Introduction
The carob tree (Ceratonia siliqua L., Fabaceae) is one of the most important plant species in the Mediterranean region. It is noted for its high capacity to adapt to different drought stress conditions. The economic importance of this species arises from the industrial use of the locust bean gum extracted from its seeds, which is widely used as a natural additive (E-410) in the food industry to function as a stabilizer, thickener, and flavorant (El Bouzdoudi et al., 2017; Fadel et al., 2011). The worldwide production of carob is about 156 800 t·year-1. The main producers are Portugal (25.46 %), Italy (18.24 %), Spain (16.51 %) Morocco (13.89 %), and Turkey (8.45 %) (FAO, 2016). It was also successfully introduced in other Mediterranean-like regions such as the southern United States, Mexico, Chile, Argentina and southern Australia (Benković et al., 2016).
As traditional carob propagation methods have failed to meet market demand, the use of in vitro techniques seems appropriate to fulfill the increased demand for plant material (Shahzad, Akhtar, Bukhari, & Perveen, 2017). One of the methods to develop a reproducible system for plantlet regeneration is through callus, which is also considered the most suitable material for somatic embryogenesis, genetic transformation and production of secondary metabolites (Azeez et al., 2017; Mujib, Ali, Tonk, & Zafar, 2017; Reyes-Díaz, Arzate-Fernández, Piña-Escutia, & Vázquez-García, 2017). The efficiency of callus induction is related to plant material (explants and genotypes) and culture medium composition (salt composition, plant growth regulators and carbohydrates). The most widely used explants for callus induction, in C. siliqua as well as other species, are immature embryos and cotyledons (Canhoto, Rama, & Cruz, 2006; Custodio & Romano, 2006). This material is laborious to obtain and available only during a very short period of the plant growth cycle. Callus induction from mature seed cotyledons has been successfully achieved in our previous experiments (Lozzi, Abousalim, & Abdelwahd, 2015). This material has the advantage of being easily excised and readily available throughout the year. Furthermore, cotyledon-derived calli from mature seeds are considered a suitable starting material of undifferentiated cells for in vitro regeneration into fertile plants (Din et al., 2016).
Like other woody plants, carob is difficult to propagate under in vitro conditions and only some success has been achieved so far. Although there are a few reports on embryonic callus induction in C. siliqua (Canhoto et al., 2006; Custodio & Romano, 2006; Ksia et al., 2008; Lozzi et al., 2015), all previous studies have concentrated on the influence of plant growth regulators, and little, if anything, is known about the effect of genotype, mineral nutrients and carbohydrates on callus induction in this plant. Mineral composition of the culture medium is one of the most important factors governing plant growth and morphogenesis (George & De Klerk, 2008; Kim, Gopal, & Sivanesan, 2017). Murashige and Skoog (MS) (Murashige & Skoog, 1962) medium (developed for optimal growth of tobacco callus) is the most widely used in carob as in other plant species (Canhoto et al., 2006; George & de Klerk, 2008). However, this medium is not optimal for all plant tissues (Varshney & Anis, 2014). Carbohydrates are another major component of tissue culture media (Khorsha, Alizadeh, & Mashayekhi, 2016). Sucrose is the most widely used carbon source due to its highly favorable effect on growth and relatively low cost (Sumaryono, Wirdhatul, & Ratnadewi, 2012). All studies on carob have used sucrose as the carbon source at a concentration of 81 mM. However, it has been reported that the optimum concentration of sucrose differs among plant species (Thorpe et al., 2008). Mannitol is a naturally occurring 6-carbon sugar alcohol that is widely used in plant tissue culture as an osmotic agent to modify the water potential of a culture medium (Tholakalabavi, Zwiazek, & Thorpe, 1994). This carbohydrate has been applied successfully in several plant species such as in Cucumis sativus L. (Lou & Kako, 1995), Cucumis melo L. (Nakagawa et al., 2001), Phoenix dactylifera L. (Shibli, Subaih, & Abdelrahman, 2005), and Olea europaea L. to increase the efficiency of callus induction and somatic embryogenesis (Brhadda, Walali, & Abousalim, 2008).
In this context, the aim of this study was to assess the effects of five genotypes of C. siliqua on callus induction and to optimize culture medium composition for better growth of embryonic callus. This in order to provide baseline information for the development of an appropriate protocol of interest for mass propagation of carob via somatic embryogenesis. Histological analysis of induced calli and differentiated structures are also considered in this study.
Materials and methods
Plant material
Mature seeds of selected C. siliqua trees of the productive variety “Dkar” growing in five regions of Morocco were used as sources of cotyledonary explants (Table 1).
Table 1 Geographic origin of Moroccan carob (Ceratonia siliqua) trees used in this study.
| Regions | Code | Geographic region | Latitude | Longitude | Elevation (m) |
| El Hoceima | GH | North coastal | 35° 11’ | 3° 57’ | 50-250 |
| Ouazzane | GO | Rif mountain | 34° 58’ | 5° 29’ | 170-230 |
| Marrakech | GM | High Atlas mountain | 31° 37’ | 7° 00’ | 700-1 000 |
| Azilal | GA | Middle Atlas mountain | 32° 12’ | 6° 28’ | 400-700 |
| Bni Mellal | GB | Middle Atlas mountain | 32° 30’ | 6° 03’ | 500-800 |
Preparation of explants
Accessions were stored at 5 °C throughout the experimental period. Seeds were treated with concentrated sulfuric acid (98 %) for 60 min, washed with sterile water 3-4 times and then soaked in sterile distilled water for 24 h. Cotyledons were aseptically excised. The endosperm and the embryo axis were removed, and each cotyledon was transversely segmented into four portions of 4-6 mm in length. The explants were placed with the abaxial surface in contact with the culture medium.
Callus initiation medium and culture conditions
For callus initiation, explants were cultured in 90 mm-diameter Petri dishes containing 20 mL of MS medium supplemented with 90 mM sucrose and 2,4-dichlorophenoxyacetic acid (2,4-D) at the optimal concentration reported in our prior study (10 µM) (Lozzi et al., 2015). Medium was solidified with 0.7 % agar (SIGMA) and the pH was adjusted to 5.8 with 1 M NaOH before autoclaving at 121 °C for 20 min.
Explants of the five carob genotypes were cultivated on callus initiation medium to test their callogenesis capacities. The genotypes showing the best callogenesis response were grown on four culture media: MS, Gamborg (B5) (Gamborg, Miller, & Ojima, 1968), Woody Plant Medium (WPM) (Lloyd & McCown, 1980) and Driver and Kuniyuki (DKW) (Driver & Kuniyuki, 1984) supplemented with three 2,4-D doses (2.5, 5, 10 µM). Combinations of sucrose and mannitol (0, 45, 90, 120, and 180 mM) were also compared.
Cultures were maintained completely in the dark in the culture room at 25 ± 2 °C for five weeks. Callus samples of known fresh mass were dried to constant weight in an oven set at 60 °C for 24 h. The morphology was observed using a stereomicroscope (SZ2-ILST, Olympus, Japan).
Histological analysis
For histological examination, callus was fixed for at least 24 hours in Formaldehyde-Acetic acid- Ethanol (5:5:90/v:v:v) mixture, dehydrated in ethanol-butanol series and embedded in paraffin wax as described by Cob-Uicab et al. (2011). Sections (7 µm) were cut using a rotary microtome (Leica) and stained with 0.05 % Toluidine blue O. The sections were dried and observed under a Euromex ZE-1657 stereomicroscope.
Statistical analysis
A completely randomized factorial design was used in this study. The results were expressed as means ± standard deviation calculated from three independent experiments, each consisting of at least 16 explants. The data obtained were analyzed statistically by analysis of variance (ANOVA) for the three repeated measures using SPSS software, V. 21 (SPSS, 2012) and differences among means were evaluated by Duncan’s multiple range test (P = 0.05). Percentage data were transformed to arcsin before analysis.
Results
Mature cotyledons started to swell after one week in the callus induction medium. During the second week of culture, calli started to emerge mainly from the cut ends of explants that were not in contact with the medium (Figure 1). According with Table 2 the five genotypes induced a high rate of callus (75 to 100 %) and, genotypic differences in dry weight were observed. The highest values were recorded in ‘GH’, ‘GM’ and ‘GB’ calli. With regard to callus color, ‘GB’ produced a higher creamy-white biomass (27.3 mg), whereas the other genotypes produced mainly creamy-brown callus. Thus, this genotype was used in the subsequent experiments. Some explants from ‘GH’, ‘GM’ and ‘GB’ genotypes showed root initiation (2 - 6.7 %) from induced calli.
Table 2 Callus induction from mature cotyledons of five different genotypes of Ceratonia siliqua after five weeks in Murashige and Skoog medium supplemented with 10 µM of 2,4-D.
| Genotype | Callus induction (%) | Callus dry weight (mg) | Callus color | Morphology | Root induction (%) |
|---|---|---|---|---|---|
| GH | 100 ± 0 | 26.5 ± 3.0a | Creamy-Brown | Compact | 6.7 ± 4.4 |
| GO | 100 ± 0 | 16.5 ± 1.1bc | Creamy-White | Compact | 0.0 |
| GM | 75 ± 6.9 | 24.9 ± 3.5a | Creamy-Brown | Compact | 4.2 ± 6.3 |
| GA | 87.5 ± 4.2 | 21.6 ± 3.5ab | Creamy-Brown | Compact | 0.0 |
| GB | 100 ± 0 | 27.3 ± 5.3a | Creamy-White | Compact | 2.0 ± 2.2 |
GH = El Hoceima, GO = Ouazzane, GM = Merrakech, GA = Azilal, GB = Elksiba. Mean values followed by the same letters are not significantly different from each other according to Duncan’s Multiple Range Test (P = 0.05).
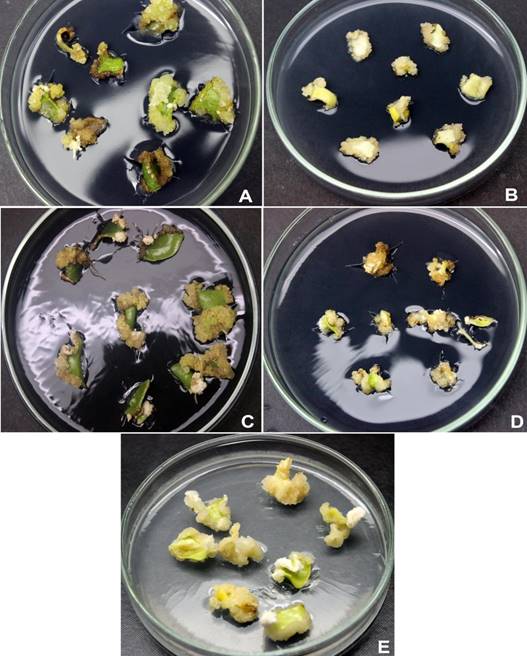
Figure 1 Callus induction from mature cotyledons of five genotypes (A = El Hoceima, B = Ouazzane, C = Merrakech, D = Azilal, E = Elksiba) of Ceratonia siliqua after five weeks in Murashige and Skoog medium supplemented with 10 µM of auxin 2,4-D.
According to Table 3, the experiment designed to compare basal media showed that explants were able to induce calli on all tested salt compositions. Noticeably, only B5 and WPM media permitted the development of friable calli, but WPM scored the lowest dry weights for all auxin concentrations tested. Maximum increase in dry weight (39.1 mg) was recorded when B5 medium supplemented with 10 µM 2,4-D was used, whereas there were no significant differences with lower concentrations. With the same medium, creamy-white calli were obtained with 2.5 µM 2,4-D and higher concentrations turned the calli color to brown (Table 3). With regard to root induction, there was a significant interaction (P ≤ 0.001) between salt media and 2,4-D concentration. MS medium containing 2.5 µM 2,4-D showed the highest rate root induction (40 %), which declined significantly with the increase of the 2,4-D concentration. No roots developed on B5 medium.
Table 3 Effect of culture medium and 2,4-D on callus formation of Ceratonia siliqua after five weeks in the induction medium.
| Culture medium | 2,4-D (µM) | Callus induction (%) | Callus dry weight (mg) | Color | Morphology | Root induction (%) |
|---|---|---|---|---|---|---|
| MS | 2.5 | 100 | 15.5 ± 2.2 ef | Creamy-White | Compact | 40.0 ± 2.32 a |
| MS | 5 | 100 | 16.2 ± 8.9 ef | Creamy-White | Compact | 11.1 ± 0.32 b |
| MS | 10 | 100 | 24 ± 3.4 cde | Creamy- White | Compact | 0.0b |
| WPM | 2.5 | 100 | 9.8 ± 5.1 f | Creamy-White | Friable | 2.3 ± 1.6 b |
| WPM | 5 | 100 | 14.2 ± 5.0 ef | Creamy-Brown | Friable | 4.2 ± 0.48 b |
| WPM | 10 | 100 | 15.6 ± 1.9 ef | Creamy-Brown | Friable | 0.0b |
| DKW | 2.5 | 100 | 22.2 ± 5.3 de | Creamy-White | Compact | 0.0b |
| DKW | 5 | 100 | 24.3 ± 5.8 cde | Creamy-White | Compact | 5.6 ± 0.32 b |
| DKW | 10 | 100 | 27.8 ± 6.1 bcd | Creamy-White | Compact | 0.0b |
| B5 | 2.5 | 100 | 32.5 ± 5.3 abc | Creamy-White | Friable | 0.0b |
| B5 | 5 | 100 | 34.2 ± 6.9 ab | Creamy-Brown | Friable | 0.0b |
| B5 | 10 | 100 | 39.1 ± 4.5 a | Brown | Friable | 0.0b |
| Significance of differences in two-way ANOVA | ||||||
| Medium | NS | P ≤ 0.0001 | P ≤ 0.0001 | |||
| 2,4-D | NS | P ≤ 0.05 | P ≤ 0.001 | |||
| Medium ×2,4-D | NS | NS | P ≤ 0.0001 | |||
Means followed by the same letters are not significantly different from each other according to Duncan’s Multiple Range Test. Culture medium: Murashige and Skoog (MS), Gamborg (B5), Woody Plant Medium (WPM) and Driver and Kuniyuki (DKW). Auxin 2,4-D (2,4-dichlorophenoxyacetic acid)
For the carbohydrate combination experiment, all explants induced calli when B5 medium was supplemented with various concentrations of sucrose, regardless of the presence of mannitol (Figure 2). On the other hand, no callus was induced with mannitol alone even after five weeks. Generally, the calli formed were creamy-white and friable. Sucrose concentration significantly (P ≤ 0.05) affected the callus dry weight with the highest result (38.4 mg) in the presence of 90 mM sucrose and in the absence of mannitol; consequently, the lowest weights were recorded in all media supplemented with mannitol.
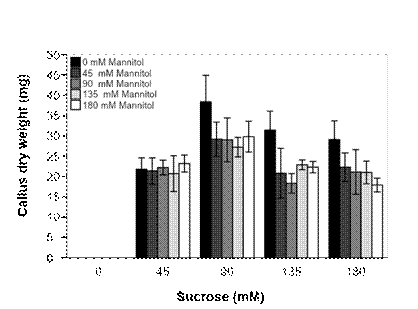
Figure 2 Effect of combinations of sucrose and mannitol on Ceratonia siliqua callus induction after five weeks in Gamborg (B5) medium supplemented with 2.5 µM of auxins 2,4-D.
Histological observations of the friable creamy-white calli showed the formation of meristematic centers with isodiametric cells, smaller than surrounding cells, having densely stained cytoplasm and nucleus and undergoing anticlinal and periclinal divisions (Figure 3a). After four weeks of culture, small embryogenic masses and globular proembryos were seen on the surface of the calli (Figure 3b). Histological examinations also showed the development of roots with vascular bundles in some induced calli.

Figure 3 Microscopic observations of calli induced from mature cotyledons of Ceratonia siliqua. Meristematic center (MC) initiation with actively dividing cells having densely stained cytoplasm and nucleus (N) surrounded by highly vacuolated cells (VC). Detail of the cells in the globular structures (GPe) obtained on the surface of the calli developed at the cut ends of the explants.
Discussion
This research evaluated the effect of five genotypes of explants and culture medium composition (mineral nutrients, 2,4-D and carbohydrate concentrations) on callus induction and proliferation from mature cotyledons of C. siliqua. This plant material has the advantage of being easily excised and available throughout the year.
Studies report that callus proliferation is genotype dependent. This dependency was seen in Castanea sativa Mill. (Hoque, Biswas, & Alam, 2007), Vigna subterranea (L.) Verdc. (Konaté, Koné, Kouakou, Kouadio, & Zouzou,2013) and Theobroma cacao L. (Ramírez, Vasquez, Osorio, Garcés, & Trujillo, 2018). The genotype effect can also induce changes in color, texture and morphogenetic potential of callus and this could occur even among related varieties of the same species (Saeed & Shahzad, 2015; Schween & Schwenkel, 2003; Shahnewaz, Bari, Siddique, & Rahman, 2004). Over the last few years, phenotypic and genetic studies have revealed a high degree of diversity of carob pods and seeds collected from different areas of Morocco (El Kahkahi, Zouhair, Ait Chitt, & Errakhi, 2014; Konaté, Filali-Maltouf, & Berraho, 2007; Sidina et al., 2009). In the present investigation, five different regions across Morocco were used as sources of cotyledonary explants. All compared genotypes showed high callus induction levels (75 to 100 %). However, callus morphology varied widely among genotypes. The difference could be attributed to the difference in the endogenous hormone concentration within genotypes (Han, Jin, Wu, & Zhang, 2011; Salehi & Khosh-Khui, 2005).
Salt composition of the medium is another important factor influencing callus induction and proliferation (George & de Klerk, 2008). In this study, all basal media were able to produce calli from mature cotyledon explants of carob. Calli dry weight presented significant differences with the highest results being observed on B5. WPM produced the lowest amount of calli. This medium has a poor salt composition with a lower content of nitrogen, potassium and chloride. It has also a lower total ionic amount compared to the other media. Notably, friable calli were obtained only with B5 and WPM. Development of this callus morphology could be due to the low nitrogen concentration in these media. Similar to the present findings, calli of Barringtonia racemosa (L.) Spreng. were friable on WPM and B5 media, and compact on MS medium (Behbahani, Shanehsazzadeh, & Hessami, 2011). In B5 medium, increasing the auxins 2,4-D concentration from 2.5 to 10 µM improved callus dry weight. Noticeably, creamy-white calli were obtained at the lowest concentration while slightly brownish ones were observed at the highest concentration. The interaction between salt media and 2,4-D concentration was significant in the case of root induction. MS basal medium supplemented with 2.5 µM 2,4-D showed the highest rate. The percentage of induced roots declined as the concentration of the auxin increased. A high 2,4-D concentration (10 µM) inhibited the growth of roots for all the media tested. This could be explained by the fact that synthetic auxin at high concentration may produce herbicidal effects which could block the adventitious root initiation (Evans, Coleman, & Kearns, 2003).
In the present study, callus growth was also affected by different concentrations of sucrose and mannitol. B5 medium supplemented with 90 mM of sucrose produced the highest amount of calli. Higher sucrose concentrations appear to reduce callus proliferation, which can be explained by a decrease in the osmotic potential of the medium (Lipavská & Vreugdenhil, 1996). In similar studies conducted in O. europaea, callus induction increased when the sucrose concentration was increased from 54 to 243 mM (Brhadda et al., 2008). By contrast, sucrose at 43.8 mM produced the highest amount of callus in Aquilaria malaccensis Lam. while a higher level of sucrose had lower results (Jayaraman, Daud, Halis, & Mohamed, 2014). Adding mannitol to medium containing sucrose decreased callus dry weight, whereas no callus was induced in the presence of mannitol alone. This decline could be caused by an excessive osmotic contribution (Thorpe et al., 2008). In the experiments reported here, the use of mannitol with sucrose yielded low results as compared to the use of sucrose alone in the culture medium. Similar results were obtained with callus of Vitis vinifera L. (Yancheva & Roichev, 2005). However, when mannitol was used as the sole carbohydrate source, no callus was induced, indicating that sucrose is essential for callus induction in C. siliqua. The inability of carob cultures to use this sugar alcohol could be attributed either to the uptake inhibition of these carbohydrates or the absence of genes encoding enzymes involved in polyol catabolism in C. siliqua (Steinitz, 1999).
Calli induced in B5 medium assessed by histological analysis showed active centers with embryogenic characteristics. Unlike the non-embryogenic cells which are large, elongated and vacuolated, embryogenic cells are generally small, densely filled with cytoplasm and isodiametric (Brhadda, Walali, & Abousalim,2007; Kumar, Suri, Sonie, & Ramawat, 2003). After four weeks, these meristematic cells evolved into embryogenic masses and globular proembryos.
Conclusions
This study confirms the high potential of mature cotyledons of C. siliqua for embryonic callus induction and proliferation. The expression of this potential is influenced by the genotype, 2,4-D concentration, salt composition of the basal medium and carbohydrates. The obtained callus showed varied texture and color. Histological investigation for induced calli showed active centers with embryogenic characteristics. This study is the first step towards establishing an efficient protocol for carob mass propagation via somatic embryogenesis and shoot formation from mature cotyledons. Further studies including critical factors that would improve the transition from proembryos to somatic embryos and subsequent plant regeneration are being considered.











 text in
text in 


