Introduction
White-tailed deer (Odocoileus virginianus) is a species of great ecological and socioeconomic importance in Mexico (Clemente et al., 2015). That is why aspects like productivity and hormonal levels have to be studied for a better management of the species (Shipka et al., 2007), both for its conservation or exploitation (Pelletier et al., 2003). Hormones regulate individuals’ physiology and behavior, as well as their growth, body condition, social behavior, and above all, reproduction (Morden et al., 2011). In deer present in temperate and cold areas, photoperiod affects hormonal production, since it is responsible for the adaptation of deer to high latitudes, being the reproductive season when photoperiod decreases and reproductive hormones increase (Garcia et al., 2005). In most mammals, photoperiod is the most important environmental stimulus for determining the reproductive season, and in species with seasonal reproduction, light is the determining environmental factor of their reproductive cycles since these animals use the variation of daylight hours to know the time of the year they are in (Pérez, 2014).
Seasonal changes in vegetation availability and quality as well have been observed to possibly affect glucocorticoid production level in white-tailed deer (Millspaugh & Washburn, 2004). White-tailed deer is a very sensitive species and gets easily stressed by disturbances caused by human activities, including those associated to hunting activities, entertainment or ecotourism, provoking alterations in their hormonal production (Brown et al., 2012). This situation has economic and conservative implications; therefore, there is interest in knowing which factors influence hormonal production and for mitigating those causing stress or having deleterious effects on populations (Millspaugh & Washburn, 2004).
Although the effects of stress on wild fauna are not well known, some research studies suggest a possible long-time deleterious physiological effect (Brown et al., 2012). Glucocorticoid secretion occurs during the response to stress, which enables the animal to face stress, mobilizing energy required for this response and minimizing the energy cost in tissues which are not necessary for immediate survival (Huber et al., 2003a). Regarding the relation between glucocorticoids and testosterone, glucocorticoids are believed to possess a suppressive effect on androgens production (McCoy & Ditchkoff, 2012). Testosterone, together with estradiol, constitute a group of essential sexual steroid hormones, which regulate not only reproductive patterns in females and males during the reproductive season, but also regulate sexual and physiological behavioral aspects (for instance antlers production) during the life of individuals (Soto et al., 2004). Previously, hormonal levels have been measured through blood samples, which require capturing and restraining the animals; however, these activities are triggering stress (Kapke et al., 1999). Stress caused by capture can affect blood sampling by modifying hormones concentrations; consequently, hormonal measurements can be biased (McCoy & Ditchkoff, 2012). Since feces can be collected without disturbing and stressing individuals, it has become a very common sampling technic in behavioral ecological and genotyping studies (Huber et al., 2003b).
White-tailed deer populations present in the Sierra Madre Occidental of the state of Durango have been little studied from the hormonal point of view. At the same time, some producers have placed enclosures with the purpose of initiating or intensifying deer production or hunting activity in their properties. It is believed that a captive population and its frequent contact with people and their activities, could produce major cortisol levels in feces, and, in turn, affect reproduction. On its behalf, vegetation cover, translated as the food and protection availability for deer, could be one of the leading environmental variables triggering stress in white-tailed deer populations, as in free-living as in an enclosure. Therefore, the objectives of the present study were: 1) to identify the sex of the individuals producing feces by means of fecal Deoxyribonucleic Acid (DNA) amplification, 2) to determine cortisol, testosterone and estradiol levels in white-tailed deer feces in two populations, as in free-living as in enclosure, 3) to perform a correlational analysis between hormones and environmental variables of each site to know whether some environmental factor is fostering stress and, 4) to compare hormonal levels among populations, sexes and time of the year.
Material and Methods
Study area
White-tailed deer feces were collected in two Management Units for Wildlife Conservation (UMA) located in the municipality of Durango, Durango, Mexico. Wildlife population was present in Salvador Allende (SA). Estimated population density during winter was of 2.52 deer/km2 (Vega et al., 2016). This UMA was located at coordinates 24° 71’-24° 05’ N and 104° 51’-104° 56’ W, with an altitude of 2,200-2,680 masl, and a temperate semi-cold and temperate semi-humid climate. This UMA had a 3,200 ha area of free movement of white-tailed deer. Main types of vegetation in this UMA were pine tree-holm oak forest, pine trees forest, and holm oak forest (González et al., 2007). The population in natural enclosure was present in Molinillos (MO). Estimated population density during winter was of 4.58 deer/km2 (Vega et al., 2016). This UMA was located at coordinates 23° 36’- 23° 39’ N and 104° 59’- 105°06’ W, with an altitude of 2,000-2680 masl. This UMA had a 300 ha area enclosed with a deer fence. The climate in this site was temperate sub-humid and temperate semi-cold. Main types of vegetation were pine trees forest, pine tree-holm oak forest, holm oak forest, and pasture land (Rosales & Villanueva, 2014).
Feces sampling and classification
Fresh feces were collected every two weeks for 13 months in each UMA covering: spring (March 21st-June 21st), summer (June 22nd-Septembre 22nd), autumn (September 23rd-December 21st) and winter (December 22nd-March 20th). Sampling period in Salvador Allende spanned from March 1st of 2015 to March 31st of 2016, and in Molinillos from October 1st of 2015 to October 31st of 2016. The sites with higher activity of white-tailed deer were established for each UMA, in sampling taken previous to the sampling period. Two 1,000-m-long transects were gone through during each sampling visit. Twenty-thirty superior fecal pellets of each group found during sampling were placed in 50 mL Falcon ® tubes with ethanol 96 % to be used in DNA extraction. The other part of the pellets was conserved in plastic bags at -20 °C. Length and width of fecal pellets of each group were measured before storing them in freezing conditions. The volume of fecal pellets was calculated from these data, and the mean was obtained per fecal group to assign them to a category of age and sex. For this, belonging values were calculated for three groups (adult females, adult males, and juveniles) through fuzzy K-means clustering algorithm (Sánchez-Rojas et al., 2004; Sánchez-Rojas et al., 2009). Fuzzy Clustering Tool (Equihua, 2000) software was used. The identification of sex was performed through molecular methods, to obtain the sex of fecal groups of juveniles, as well as to confirm sex classification in fecal groups of adults.
DNA extraction in white-tailed deer feces
DNA was isolated through CTAB-acetate method from two to four pellets maintained in ethanol 96 %. Firstly, alcohol was evaporated for 45 min at room temperature in Petri dishes. Once dried, they were chopped with sterile bistouries, and 0.5 g of the sample was placed in 1.5 mL micro-centrifuge tubes. One mL of extraction buffer (2 % CTAB, 100 mM TRIS, pH 8, 20 mM EDTA, pH 8, 1.4 M NaCl and 1 % PVP) and 5 µL of 2-mercaptoethanol were added to each tube. Tubes were shaken in vortex (Labnet ®) at 3,400 rpm for 10 s and placed one hour in digestion in a thermos-shaker (TermoFisher® mod. MSC-100) at 65 °C. After one hour of digestion, 50 µL of Proteinase K were added and placed in digestion one more hour at 65 °C. To separate the solid part of the sample, tubes were placed in a centrifuge (Eppendorf® 5415) for 15 min at 13,200 rpm. The supernatant was placed in new tubes, and 400 µL of chloroform-isoamyl alcohol (24:1) were added, they were shaken through vortex at 3,400 rpm for 10 s and placed in the centrifuge for 6 minutes at 13,200 rpm. The supernatant was placed in new tubes, and 300 µL of ammonium acetate (3 M, pH 5.2) and 600 µL of isopropanol were added. Tubes were maintained at -20 °C overnight. Afterward, tubes were centrifuged for 15 min at 13,200 rpm. The supernatant was thrown away, and a DNA pellet remained at the bottom of the tube. DNA pellet was washed by adding 500 µL of ethanol 80 %, was shaken in vortex for 10 s at 3,400 rpm and was centrifuged for 6 min at 13,200 rpm. The washing step was performed twice. Finally, ethanol 80 % was thrown away, and the tubes were dried. DNA was hydrated with 55 µL of molecular grade water (Cellgro Corning® 46000). Purity and quantity of DNA in ng/μL was obtained through a Nanodrop® 2000 (Thermo Scientific®) spectrophotometer. The DNA quality was observed through 1 % agarose gels dyed with ethidium bromide.
Sex identification from DNA in feces
To identify sex in individuals that fecal groups produced, a fragment of SRY gene was amplified utilizing Polymerase Chain Reaction (PCR) with the primers pair: SRY forward CAT CTT GTC TGT GTG TCG TG and SRY reverse: CGG GTA GTG TCG TTT GTC TA (Lounsberry et al., 2015). Three samples of tissue coming from adult male deer hunted in Salvador Allende UMA were used as positive controls. Final concentrations for PCR reaction were 100-150 ng/μL of DNA, 2.5 mM MgCl2, 0.5 mM dNTP’s, 0.6 pM of PCR primers and 0.750 unities of Taq DNA polymerase. PCR conditions were 3 min at 95 °C of initial denaturalization, 35 cycles of 30 s at 94 °C, 1.30 min at 58 °C, 1 min at 72 °C, finally, 10 min at 72 °C of final elongation. Each event of PCR reaction includes a positive control and negative control (without DNA). PCR products were observed in 1 % agarose gel dyed with ethidium bromide. A molecular weight marker, as well as positive and negative controls, were added in each gel. To ensure the quality of the results, DNA extractions, PCR reactions, and the observation of PCR products in gel were performed in duplicate. Samples that did not present amplification were taken as females, while samples displaying one band (~200 pb) were taken as males (Figure 1). Samples showing diffuse bands were not taken into account for the study.
Hormonal analysis
0.5 g of the frozen stored feces were taken to realize total steroid extraction. For total steroid extraction, Pavitt et al. (2015) method was used with some modifications. Samples were defrosted and placed in 2 mL micro-centrifuge tubes, and 720 µL of methanol and 80 µL of distilled water were added. Tubes were shaken in vortex for 10 min at 3,400 rpm and maintained in a plate shaker overnight in constant movement. Tubes were centrifuged for 15 min at 2000 rpm, and the supernatant was transferred to new sterile tubes. These extracts of total steroid were diluted 1:20. 20 µL of the diluted extracts were used for hormone quantification. For this, Cortisol ELISA Kit, Estradiol Elisa Kit, and Testosterone Elisa Kit commercial kits, from Cayman® brand, were used, according to manufacturer indications. For cortisol, the sensitivity was of 0.07 ng/g and intra- and inter-assay coefficients of variation were of <15 and <25 %, respectively. For estradiol, the sensitivity was of 0.03 ng/g and intra- and inter-assay coefficients of variation were of <18 and <30 %, respectively. For testosterone, the sensitivity was of 0.012 ng/g and intra- and inter-assay coefficients of variation were of <20 and <15 %, respectively. Samples were analyzed in an ELISA plate-reader, EliRead brand of KontroLab®, and the concentration of each sample was determined by using the equation obtained when plotting standards for each hormone.
Vegetation and environmental variables sampling
Canfield lines and point-centered quadrants were performed to estimate vegetation cover at each season, in parallel sites to sampling sites of fecal groups. Canfield lines were realized in a portion of 2 m until reaching 20m of long for a higher sampling representation (Mostacedo & Fredericksen, 2000). In addition, ten sites were randomly sampled in point-centered quadrants, for bushy species (Mostacedo & Fredericksen, 2000). Data on temperature, precipitation, and photoperiod were obtained from governmental institutions, Water National Commission (CONAGUA) and Mexican Institute of Water Technology (IMTA).
Statistical analysis
To determine differences in steroid hormone levels between sexes and between UMA at each season of the year, a factorial analysis of variance (ANOVA) was performed, where factors were UMA, season and sex (Proc GLM, SAS Institute 9.2, Cary, NC, USA). Besides, Pearson coefficient of correlation was obtained to connect hormone quantity (cortisol, testosterone or estradiol) for each UMA with its environmental variables as temperature, precipitation, photoperiod and vegetation cover (Proc Corr, SAS Institute 8.0, Cary, NC, USA).
Results and Discussion
A total of 385 fresh feces were collected in both UMA, covering all seasons (Table 1). Through the amplification of SRY gene fragment, 219 feces were identified as female and 166 as males (Table 1). Male: female relationship for Salvador Allende was 1:2.02 and for Molinillos 1:1.12. Generally, cortisol, testosterone, and estradiol levels in feces increased as seasons progressed to winter, and photoperiod decreased (Figure 2). Significant negative correlations were found between estradiol with temperature and photoperiod in SA (Table 2). On the other hand, in MO, a negative correlation was observed between cortisol with temperature and photoperiod, testosterone with temperature and photoperiod, estradiol with temperature and photoperiod (Table 2). Seasonal environmental characteristics for each UMA were shown in Table 3. The increase in hormonal levels in feces coincided with the reduction of photoperiod and the presence of the reproductive season (autumn-winter). This is because photoperiod was the primary environmental mechanism which controls seasonal changes through the regulation of the hypothalamus-pituitary-gonads axis (Li et al., 2001), since the pineal gland functioned as a neuroendocrine translator of the circadian and circannual variation of the photoperiod and produced melatonin in response to darkness, and high levels of melatonin rise steroid reproductive hormone production (Bubenik, 2006). The adaptive strategy to photoperiod ensured that pregnancy and lactation period coincide with the period where there were the highest source and variety of food (Li et al., 2001).
Table 1 Number faecal groups collected by season in two Wildlife Management and Conservation Units (UMA) in Durango, Mexico, categorized by the age (obtained by faecal morphometry) and sex (by SRY gene marker amplification). (F = females, M = males).
| Salvador Allende | Molinillos | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Yearlings | Adults | Yearlings | |||||||
| Season | F | M | F | M | Total | F | M | F | M | Total |
| Spring | 10 | 2 | 15 | 9 | 36 | 16 | 3 | 19 | 18 | 56 |
| Summer | 6 | 1 | 2 | 4 | 13 | 3 | 6 | 6 | 21 | 36 |
| Autumn | 8 | 1 | 6 | 8 | 23 | 40 | 13 | 26 | 51 | 130 |
| Winter | 16 | 3 | 12 | 9 | 40 | 20 | 2 | 14 | 15 | 51 |
| Total | 40 | 7 | 35 | 30 | 112 | 79 | 24 | 65 | 105 | 273 |
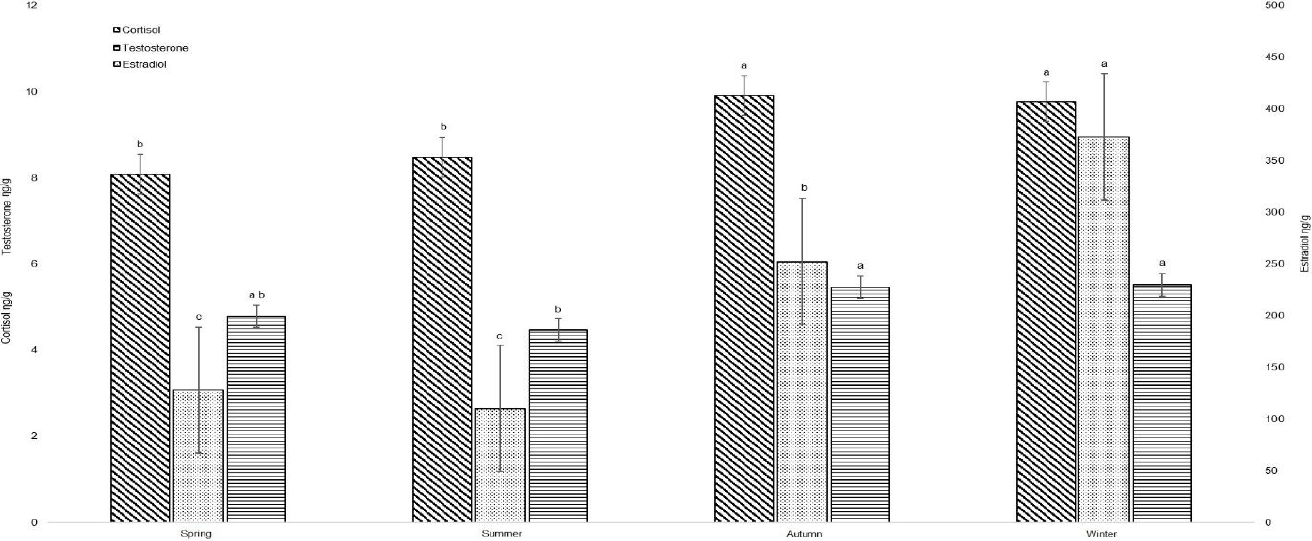
Figure 2 Cortisol, testosterone, and estradiol mean levels in feaces from white-tailed deer in two Wildlife Management and Conservation Units (UMA) in Durango, Mexico. The same letter on the bars with the same pattern indicates no significant differences. (Cortisol F3,103 = 16.28, p<0.0001, testosterone F3,103=3.73, p=0.0136, estradiol F3,103=6.68, p=0.0004).
Table 2 Pearson correlation coefficients matrix between cortisol, testosterone (T), estradiol (E2), and environmental variables in two Wildlife Management and Conservation Units (UMA) in Durango, Mexico.
| Salvador Allende | |||||||
|---|---|---|---|---|---|---|---|
| Cortisol | T | E2 | Temperature | Precipitation | Photoperiod | Cover | |
| Cortisol | - | 0.37300 | 0.16748 | -0.14172 | 0.16823 | -0.26218 | 0.33104 |
| p | 0.0463 | 0.3763 | 0.4550 | 0.3742 | 0.1616 | 0.0740 | |
| T | - | - | -0.31834 | -0.01084 | 0.09551 | -0.13959 | 0.34732 |
| p | 0.0924 | 0.9555 | 0.6221 | 0.4702 | 0.0649 | ||
| E2 | - | - | - | -0.60536 | -0.35185 | -0.34895 | -0.23595 |
| p | 0.0004 | 0.0566 | 0.0588 | 0.2094 | |||
| Molinillos | |||||||
| Cortisol | T | E2 | Temperature | Precipitation | Photoperiod | Cover | |
| Cortisol | - | 0.43491 | 0.38241 | -0.51328 | -0.21410 | -0.73204 | -0.58998 |
| p | <0.0001 | 0.0002 | <.0001 | 0.0372 | <.0001 | <.0001 | |
| T | - | - | 0.20859 | -0.34290 | -0.08039 | -0.33039 | -0.32017 |
| p | 0.0498 | 0.0009 | 0.4488 | 0.0014 | 0.0020 | ||
| E2 | - | - | - | -0.44417 | -0.12131 | -0.51178 | -0.40643 |
| p | <.0001 | 0.2441 | <.0001 | <.0001 | |||
p=significance; Salvador Allende=free-living population; Molinillos=enclosured population.
Table 3 Environmental characteristics by season in two Wildlife Management and Conservation Units (UMA) in Durango, Mexico, during 2015 and 2016.
| Salvador Allende | Molinillos | |||||||
|---|---|---|---|---|---|---|---|---|
| Season | T (°C) | PP (mm) |
Photoperiod (h) |
Cover (%) | T (°C) | PP (mm) |
Photoperiod (h) |
Cover (%) |
| Spring | 9.2 | 2.6 | 13.31 | 36.5 | 2.9 | 1.1 | 12.92 | 57.8 |
| Summer | 11.2 | 13.8 | 12.95 | 75.2 | 11.3 | 8.9 | 12.99 | 85.8 |
| Autumn | 4.0 | 0.0 | 11.15 | 66.5 | 4.8 | 0.3 | 11.28 | 54.4 |
| Winter | 2.7 | 0.0 | 11.09 | 57.4 | 0.3 | 0.0 | 11.15 | 51.9 |
T = mean temperature by season. PP=mean pluvial precipitation by season. Photoperiod=mean hours of daylight by season. Cover=percentage of vegetal cover of browse-shrub stratus by season.
Regarding vegetation, no correlation was found between steroid hormones and vegetation cover in SA. However, a negative correlation was observed between vegetation cover and cortisol, testosterone, and estradiol in MO (Table 2). Cortisol levels could be influenced by diet and vegetation type. During winter, deer living in temperate areas had to deal with a reduced abundance of plants and a low forage quality, and, in turn, with high energy demands caused by thermoregulation and locomotion (Taillon & Côté, 2008). When body condition is low, herbivores used body reserves and muscle proteins to survive, rising glucocorticoids secretion, since they mobilized energetic reserves required to face environmental conditions (Huber et al., 2003a). In this study, cortisol levels in feces were not different between populations for autumn and winter. However, in spring and summer, the free-living population had higher cortisol levels in feces than the population in the enclosure and the vegetation cover was lower during this period, although the correlation was not statistically significant (Table 4).
Table 4 Cortisol, testosterone and estradiol levels in feaces from white-tailed deer in two Wildlife Management and Conservation Units (UMA) in Durango, Mexico.
| Season | UMA | Females | Males | ||||
|---|---|---|---|---|---|---|---|
| Cortisol ng/g |
T ng/g | E2 ng/g | Cortisol ng/g |
T ng/g | E2 ng/g | ||
| Spring | SA | 9.64 ± 0.50 | 3.43 ± 1.00 | 287.87 ± 125.50 | 8.64 ± 0.94 | 3.75 ± 1.00 | 153.49 ± 115.93 |
| M | 8.07 ± 0.67 | 5.02 ± 1.15 | 112.91 ± 83.68 | 7.76 ± 0.47 | 4.90 ± 1.07 | 125.39 ± 84.61 | |
| Summer | SA | 10.18 ± 0.84 | 5.06 ± 0.99 | 181.62 ± 67.37 | 9.57 ± 0.77 | 4.18 ± 0.95 | 203.91 ± 80.45 |
| M | 8.36 ± 0.62 | 4.94 ± 0.75 | 103.83 ± 42.72 | 7.63 ± 0.56 | 4.29 ± 0.70 | 79.10 ± 39.20 | |
| Autumn | SA | 9.88 ± 0.49 | 4.99 ± 1.16 | 148.80 ± 163.33 | 10.33 ± 1.20 | 5.04 ± 0.99 | 321.10 ± 150.25 |
| M | 9.92 ± 0.55 | 4.97 ± 1.44 | 279.60 ± 235.47 | 9.89 ± 1.18 | 5.70 ± 1.26 | 241.10 ± 198.64 | |
| Winter | SA | 10.09 ± 0.83 | 3.92 ± 1.15 | 392.37 ± 162.45 | 9.48 ± 1.15 | 4.30 ± 0.51 | 319.60 ± 144.98 |
| M | 10.12 ± 0.66 | 4.39 ± 1.64 | 397.19 ± 177.81 | 9.52 ± 0.88 | 5.54 ± 0.96 | 344.60 ± 168.29 | |
T=testosterone. E=estradiol. SA=Salvador Allende, free-living population. M=Molinillos, enclosured population.
Average cortisol level in feces was higher in SA than in MO (F1,103=31.87, p<0.0001) (Figure 3). These differences were more evident in spring and summer (F3,103=8.17, p<0.0001) (Table 4). For enclosure conditions, cortisol levels were expected to be higher in MO. Millspaugh & Washburn (2004) observed that wild animals which were submitted to enclosures were able to produce a higher glucocorticoid quantity than free-living animals. This is because a bit before a stressing situation was produced, the hypothalamus-pituitary-adrenal axis was activated, and the results were the high glucocorticoid production (He et al., 2004), but, as time progressed and stress-causing conditions carried on, the body may create a regulatory response in such a way that glucocorticoids production decreases below normal levels and may reflect the final step of stress in animals that have been reared in captivity since they were born (Linklater et al., 2010).
This phenomenon could be an explanation for the population in Molinillos, since this population has been maintained for 13 years in natural enclosure. However, He et al. (2004) observed that in reproduction center of musk deer (Moschus sp.) in semi-captivity or natural enclosure, individuals showed less stress-associated behavior (for instance anxiety and hypersensitivity) than those who lived in captivity. Therefore, environments created in natural enclosures were suggested not to be detrimental for musk deer (Liu et al., 2010). Contrary to MO, hunting activity was allowed in SA, and therefore cortisol levels in feces in this UMA could have been affected by this activity. This may suggest that natural enclosure conditions maintained in Molinillos were not generating high stress levels, and probably that the population density was not high enough yet to affect stress hormones production.
Cortisol was observed to decrease in males as testosterone decreased as well in both UMA (Table 4). A significant correlation was observed as well between testosterone and estradiol in MO (Table 2). McCoy & Ditchkoff (2012) reported that reproductive activity rose glucocorticoid levels in white-tailed deer males, as observed in the present study; Pelletier et al. (2003) observed the same in bighorn sheep (Ovis canadiensis) and Chunwang et al. (2004) in Père David’s deer (Elaphurus davidianus). In this study, the highest cortisol levels in both UMA were observed to be present before the reproductive season and to be maintained in winter. Similarly, these high cortisol levels correspond to testosterone levels (Table 4). The increase in cortisol was attributed to the increase of testosterone that, in turn, promote aggressive interactions among males with the purpose of establishing dominance (Chunwang et al., 2004). In white-tailed deer, competition among males to mate with a higher number of females in estrum, through the intimidation of other males, occurred at the beginning of the reproductive season to establish or maintain hierarchy of dominance and to be able to reproduce (Ronsberry et al., 2001; Garcia et al., 2005). As animals which exerted dominance as those which were subordinates have been observed to present high levels of stress and cortisol production rose a bit at the beginning of the reproductive season when the hierarchy was established (Bartos et al., 2010). In this study, this was corroborated while observing a positive correlation between cortisol and testosterone in feces in both populations.
Regarding cortisol levels between sexes, females were observed to have higher levels than males (F1,103=27.85, p<0.0001), especially during summer and winter (F3,103=3.46, p=0.0192) (Table 3). Huber et al. (2003a) observed higher levels of cortisol metabolites in winter (December and January) than in the rest of the year in red deer, but they did not find any significant differences between females and males in a month in particular. On the other hand, He et al. (2014) found differences in cortisol levels between sexes for musk deer, males had significantly less fecal glucocorticoids than females. McCoy & Ditchkoff (2012) observed higher levels of fecal glucocorticoids in females of white-tailed deer before and during the reproductive season. Yoshimura et al. (2003) mentioned that the difference in adrenocortical activity between females and males was common in various species. These differences may be caused by reproductive hormones, glucocorticoids receptors, and their binding proteins. Differences between sexes may reflect a difference in steroid metabolism, extraction routes, and response to the pituitary gland (Huber et al., 2003a).
In this study, females were observed to show higher testosterone levels in feces than males in summer (F3,103=5.79, p=0.0011) (Table 3). López & Montes (2016) observed a higher quantity of metabolites of fecal testosterone in females in white-tailed deer, although it was not significantly different. Also, they found that males produced more estradiol during the not reproductive season, while females produced more estradiol during the reproductive season. High estradiol levels in males before or during the reproductive season were explained by the importance of this hormone in spermatogenesis (Garcia et al., 2005). During the not reproductive season (spring and summer), estradiol can be associated with antlers production, development, and growth. Bubenik et al. (1997) observed that antlers growth occurred when blood testosterone concentrations were minimal, but the hormone rapidly increased during the phase of antlers mineralization, reaching a maximum a bit before the reproductive season. Therefore, estradiol was the most critical hormone for antlers growth and maturation. Although the metabolic pathway is not precisely known, estradiol is believed to be produced from testosterone aromatization (Bubenik et al., 2005), that is why testosterone levels in feces were reduced.
The values of cortisol and testosterone levels in this study were low, even though inside the ranges reported in various studies performed in white-tailed deer and other deer species (Pelletier et al., 2003; Chunwang et al., 2004; McCoy & Ditchkoff, 2012; López & Montes, 2016). This could have occurred by the microbial activity in feces that metabolizes steroid hormones to metabolites, with which there were no crossed reaction with antibodies used in this study. For this work, feces were collected fresh, but it took a few hours to transfer them into freezing conditions. Millspaugh & Washburn (2004) commented that hormonal metabolites might change in short periods of time, especially when they get hot, frozen, or defrosted. When Li et al. (2001) obtained low fecal estradiol levels in their work, they attributed this result to the sensitivity of the technic used or to the fact that the hormone had been metabolized in feces to another steroid that did not present crossed reaction with the antibody they used.
Conclusion
Reproductive hormones, testosterone, and estradiol rose during the reproductive season (autumn-winter) and when photoperiod decreased. Fecal cortisol increased in winter due to the reproductive season and activities related to this season as aggressiveness among males, low temperatures, as well as low quantity and quality of available food. Fecal cortisol was higher in the free-living population, indicating that populations in natural enclosure were not always the cause of high levels of stress when management conditions were adequate. Under a correct management and monitoring, natural enclosures may be a good strategy for white-tailed deer management and reproduction, without causing deleterious effects in these populations. Finally, there were various factors as dietary composition, intra- and inter-species social relationships and human activities which fostered the rise in cortisol levels in the free-living population that should be studied.











 text in
text in 




