
According to Bernabé-Santiago, Ávila-Calderón, and Rutiaga-Quiñones (2013), and Cárdenas-Gutiérrez et al. (2018), 32 mL of distilled water was mixed with 1.0 g of extractable grinded wood, 0.3 g of sodium chlorite and two drops of glacial acetic acid in a beaker. The sample was subjected to heat bath in water at 75 °C. The addition of sodium chlorite and acetic acid was repeated every hour for four hours. After chlorination, the solution was filtered and washed with distilled water at room temperature; subsequently, 10 mL of acetone was added and the residue was placed in a drying oven (ECOSHEL, Mod HV-20) at 40 °C until constant weight, with measurements every 24 h. The holocellulose content was calculated by dividing the weight of the anhydrous residue by the weight of the anhydrous wood free of extractables.
Cellulose determination
One gram of anhydrous wood free of extractives was mixed with 10 mL of 17.5 % NaOH at room temperature. The mixture was incubated for five minutes and then 5 mL of 17.5 % NaOH was added to let it stand for five minutes. This last procedure was repeated to let it stand for 30 minutes, then 33 mL of distilled water was added to the generated product, letting it stand for one hour. The solution was then filtered and washed with 35 mL of 8.3 % NaOH, distilled water and 10 % acetic acid and allowed to stand for three minutes. The reaction was then neutralized with distilled water (approximately 250 mL) and finally allowed to stand in an oven for 12 h at 103 °C for subsequent weighing (ASTM, 1977).
Lignin determination
One gram of free anhydrous extractable wood was mixed, by stirring, with 50 mL of 72 % sulfuric acid and 5 mL of 40 % hydrobromic acid and allowed to stand for two hours. Then, 200 mL of distilled water was added, and the mixture was boiled for 5 min. Finally, the mixture was filtered through a Büchner funnel to wash the samples to remove the acid residues. The product was dried in an oven at 103 °C to constant weight. The lignin content was calculated by dividing the weight of the anhydrous sample by the weight of the anhydrous ground wood free of extractives, according to Cárdenas-Gutiérrez et al. (2018).
Fourier Transform Infrared Spectroscopy (FTIR) analysis of the wood
From the wood probes used in the mechanical tests, samples were selected from both the control treatment and the fungal inoculation treatment at nine months. The samples were conditioned to a particle size of 425 µm (wood powder) in a pulverizing mill (MICRONTM, model K20F, Mexico) (Chen et al., 2010; Traoré, Kaal, & Cortizas, 2016). FTIR analysis was performed on a Thermo Scientific spectrometer equipment (model Nicolet iS10-Waltham, MA, USA). Using the ATR (Attenuated Total Reflection) technique, the data were expressed in wavenumbers (cm-1) for the main absorption maxima, the wood dust came into contact with the ATR crystal and the evanescent wave could be absorbed by the dust (Durmaz, Özgenç, Boyacı, Yıldız, & Erişir, 2016). The resulting attenuated radiation produced an ATR spectrum that is similar to a conventional absorption spectrum. The spectrum was obtained using 50 scans in the range of 4 000 cm-1 to 400 cm-1 with a resolution of 4 cm-1, the magnitude of the spectrum was normalized to 600 cm-1 to 1 800 cm-1.
Microscopic analysis
Samples from control treatments (free of G. trabeum) and from nine months of exposure to the fungus were used. The wood probe had dimensions of 2 cm2, and with a sliding microtome (Leitz WETZLAR 440473, Germany) 20 µm thick cross-sections were made. The sections were prepared according to Bari et al. (2015) using safranin stain for 12 hours. Finally, the samples were placed on a slide for observation under a Zeigen ZEINF139A optical microscope (10x and 40x).
Results and discussion
Damage of Gloeophyllum trabeum in wood
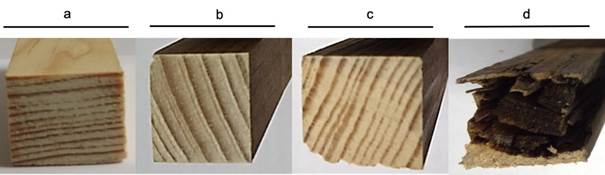
The fungus G. trabeum degraded pine wood at a different rate depending on substrate size (PC and SB wood). At three months of fungal exposure, wood weight loss was 5 % (SB and CPG) and 4.8 % (PC), while at nine months, it was 8.5 % (SB) and 4.5 % (PC) (Table 1). The wood specimen showed no surface damage at zero, three and six months (Figure 1).
The physiological versatility of G. trabeum to nourish hemicelluloses is interesting. In the early stages of fungal growth, hemicelluloses depolymerize more rapidly than cellulose. When pine wood is used as substrate, cellulose depolymerization is high in microfibrils, due to the removal of hemicellulose, increasing cellulosic enzymatic hydrolysis (Monrroy, Ortega, Ramírez, Baeza, & Freer, 2011).
Although, the data showed unremarkable weight loss in the samples, in SB, after three months a significant decrease of 14 % in modulus of rupture (MOR) strength occurred, reaching 44 % after nine months, which corresponds to a reduction from 100 N∙mm-2 to 56 N∙mm-2 (Table 1). This indicates that, despite the low weight loss in the pine wood probe (8.5 %), the decrease in strength was significant when subjected to stress. The MOE, which represents the elastic behavior of pine wood, was affected by fungal degradation, decreasing 54.7 % with respect to the wood used in the control treatment.
It is interesting to note that although weight loss in PC was low (4.5 %), this was sufficient for the fungus to weaken the resistance of pine wood (Table 1b). MOR losses in pine wood were 16.7 %, 21.7 %, and 35 % at three, six, and nine months of fungal exposure, respectively, which showed decreases from 60 N∙mm-2 to 39 N∙mm-2. Low weight loss and decrease in MOR are associated with delignification suffered by pine wood due to fungal degradation. The proportional limit (R) in perpendicular compression was the most affected with a decrease of 42.5 %, 49.1 % and 52.2 % for three, six and nine months, respectively, where the resistance value of the pine wood control is the reference (Table 1).
Table 1 Weight loss, Static bending, Parallel compression and Compression perpendicular to the grain in Pinus pseudostrobus wood, after zero, three, six, nine months of exposure to fungi Gloeophyllum trabeum.
| Time (months) | Weight loss (%) | MOR (N∙mm-2) | MOE (N∙mm-2) | |
|---|---|---|---|---|
| Static Bending | ||||
| 0 | 0 | 100.0 ± 14.0 a | 9 412 | |
| 3 | 5.0 ± 0.3 a | 86.1 ± 16.0 b | 9 361 | |
| 6 | 6.5 ± 1.0 b | 81.7 ± 8.5 b | 7 871 | |
| 9 | 8.5 ± 8.4 b | 56.2 ± 26.3 b | 5 156 | |
| Parallel Compression | ||||
| 0 | 0 | 60.0 ± 5.0 a | ||
| 3 | 4.8 ± 1.2 a | 50.0 ± 4.5 b | ||
| 6 | 7.3 ± 2.7 b | 47.0 ± 2.2 b | ||
| 9 | 4.5 ± 1.1 a | 39.8 ± 2.0 b | ||
| Compression perpendicular to the grain | ||||
| 0 | 0 | 42.2 ± 8.0 a | ||
| 3 | 5.0 ± 0.3 a | 24.3 ± 6.0 b | ||
| 6 | 6.5 ± 1.0 b | 21.5 ± 4.6 b | ||
| 9 | 8.5 ± 8.4 b | 20.2 ± 4.0 b | ||
± Standard deviation. MOR: Modulus of Rupture, MOE: Modulus of Elasticity. Zero corresponds to Control treatment (Undecayed wood). Values with different letters show significant difference according to the Duncan’s means separation test for multiple comparisons (P < 0.05).
Another interesting contribution of this study is found in the first phase of fungal growth, since no mechanical or aesthetic damage was observed in the pine wood probes, although there was a slight loss of weight compared to the control treatment. On the other hand, low cellulose degradation was detected in the three- and six-month stages of exposure to fungi. The fungi use an extracellular and superior enzymatic system (Hosseinpourpia & Mai, 2016; Krueger, Hofmann, Moeder, & Schlosser, 2015) that explains the efficient depolymerization of pine wood polysaccharides and polyphenols. For this reason, it can be assumed that pine wood probes lose strength when subjected to SB and CPG, despite little weight loss and visual evaluation without damage (three and six months). This information is transcendental in the construction industry, because visual evaluations are performed on pine wood structures, ignoring exposure to G. trabeum and other fungal organisms with similar characteristics, putting safety at risk as the mechanical performance of the material is compromised.
Chemical analysis of pinewood biodegraded by Gloeophyllum trabeum
Table 2 shows the results of the chemical composition of P. pseudostrobus wood. After three months of fungal exposure, hemicellulose was the structural component that decreased the most: 59.3 % for the pieces evaluated in PC and 63.1 % for SB. It is important to note that, after six months of fungal exposure, wood pieces continued to decrease to 91.6 % (SB) and 63.1 % (PC), considering pine wood as 100 % of the control value. This result agrees with previous publications, where it is shown that brown rot fungi feed mainly on hemicellulose, as mentioned by Hastrup et al. (2012). Hemicellulose is incorporated into cellulose microfibrils and in the cellulose degradation process, hemicellulose is the first component to be removed. Considering the chemical characteristics, cellulose decreased in smaller proportion after six months: 4.4 % in SB and 9.7 % in PC. When analyzing the pine wood specimens after nine months of exposure to fungi, the decrease in hemicellulose and cellulose was not detected in the chemical analysis, since the degradation was concentrated at the lower end of the pine wood specimens (Figure 1). Therefore, to complement this information, results derived from the Fourier transform infrared spectroscopy (FTIR) showed significant reduction of hemicellulose and cellulose. Initially, lignin had no significant losses in its percentage value, but after nine months, it decreased 43.4 % in the case of SB and 29.8 % in the case of PC in relation to the value of the control.
Table 2 Chemical analysis of Pinus pseudostrobus wood exposed to Gloeophyllum trabeum fungi.
| Component | Control (%, 0 months) | 3 months | 6 months | 9 months | |||
|---|---|---|---|---|---|---|---|
| SB (%) | PC (%) | SB (%) | PC (%) | SB (%) | PC (%) | ||
| Inorganic | 0.2 ± 0.0 a | 0.3 ± 0.1 b | 0.4 ± 0.2 b | 0.3 ± 0.1 b | 0.5 ± 0.0 b | 0.4 ± 0.0 b | 0.5 ± 0.2 b |
| Total removable | 2.7 ± 0.0 a | 1.9 ± 0.3 a | 2.6 ± 0.5 a | 2.3 ± 0.5 a | 2.4 ± 0.6 a | 3.1 ± 0.0 a | 3.7 ± 0.0 b |
| Lignin | 29.5 ± 3.0 a | 25.9 ± 0.8 a | 28.0 ± 0.3 a | 28.3 ± 0.8 a | 29.5 ± 1.0 a | 16.7 ± 0.2 b | 20.7 ± 2.0 b |
| Holocellulose | 80.0 ± 0.0 a | 72.1 ± 0.0 b | 69.2 ± 0.0 b | 65.4 ± 0.0 b | 65.5 ± 0.0 b | 78.0 ± 0.0 a | 79.0 ± 0.0 a |
| α-cellulose | 67.2 ± 0.0 a | 67.0 ± 0.7 a | 63.3 ± 0.6 b | 64.3 ± 1.2 a | 60.7 ± 0.8 b | 65.0 ± 0.0 a | 67.0 ± 0.0 a |
| Hemicellulose | 13 | 4.8 | 5.3 | 1.1 | 4.8 | 13 | 12 |
± Standard deviation. Static bending (SB) and Parallel compression (PC) after zero, three, six, nine months of exposure to wood decay fungi G. trabeum. Values with different letters show significant difference according to the Duncan’s means separation test for multiple comparisons (P < 0.05).
Analysis of structural components using Fourier Transform Infrared Spectroscopy
Figure 2 shows the FTIR spectrum of the pine wood fungus degraded for nine months. The spectrum showed interesting changes, especially in the absorbance peaks corresponding to the vibrations of the cell wall groups at 1 800-800 cm-1. Signals corresponding to C-H deformation in cellulose and hemicellulose, phenolic group of cellulose and C-O-C in carbohydrates were found in these bands of the spectrum (Chow & Ting, 2019; Pandey & Pitman, 2003; Poletto, Zattera, & Santana, 2012).
On the other hand, according to the degradation profile produced by G. trabeum, differences in wood components were observed. The absorbance peaks assigned to bands 1 732 and 894 cm-1 corresponded to hemicelluloses and showed differences in intensity, being lower in pine wood exposed to the fungus (Figure 2); pine wood probes subjected to SB affected the percentage of MOR reduction. Bands at 1 508, 1 423 and 1 266 cm-1, corresponding to lignin molecules, increased in degraded pine wood, due to the effect of G. trabeum leaving lignin residues. For cellulose, bands were assigned in the regions of 1 316 and 1 369 cm-1, where loss of material is shown, since the intensity of cellulose in degraded pine wood is below the control treatment (Table 3).
The data corresponding to lignin complement the previous argument, since concentration in wood pieces showed no considerable changes, until after nine months of exposure to the fungus it significantly decreased 43.4 % (SB) and 29.8 % (PC) (Table 2). The analysis of the structural components of wood by FTIR allowed integrating the information seen previously, since the wood suffered significant loss of lignin and chemical modification by the fungus. Cellulose and hemicellulose were removed in the degraded wood, but a residue of modified lignin was found (Durmaz et al., 2016), which also translates into decreased mechanical properties of the material after nine months of exposure to the fungus.
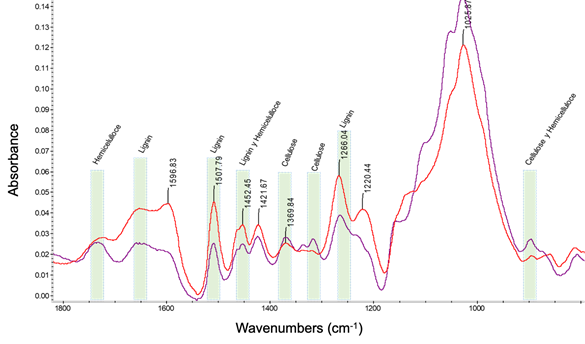
Figure 2 Fourier transform infrared spectroscopy analysis, showing the bands of the spectrum of the control treatment (undeteriorated wood, purple line) and of the decayed wood after nine months (red line) of exposure to Gloeophyllum trabeum fungi.
Table 3 Fourier transform infrared spectroscopy analysis shows the position and intensity of the peaks recorded between the regions (wave number) 1851-649 cm-1. Results for control wood (undecayed wood) and decayed wood, after nine months of exposure to Gloeophyllum trabeum fungi.
| Control wood | Decayed wood | Attribution | References | ||
|---|---|---|---|---|---|
| Position (cm-1) | Intensity | Position (cm-1) | Intensity | ||
| 894.73 | 0.0260 | -- | -- | Cellulose and Hemicellulose | |
| 1 264.49 | 0.0379 | 1 266.04 | 0.0578 | Lignin | Pandey and Pitman (2003) |
| 1 316.38 | 0.0275 | -- | -- | Cellulose and Hemicellulose | Durmaz, Özgenç, Boyacı, Yıldız, and Erişir (2016) |
| 1 367.96 | 0.0285 | 1 369.84 | 0.0252 | Cellulose and Hemicellulose | Chow and Ting (2019) |
| 1 423.10 | 0.0285 | 1 421.66 | 0.0341 | Lignin and hemicellulose | Bari et al. (2015); Poletto, Zattera, and Santana (2012) |
| 1 508.35 | 0.0253 | 1 507.78 | 0.0451 | Lignin | Curling, Clausen, and Winandy (2002) |
| 1 732.11 | 0.0250 | -- | -- | Xylan in hemicellulose | Curling, Clausen, and Winandy (2001); Chow and Ting (2019) |
Internalization of Gloeophyllum trabeum mycelium in pinewood
Figure 3 shows the microscopic evaluation of the damage produced by the fungus G. trabeum on pine wood after six and nine months of exposure. Pine wood exposed to the fungus for six months only suffered thinning of the cell walls without breaking them (Figure 3b), which is due to the loss of hemicelluloses, reduction of lignin and cellulose masses, coinciding with the data obtained in the chemical analysis (Table 2). The decrease in the main polymers of the cell wall of the degraded wood (nine months) could be observed in the rupture and deformation caused by the fungus (presence of hyphae in cutting wood) (Figure 3c). This has a direct impact on the reduction of resistance to mechanical stress, especially in pine wood subjected to SB.
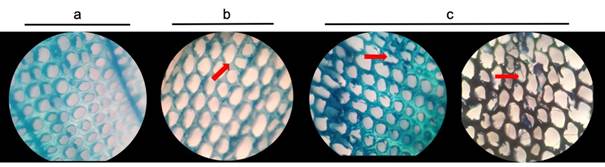
Figure 3 Microscopic analysis of Pinus pseudostrobus wood exposed to Gloeophyllum trabeum fungi. Transversal cuts: a) control treatment (undeteriorated wood), b) six months of exposure and c) nine months of exposure. Images (40x) show colonization and thinning (red arrows) of tracheid cell walls.
The mechanisms of development and degradation of G. trabeum are reflected in the thinning and rupture of plant tissue cells (tracheids) (Donaldson, Radotić, Kalauzi, Djikanović, & Jeremić, 2010). This has a direct impact on the mechanical properties of the material. Another interesting contribution of this study is that lignocellulosic material subjected to mechanical stress reduces its mechanical properties, because SB and CPF suffered the highest losses due to the fungus. After nine months of exposure, there is significant fungal attack on pine wood structures; previous reports indicate that, under optimal growth conditions, brown rot fungi cause similar early-stage strength reduction (a ratio of 50 % strength reduction per 7 % mass loss [Witomski et al., 2016]). This shows that damage caused by G. trabeum on pine wood (e.g., P. pseudostrobus), which is widely distributed throughout the world, has no significant impact on durability, and such damage is not reflected in the aesthetics of the material, which is alarming, as the common user of pine wood would not be able to notice the problem.
Finally, it is important to mention that the behavior of the fungus depends on the size of the exposed wood, since those with larger dimensions suffered greater weight loss. This reduction allowed classifying the wood as highly resistant to degradation by G. trabeum after nine months of interaction with the fungus, but with repercussions on the integrity of the material for use in wood structures.
Conclusions
After nine months, degradation of Pinus pseudostrobus wood in contact with the fungus Gloeophyllum trabeum was minimal if only weight is considered. However, chemical and mechanical properties showed that the fungus causes low resistance to mechanical stresses by decreasing static bending (100 to 56 N∙mm-2 [modulus of rupture]) and compression perpendicular to the grain (42.2 to 20.2 N∙mm-2 [limit of proportionality]). Therefore, these parameters should be considered when evaluating wood in contact with any xylophagous fungus. P. pseudostrobus wood that has been exposed to fungal degradation is significantly compromised in its mechanical properties, making it unfit for construction.











 text in
text in