Introduction
Encapsulation is the retention of an active compound called a “core” by a coating material called a “wall” (Ray et al., 2016). The core material must be compatible with the wall material to achieve encapsulation (Nava-Reyna et al., 2015; Costamagna et al., 2017) and the wall material will influence the morphology of the final package (Valenzuela et al., 2013). A widely used wall material within carbohydrates is sodium alginate (SA) (Dias et al., 2017). SA is a polymer formed by a polyanionic linear structure, made up by D-mannuric and L-guluronic acids. The polymer is extracted from algae, non-toxic, biocompatible and biodegradable; it has a good capacity to form gels, spheres, micro and nanoparticles. These characteristics make SA be used as an encapsulating material (Hernández-Gálvez, 2015; Hernández-Torres et al., 2016; Babazadeh et al., 2016).
One encapsulation technique that is widely reported in the literature is coacervation (Nazzaro et al., 2012; Zhao & Wang, 2017). Coacervation is defined as the separation of a soluble colloid into two liquid phases, one of which contains a high proportion of colloid (coacervate layer) (Giro-Paloma et al., 2016). During coacervation the core is wrapped by a fluid and viscous film of the polymer. The fluid film is hardened and solidified to form a hard capsule around the core with the help of a crosslinker solution (CaCl2) (Dias et al., 2017). One of the advantages of the coacervation technique is its ease of implementation, as well as its low cost, however, a disadvantage is that particles of millimetric and micrometric size are obtained.
Another technique that has allowed the development of micro and nano-sized spherical particles or capsules is electrospinning (Soto-Martinez, 2018). Electrospinning is a technique that uses electrostatic forces to generate microfilaments and macroparticles. Electrospinning is widely used in both industrial processes and scientific research to obtain different products for several applications in the pharmaceutical field, metallurgy, automotive paint, fuel injection, cosmetics and food (Zhang et al., 2013; Bock et al., 2011). Currently, many biopolymers such as lipids, proteins and carbohydrates have been studied by means of electrostatic atomization techniques as electrospinning to produce nanofibers and wall materials for encapsulation processes (Lopez-Rubio et al., 2009) but none of them have reported to use electrospinning and coacervation for produc producing Fe microcapsules.
Zhu et al. (2017), reported electrostatic coacervation as the combination of classical coacervation using sodium alginate/calcium chloride with electrostatics. The aim of joining both techniques is to obtain capsules of micrometric and nanometric size taking advantage of the electrostatic attraction between the core material and the wall material allowing a decrease in the particle size. The result of this combination generates a product in the form of micro-drops or fibers from 1000 µ to 0.5 µ (Soto-Martinez, 2018; Perez-Calderon et al., 2018). Currently, the development of nanofibers has been very successful, due to the different fields in which they can be used, from the textile industry to the biomedical industry. However, in the area of food, few studies have been carried out in this area and are aimed at the use of compounds of natural and edible origin, such as amaranth protein. In the food industry, nanofibres can be very useful for the encapsulation of assets that are sensitive to pH or that interact with food components and reduce their biological activity. In this project, two bacteriocins (pediocin and nisin) were incorporated into amaranth and pullulan nanofibers. Solution properties, process parameters and some environmental conditions may affect the size and characteristics of the micro-droplets (Wen et al., 2017).
It has been reported that microcapsules increase the absorption and efficiency of the encapsulated product and decrease the sensory perception of the encapsulated material when ingested (Zhao and Wang, 2017; Wu et al., 2016; Appelqvist et al., 2015; Valenzuela et al., 2013). The advantage of the microencapsulation by coacervation (Sodium Alginate/Calcium Chloride) is that it protects the compounds in the nucleus from external factors affecting them, such as light, oxygen, pH and temperature, among others (Zhu, 2017; Dias et al., 2017; Ray et al., 2016; Aditya et al., 2017).
Iron is a micronutrient that is vulnerable to digestive conditions. It is affected by gastric pH, which decreases its activity before reaching the intestine, where it is absorbed (Galán et al., 2014). When encapsulating Fe to be used as an additive for food fortification, it must be taken into account the stomach pH and additionally its release into the small intestine. Fe Encapsulation has been reported using starch as a wall material by spray drying (Duran et al., 2017). On the other hand, Valenzuela et al. (2014) reported iron encapsulation, obtained from bovine erythrocytes, using classical coacervation. The authors reported capsules sizes from 830 µm to 1.57 mm and a low encapsulation performance.
Therefore, the objective of this work was to obtain Fe microcapsules by electrostatic coacervation to be used as a food additive. The work was developed in 2 stages: obtaining the suspension of the core material: sodium alginate/ferrous sulfate (SA/FS) in a first stage and obtaining the microcapsules by electrostatic coacervation using calcium chloride as a crosslinker material in a second stage. Once obtained the micro capsules of SA/ FS, they were physically and chemically characterized by measuring the particle size by means of SEM, Fe content, encapsulation efficiency, thermal stability, release profile and SA/FS solution viscosity.
Material and Methods
For iron encapsulation, ferrous sulfate (FS) in the form of heptahydrated crystals (CAS 7782-63-0) from Meyer, Química Suastes, S.A. de C.V. was used in different concentrations; sodium alginate (SA) (CAS 9005-38-3) (SA) was used as the wall material and calcium chloride was used as a crosslinker solution (CAS 10043-52-4) (CaCl2); both reactive from Deiman S.A. de C.V. (Mexico).
Preparation of Sodium Alginate/Ferrous Sulfate suspension and of the crosslinker solution
Five suspensions were prepared using 1.5 g SA in 60 mL of water. Suspensions were stirred on a magnetic plate (Model SP46925, Barnstead Thermolyne, USA) for 30 minutes at 25 °C, and then they were completed to a final volume of 90 mL. Subsequently, FS was solubilized in 10 mL of water. The solution was mixed with 90 mL of SA suspension to reach the following concentrations: 16, 25, 33, 50 and 75 % wall material. Suspensions were stirred (Model SP46, Barnstead Thermolyne, USA) for 5 min. The crosslinker solution was prepared with a concentration of 1N CaCl2.
Viscosity and conductance of SA/FS suspensions.
Viscosity of the SA/FS suspensions (16, 25, 33, 50 and 75 % in relation to the wall material) was determined by using a rheometer (model MCR-101, Anton Parr, Austria) at 25 °C, adapted with a ST242D/2v/2v-30 geometry. In addition, conductance was measured, by using a conductivity meter (model PCECM 41, PCE Ibérica, Spain) at 25 °C.
Electrostatic coacervation equipment
The electrostatic coacervation equipment was designed and built at CICATA-IPN Campus Querétaro (Figure 1). The equipment consists of a motorized syringe pump, built in the laboratory, where the SA/FS suspension is placed, a 30 G needle connected to the syringe pump through a silicone hose, a high voltage source variable from 1 to 30 KV to generate electrostatic attraction forces (model 30A24-P30, Ultravolt, USA), 1 to 12 VDC variable low voltage source (LM317) for syringe pump control, a magnetic stirrer (model PC-320, CORNING, USA) with a 250 mL beaker for the CaCl2 solution. The high voltage positive end was connected to the 30G needle and the high voltage negative end was placed under the beaker as shown in Figure 1.
Obtaining microcapsules through electrostatic coacervation process
SA/FS suspensions were placed in a 10 mL syringe with a 30 G needle, which was attached to the electrostatic coacervation equipment (Figure 1). The variables assessed for obtaining microcapsules were: high voltage, forward flow rate and distance between electrodes. The SA/FS suspension was deposited in a syringe, and by means of a syringe pump, drops were formed, forming capsules by coacervation with sodium alginate when falling into CaCl2. The flow rate ranged from 0.01 mL/min to 0.02 mL/min. The high voltage was connected to the needle, polarizing the SA/FS solution and, by electrostatic attraction forces, smaller drops were formed that fell into the crosslinker solution (CaCl2) in constant agitation and at a distance of 10 cm (Figure 1). The microcapsules formed by electrostatic coacervation were filtered and rinsed with water to remove excess CaCl2. The microcapsules of each treatment were stored in 50 mL glass bottles in refrigeration and protected from light until analysis.
Characterization of SA/FS microcapsules
Iron content and encapsulation efficiency
The iron content in the microcapsules was determined by using the volumetric technique using 1N potassium permanganate (Verde et al., 2013). 2 mg of microcapsules, 3 mL of distilled water and 1 mL of 10 % diluted sulfuric acid were placed and then stirred for 2 min. To determine the encapsulation efficiency, the following formula was applied, where Fet is the total added iron and Fes is the iron contained in the microcapsules (Equation 1).
Size and morphology of the microcapsule
For the study of the morphology of the microcapsule, a Phenom Pro environmental scanning electron microscope (Desktop SEM, Eindhoven, The Netherlands) was used, which was conditioned to work in a low-vacuum module, with the electron beam adjusted to 5 kV. Pictures were taken at 150X and 500X. To determine the size, a digital optical microscope with 2MP sensor was used in a range of 300X - 1000X in magnification. Sixty microcapsules per treatment were evaluated, showing the average size ± standard deviation. This test was performed in triplicate.
Microcapsule thermal stability
Thermal stability was analyzed in a Mettler Toledo Differential Scanning Calorimeter (model DSC1, Switzerland), 3 g of sample were weighed in a 40 µL aluminum crucible, which was sealed by mechanical pressure (ME-00119410 model). The sample was left to stand for 15 minutes. Subsequently, an isotherm was performed from 30 to 240 °C with a heating rate of 10 °C/min. An empty crucible was used as a reference. The temperature stability and the degradation enthalpy were reported. The measurements were made in duplicate. The measurement was reported ± standard deviation.
Microcapsule release profile
The release profile was evaluated by placing 12 grams of microcapsule in buffer solution at pH 2, 4 and 7.4 at 37 °C for 4 hours. The amount of iron released was analyzed every 10 minutes for the first 60 minutes, then measured every 30 minutes. The amount of iron was analyzed by volumetric titration (Cendejas & Ortega, 2017). The release percentage at a given time was reported. The test was performed in triplicate.
Statistical analysis
A randomized analysis was performed with a 33 factorial arrangement. The variables analyzed were the SA/FS ratio, the feed flow and the applied potential. Results were reported as mean ± standard deviation. Statistical analysis of the tests was performed by using an ANOVA and comparison of means by using Tukey’s test (p < 0.05) by using MINITAB 17.
Results and discussion
Characterization of SA/FS suspension
Figure 2 showed viscosity and electrical conductivity values of SA suspensions added with FS (SA/FS). The viscosity of the suspensions was between 224 ± 4.37 mPa•s (16 % FS ratio) up to 1227 ± 11.01 mPa•s (75 % FS ratio). These results were associated with SA molecular weight which influences directly on viscosity (Avendaño-Romero, 2013).
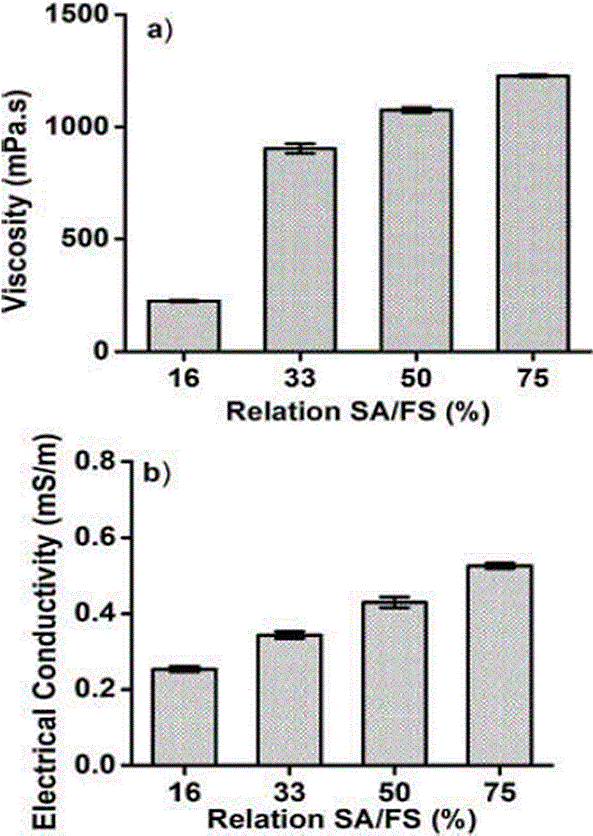
Figure 2 Viscosity. (a) and electrical conductivity (b) of sodium alginate and ferrous sulphate suspensions
The interaction of SA with FS showed a significant effect on the viscosity of the suspensions (p < 0.05). In Figure 2 (a), it is shown that as FS concentration increases, the viscosity increases. Soto-Martinez (2018) reported that the viscosity of the suspensions used is a determining factor in the formation of the microcapsules when the electrostatic technique is employed. The authors reported that at viscosity less than 800 cP, it was possible to generate droplets, which favored the formation of microcapsules. The concentration of 16 % of ferrous sulphate was the solution with the lowest viscosity and the one that allowed the best droplets formation and, consequently, the highest encapsulation efficiency.
Another determining factor for the electrostatic technique is the electrical conductivity. Figure 2(b) showed the conductivity as a function of FS concentration. The values obtained for conductivity were between 0.299 mS/m (16 % ferrous sulphate ratio) up to 0.581 mS/m (75 % ferrous sulphate ratio). The polysaccharides are not good conductors of electricity and the concentration of alginate was constant, so the change in conductivity was attributed to FS. Colin-Orozco et al. (2013) reported that conductivity is influenced by the type of solvent, the material dissolved and the availability of free ions, the latter two being associated with FS content. This coincides with the results where the increase in electrical conductivity is proportional to the increase in the amount of added iron, since FS is a salt that dissociates in water. Perez-Calderon et al. (2018), reported that water has 0.04 mS/m up to 0.2 mS/m, on the other hand, Quintero et al. (2018) reported values of 2 % chitosan-based solutions with an electrical conductivity of 0.214x10-3 mS/m. The 16 % FS suspension presented a conductivity of 0.299 mS/m, these conductivity values were higher than those reported due to the addition of FS. The latter is due to the fact that salts are dissociated in aqueous solutions causing greater conductivity.
Iron content and percentage of encapsulation efficiency
In Table 1, the effect of the variables of the electrostatic coacervation process on the formation of the microcapsules was shown. High voltage and feed low had no significant effect on microcapsules formation while the SA/FS ratio had a significant influence.
Table 1 Encapsulation efficiency and size of iron microcapsules obtained by electrostatic coacervation.
| Sample | SA-FS suspension (%) | Flow (mL/min) | Voltage (KV) | Iron content (mg) | Encapsulation efficiency (%) | Tamaño de partícula (µm) |
|---|---|---|---|---|---|---|
| 1 | 16 | 0.012 | 6 | 164.06 ± 1.82c | 65.73 ± 0.73ª | 847.60 ± 57.80ª |
| 2 | 16 | 0.014 | 6 | 162.24 ± 1.82c | 65.00 ± 0.73ª | 643.95 ± 46.65c,d,e |
| 3 | 16 | 0.016 | 6 | 165.28 ± 1.05c | 66.22 ± 0.42ª | 455.80 ± 25.71g,h,i,j |
| 4 | 16 | 0.012 | 7 | 166.49 ± 2.78c | 66.46 ± 0.73ª | 842.95 ± 45.03a,b |
| 5 | 16 | 0.014 | 7 | 164.67 ± 1.05c | 65.97 ± 0.42ª | 482.50 ± 32.19e,f,g,h,i,j |
| 6 | 16 | 0.016 | 7 | 164.06 ± 4.82c | 65.73 ± 1.93ª | 365.10 ± 35.16j |
| 7 | 16 | 0.012 | 8 | 167.71 ± 3.16c | 66.94 ± 1.11ª | 632.30 ± 29.14c,d,e,f |
| 8 | 16 | 0.014 | 8 | 161.02 ± 1.05c | 64.51 ± 0.42ª | 545.95 ± 28.35d,e,f,g,h,i |
| 9 | 16 | 0.016 | 8 | 162.85 ± 2.10c | 65.24 ± 0.84ª | 456.15 ± 29.79 g,h,i,j |
| 10 | 33 | 0.012 | 6 | 329.94 ± 1.82b | 65.73 ± 0.36ª | 676.25 ± 32.70b,c,d |
| 11 | 33 | 0.014 | 6 | 330.55 ± 2.10b | 65.85 ± 0.42ª | 538.40 ± 46.68 d,e,f,g,h,i |
| 12 | 33 | 0.016 | 6 | 328.12 ± 4.82b | 65.36±0.96ª | 454.40 ± 27.87 g,h,i,j |
| 13 | 33 | 0.012 | 7 | 330.55 ± 1.05b | 65.50±0.21ª | 646.30 ± 13.37c,d,e |
| 14 | 33 | 0.014 | 7 | 328.12 ± 1.82b | 65.36 ± 0.36ª | 576.55 ± 14.83 c,d,e,f,g,h |
| 15 | 33 | 0.016 | 7 | 328.12 ± 1.82b | 65.36 ± 0.36ª | 462.15 ± 21.70 f,g,h,i,j |
| 16 | 33 | 0.012 | 8 | 326.91 ± 1.05b | 65.12 ± 0.21ª | 615.10 ± 22.52c,d,e,f,g |
| 17 | 33 | 0.014 | 8 | 328.73 ± 2.78b | 65.61 ± 0.56ª | 585.85 ± 21.71c,d,e,f,g |
| 18 | 33 | 0.016 | 8 | 328.73 ± 1.05b | 65.73 ± 0.36ª | 409.65 ± 14.66h,i,j |
| 19 | 50 | 0.012 | 6 | 449.65 ± 1.05b | 59.82 ± 0.14b | 717.60 ± 18.55a,b,c |
| 20 | 50 | 0.014 | 6 | 452.08 ± 0.00a | 60.14 ± 0.00b | 655.30 ± 20.81c,d |
| 21 | 50 | 0.016 | 6 | 447.82 ± 2.78ª | 59.57 ± 0.37b | 517.40 ± 18.86 d,e,f,g,h,i,j |
| 22 | 50 | 0.012 | 7 | 449.04 ± 1.05ª | 59.73 ± 0.14b | 662.45 ± 21.65c,d |
| 23 | 50 | 0.014 | 7 | 450.86 ± 1.05ª | 59.98 ± 0.02b | 557.90 ± 25.28 c,d,e,f,g,h |
| 24 | 50 | 0.016 | 7 | 450.26 ± 1.82ª | 59.90 ± 0.24b | 455.50 ± 23.16 g,h,i,j |
| 25 | 50 | 0.012 | 8 | 449.04 ± 1.05ª | 59.73 ± 0.02b | 508.75 ± 20.80,d,e,f,g,h,i,j |
| 26 | 50 | 0.014 | 8 | 449.04 ± 1.05ª | 59.73 ± 0.02b | 468.25 ± 23.04e,f,g,h,i,j |
| 27 | 50 | 0.016 | 8 | 448.43 ± 1.82ª | 59.49 ± 0.37b | 381.45 ± 19.17i,j |
SA: sodium alginate, FS: ferrous sulphate. Averages with different letters in the same column indicate significant differences (p ≤ 0.05).
The results obtained showed that when FS concentration was lower (16 %), the encapsulation efficiency (EE) was higher. The EE was between 59.49 ± 0.37 % (50 % of FS) and 66.94 ± 1.11 % (16 % of FS) (Table 1). There are varied previous studies on the effect of FS concentration on EE. Valenzuela et al. (2014) reported values of 59.5 % to 75.7 % EE using hemic iron obtained from erythrocytes.
On the other hand, Table 1 showed that as FS concentration increased, the EE decreased and the iron content increased, that is, the retention capacity decreased, which indicated that the salt is found inside the alginate microparticle as well as it could be adhered to its surface. Another reason why EE decreased as the SA/FS ratio increased may be associated with a disproportionate amount of wall material, which generated material incrustations that made EE decrease (Cuatzo, 2010), and increasing viscosity, which interfered with drop formation. Iron III salts are reported in the literature to have up to 80 % EE, however, they are less bioavailable for the body (Duran et al., 2017).
Microcapsule size and morphology
The size of the microcapsules obtained was shown in Table 1. The conditions of the electrostatic potential, the SA/FS ratio had a significant effect on this parameter. This may be related to the fact that the higher the iron content, the higher the viscosity, due to the high content of iron salts, making the formation of the microcapsules more difficult.
The morphology of the iron microcapsules obtained by electrostatic coacervation was presented in Figure 3. The microcapsules had a spherical shape and their sizes ranged between 350 and 850 µm, being lower values than those reported in the literature for capsules obtained by coacervation (Valenzuela et al., 2014). Microcapsules were observed to not have a spherical shape, but with similar sizes. Samples 11, 14, 18 and 23 showed a typical effect of electro spinning, which was the incorporation of monofilaments adhered to the microcapsule (Figure 3). Other treatments showed a rough appearance on the surface of the microcapsule (Figure 3, samples 1, 8, 9, 22, 24 and 27), this may be due to the combination of the feed flow and the electrical potential applied. While in treatments 10, 16, 25 and 26 (Figure 3), cracks were present. Menger et al. (1998) indicated that ionic electrostatic bonds can form positive to negative aggregates in the formation of coacervations, appearing as deformations, which can be accentuated by the potential applied.
Treatments 19, 20 and 21 (Figure 3) showed a smooth surface appearance, possibly due to the difference of electrical potential. Treatments 2, 3, 4, 5 and 6 (Figure 3), which contained 16 % FS, were spherical and with an uniform appearance.
Valenzuela et al. (2014), reported capsules from 830 µm to 1.57 mm, using Dehydrated Bovine Erythrocytes (EBDA) as a source of iron using classical coacervation. Durán et al. (2017) reported that iron encapsulation using spray drying generated particle sizes between 200 and 600 µm, smaller than those found in the present work. However, the technique used by the authors can oxidize iron, which would affect its bioavailability in the organism.
Processing conditions of 16 % FS with a voltage of 8 KW and a flow rate of 0.012 mL/min were the ones that allowed to obtain the highest encapsulation efficiency (66.94 ± 1.11), besides, with a spherical appearance, uniform with a size of 632.30 ± 29.14 µ. Therefore, this treatment was selected for thermal stability tests and to determine the release profile of the encapsulated compound.
Thermal stability of the microcapsule
To compare the thermal stability offered by the microcapsule to the iron, free FS, a microcapsule with iron and without iron were used, both obtained with a feed flow of 0.12 mL/min and an applied voltage of 8 kw to 10 (Figure 4). The FS showed two endotherms, one between 66 °C and 80 °C, and the other at 117 °C - 131 °C (Figure 4), the first corresponding to instability (72 °C), which affected its functionality.
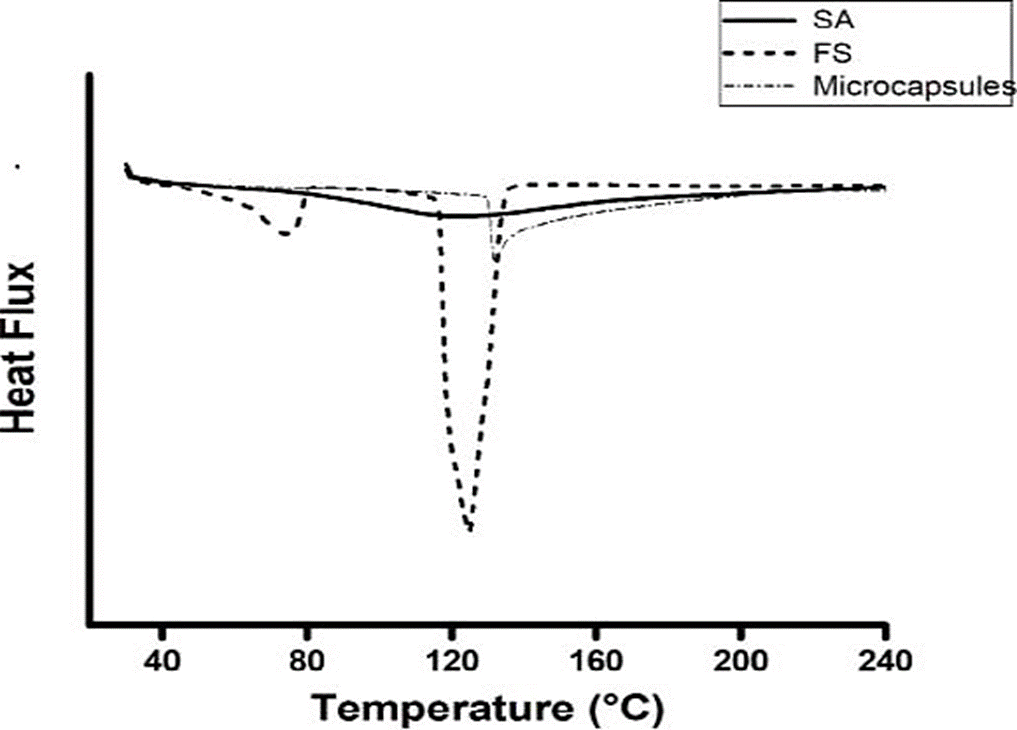
Figure 4 Thermal stability of ferrous sulphate (FS), microcapsules of sodium alginate (SA) and microcapsules of SA/FS.
SA/FS microcapsules obtained by electrostatic coacervation showed an endotherm between 130 °C and 141 °C. These results showed that the wall material (Alginate) offers a thermal protection to the encapsulated material (Ferrous sulphate). Rodríguez-Llimós et al. (2003) reported that SA as a wall material provides thermal stability to the encapsulated materials, when encapsulating paracetamol.
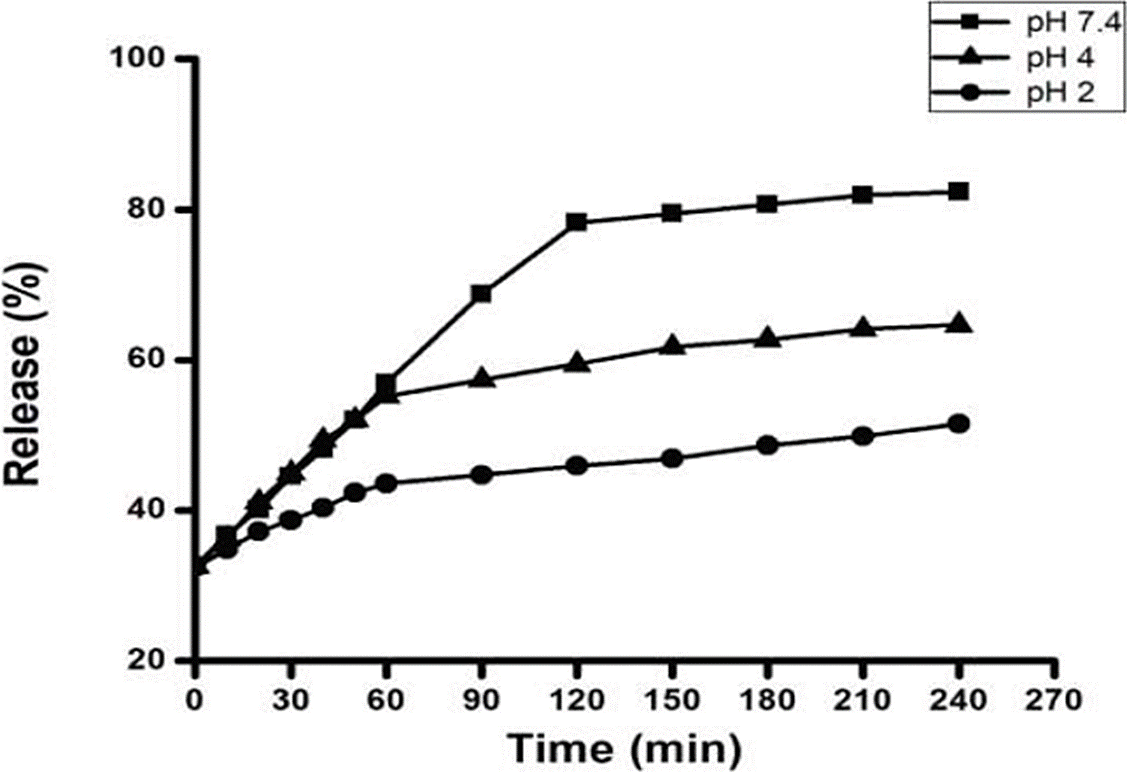
Fe release profile of SA/FS microcapsules
The mechanism of controlled release of FS was evaluated at pH 2.0, 4 and 7.2 (Figure 5). A slower release of FS was observed from coacervates at pH 2 compared to coacervates at pH 4 and 7 (p ≤ 0.05) (Figure 5). Barros et al. (2016) reported that this same combination, by passing the microcapsules from an acidic medium to a basic medium, showed an improvement in the release of the compound.
The microcapsules that were subjected to pH 7.4 showed a burst effect (large percentage of material released in a short time) in the second hour, followed by a gradual release in the rest of the time (p ≤ 0.05) (Figure 5). The release at pH 4 and 7 did not present significant differences until 60 min of exposure to pH. However, at 240 min it was 82.39 ± 0.72 % at pH 7.4, followed by pH 4 with 64.37 ± 0.28 % and at pH 2 the release was 21.52 ± 1.68 %.
Sankalia et al. (2005) described very short times of release, for SA capsules when encapsulating papain. The authors released 100 % of its content in 60 minutes of in vitro incubation in simulated intestinal solution at pH 6.8; discussing this fact as a consequence of an increase in the affinity between Ca2+ ion and Na+ phosphate buffer, weakening the crosslinker polymer, generating the total disintegration of the SA matrix.
On the other hand, Durán et al. (2017) reported a release time of three hours of the sensitive components, such as minerals and vitamins, encapsulated with SA in media with a pH above 6.5, which corresponds to the results found in this study. Based on the results found, it would be advisable to incorporate the iron microcapsules obtained by electrostatic coacervation, in foods with acidic pH to ensure their integrity and preservation of Fe for a longer time. On the other hand, the results found are encouraging since the pH of the small intestine, where the iron must be released to be absorbed, is approximately 7.
Conclusion
The electrostatic coacervation technique allowed microencapsulation of FS with high encapsulation efficiency (66.94 ± 1.11%) in average particle sizes of 600 µm, under processing conditions of 0.12mL/min power flow and an applied voltage of 8 kw at 10 cm of separation between electrodes.
The SA/FS microcapsules obtained presented a thermal stability to iron up to 130 ºC according to the measurements performed with DSC. This thermal stability of the SA/FS capsules would allow them to be added to food and cooked below 120 °C without compromising the nutritional quality of the compound.
Fe release profile of the SA/FS microcapsules obtained by electrostatic coacervation showed that Fe is released up to pH 7. This pH 7 value allowed to infer that the micronutrient could be released in the small intestine and not in the stomach.











 text in
text in